Pseudo-Eutectic of Isodimorphism to Design Biaxially-Oriented Bio-Based PA56/512 with High Strength, Toughness and Barrier Performances
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Copolymer PA56/512
2.3. Preparation of BOPA-56/512
3. Results and Discussion
3.1. Chemical Structural Characterization of PA56/512
3.2. Effect of Bidirectional Stretching on Thermal Properties of PA56/512
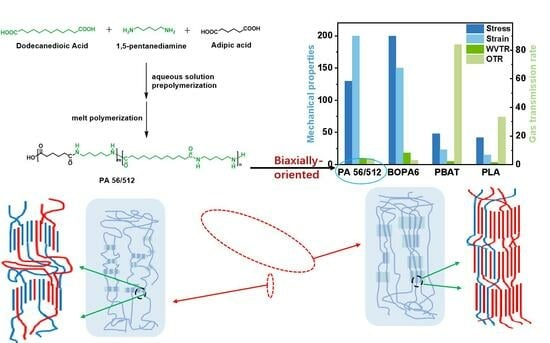
3.3. Effect of Biaxial Stretching on Mechanical and Barrier Properties of PA56/512
3.4. Effect of Biaxial Stretching on Crystal Structure of PA56/512
3.5. Effect of Biaxial Stretching on Crystal Morphology of PA56/512
3.6. Molecular Mechanism Explanation of PA56/512 with High Strength, Toughness and Barrier Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lau, W.J.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Chen, J.P.; Ismail, A.F. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef]
- Marchildon, K. Polyamides—Still Strong After Seventy Years. Macromol. React. Eng. 2011, 5, 22–54. [Google Scholar] [CrossRef]
- Zhang, X.S.; Buzinkai, J.; Quinn, E.; Rhoades, A. Key Insights into the Differences between Bimodal Crystallization Kinetics of Polyamide 66 and Polyamide 6. Macromolecules 2022, 55, 9220–9231. [Google Scholar] [CrossRef]
- Kind, S.; Wittmann, C. Bio-based production of the platform chemical 1,5-diaminopentane. Appl. Microbiol. Biotechnol. 2011, 91, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Buschke, N.; Becker, J.; Schafer, R.; Kiefer, P.; Biedendieck, R.; Wittmann, C. Systems metabolic engineering of xylose-utilizing Corynebacterium glutamicum for production of 1,5-diaminopentane. Biotechnol. J. 2013, 8, 557–570. [Google Scholar] [CrossRef]
- Buschke, N.; Schroder, H.; Wittmann, C. Metabolic engineering of Corynebacterium glutamicum for production of 1,5-diaminopentane from hemicellulose. Biotechnol. J. 2011, 6, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kang, H.L.; Guo, Y.F.; Liu, R.G.; Hao, X.M.; Qiao, R.R.; Yan, J.L. The structures and properties of bio-based polyamide 56 fibers prepared by high-speed spinning. J. Appl. Polym. Sci. 2020, 137, 49344. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Ma, J.H. Study on the unsaturated hydrogen bond behavior of bio-based polyamide 56. E-Polymers 2019, 19, 23–31. [Google Scholar] [CrossRef]
- Yan, Y.R.; Gooneie, A.; Ye, H.X.; Deng, L.L.; Qiu, Z.M.; Reifler, F.A.; Hufenus, R. Morphology and Crystallization of Biobased Polyamide 56 Blended with Polyethylene Terephthalate. Macromol. Mater. Eng. 2018, 303, 1800214. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wang, Y.; Xu, Y.H.; Liu, X.C.; Guo, W.H. Modification of biobased polyamide 56 to achieve ultra-toughening. Polym.-Plast. Technol. Mater. 2021, 60, 1585–1604. [Google Scholar] [CrossRef]
- Xu, Y.H.; Zhao, C.Y.; Guo, Z.Y.; Dong, W.J.; Liu, X.C.; Guo, W.H. EPDM-g-MAH toughened bio-based polyamide 56 to prepare thermoplastic polyamide elastomer and the performance characterization. J. Appl. Polym. Sci. 2022, 139, 52346. [Google Scholar] [CrossRef]
- Ai, T.H.; Zou, G.J.; Feng, W.T.; Ren, Z.L.; Li, F.; Wang, P.L.; Lu, B.; Ji, J.H. Synthesis and properties of biobased copolyamides based on polyamide 10T and polyamide 56 through one-pot polymerization. New J. Chem. 2021, 45, 14677–14686. [Google Scholar] [CrossRef]
- Yang, K.J.; Liu, Y.L.; Zheng, Z.K.; Tang, Z.B.; Chen, X.D. Controlled polymerization and side reaction mechanism of bio-sourced pentanediamine-derived semi-aromatic copolyamides. Polym. Chem. 2023, 14, 2390–2404. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, P.J. Crystallization of biodegradable and biobased polyesters: Polymorphism, cocrystallization, and structure-property relationship. Prog. Polym. Sci. 2020, 109, 101291. [Google Scholar] [CrossRef]
- Pan, P.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 2009, 34, 605–640. [Google Scholar] [CrossRef]
- Yu, Y.; Sang, L.; Wei, Z.Y.; Leng, X.F.; Li, Y. Unique isodimorphism and isomorphism behaviors of even-odd poly(hexamethylene dicarboxylate) aliphatic copolyesters. Polymer 2017, 115, 106–117. [Google Scholar] [CrossRef]
- Saotome, K.; Komoto, H. Isomorphism in copolyamides of long repeating chain units containing oxa- and thia-alkylene linkages. J. Polym. Sci. Part A-1-Polym. Chem. 1966, 4, 1475–1486. [Google Scholar] [CrossRef]
- Novitsky, T.F.; Lange, C.A.; Mathias, L.J.; Osborn, S.; Ayotte, R.; Manning, S. Eutectic melting behavior of polyamide 10,T-co-6,T and 12,T-co-6,T copolyterephthalamides. Polymer 2010, 51, 2417–2425. [Google Scholar] [CrossRef]
- Natta, G.; Sianesi, D.; Corradini, P. Isomorphism phenomena in macromolecules. J. Polym. Sci. 1961, 51, 527–539. [Google Scholar] [CrossRef]
- Perez-Camargo, R.A.; Arandia, I.; Safari, M.; Cavallo, D.; Lotti, N.; Soccio, M.; Muller, A.J. Crystallization of isodimorphic aliphatic random copolyesters: Pseudo-eutectic behavior and double-crystalline materials. Eur. Polym. J. 2018, 101, 233–247. [Google Scholar] [CrossRef]
- Gan, D.S.; Liu, Y.J.; Hu, T.H.; Fan, S.H.; Liu, X.C.; Cui, L.N.; Yang, L.; Wu, Y.C.; Chen, L.; Mo, Z.X. The Investigation of Copolymer Composition Sequence on Non-Isothermal Crystallization Kinetics of Bio-Based Polyamide 56/512. Polymers 2023, 15, 2345. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kang, H.L.; Wang, R.; Liu, R.G.; Hao, X.M. Crystallization of polyamide 56/polyamide 66 blends: Non-isothermal crystallization kinetics. J. Appl. Polym. Sci. 2018, 135, 46409. [Google Scholar] [CrossRef]
- Han, C.C.; Zhang, X.H.; Chen, D.; Ma, Y.H.; Zhao, C.W.; Yang, W.T. Enhanced dielectric properties of sandwich-structured biaxially oriented polypropylene by grafting hyper-branched aromatic polyamide as surface layers. J. Appl. Polym. Sci. 2020, 137, 48990. [Google Scholar] [CrossRef]
- Mrkic, S.; Galic, K.; Ivankovic, M. Effect of temperature and mechanical stress on barrier properties of polymeric films used for food packaging. J. Plast. Film Sheeting 2007, 23, 239–256. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, S.; Gao, D.; Lv, Y.; Xu, K.; Liu, J. The influence of additives on the properties of biaxial oriented polyamide film. Sci. Sin. Chim. 2014, 44, 1755–1761. [Google Scholar]
- Liu, L.C.; Lai, C.C.; Lu, M.T.; Wu, C.H.; Chen, C.M. Manufacture of biaxially-oriented polyamide 6 (BOPA6) films with high transparencies, mechanical performances, thermal resistance, and gas blocking capabilities. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2020, 259, 114605. [Google Scholar] [CrossRef]
- Lin, F.L.; Liu, Y.J.; Song, L.J.; Hao, X.H.; Liu, X.C.; Fan, S.H.; Wu, Y.C.; Mao, L. Preparation of biaxially oriented polyamide 6/polyketone/graphene oxide films with enhanced barrier and mechanical behaviors. J. Appl. Polym. Sci. 2021, 138, 50501. [Google Scholar] [CrossRef]
- Shakouri, S.; Ziaolhagh, H.R.; Sharifi-Rad, J.; Heydari-Majd, M.; Tajali, R.; Nezarat, S.; da Silva, J.A.T. The effect of packaging material and storage period on microwave-dried potato (Solanum tuberosum L.) cubes. J. Food Sci. Technol.-Mysore 2015, 52, 3899–3910. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.A.; Lee, Y.H.; Chiu, A.C.; Tsui, T.A.; Lin, K.J.; Chen, K.L.; Liu, M.W. Strengthening interfaces between biaxial oriented PET and PSMA: Effects of nitrogen plasma and bonding treatments. Polymer 2006, 47, 8583–8594. [Google Scholar] [CrossRef]
- Yuan, W.H.; Zhou, J.; Liu, K.K.; Li, X.; Xu, W.Q.; Song, H.J.; Shan, G.R.; Bao, Y.Z.; Zhao, Q.; Pan, P.J. Sequence-Rearranged Cocrystalline Polymer Network with Shape Reconfigurability and Tunable Switching Temperature. ACS Macro Lett. 2020, 9, 588–594. [Google Scholar] [CrossRef]
- Yu, Y.; Wei, Z.Y.; Zhou, C.; Zheng, L.C.; Leng, X.F.; Li, Y. Miscibility and competition of cocrystallization behavior of poly (hexamethylene dicarboxylate)s aliphatic copolyesters: Effect of chain length of aliphatic diacids. Eur. Polym. J. 2017, 92, 71–85. [Google Scholar] [CrossRef]
- Pepin, J.; Miri, V.; Lefebvre, J.M. New Insights into the Brill Transition in Polyamide 11 and Polyamide 6. Macromolecules 2016, 49, 564–573. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, P.; Qian, C.A.; Zhao, Y.; Wang, L.; Wang, D.J.; Dong, X. The Brill Transition in Long-Chain Aliphatic Polyamide 1012: The Role of Hydrogen-Bonding Organization. Macromolecules 2021, 54, 6835–6844. [Google Scholar] [CrossRef]
- Lotz, B. Brill Transition in Nylons: The Structural Scenario(#). Macromolecules 2021, 54, 565–583. [Google Scholar]
- Sangroniz, L.; Cavallo, D.; Muller, A.J. Self-Nucleation Effects on Polymer Crystallization. Macromolecules 2020, 53, 4581–4604. [Google Scholar] [CrossRef]
- Liu, X.R.; Wang, Y.; Wang, Z.F.; Cavallo, D.; Muller, A.J.; Zhu, P.; Zhao, Y.; Dong, X.; Wang, D.J. The origin of memory effects in the crystallization of polyamides: Role of hydrogen bonding. Polymer 2020, 188, 122117. [Google Scholar] [CrossRef]
- Damari, S.P.; Cullari, L.; Nadiv, R.; Nir, Y.; Laredo, D.; Grunlan, J.; Regev, O. Graphene-induced enhancement of water vapor barrier in polymer nanocomposites. Compos. Part B-Eng. 2018, 134, 218–224. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Y.; Shan, G.R.; Bao, Y.Z.; Wang, W.J.; Pan, P.J. Stretch-Induced α-to-β Crystal Transition and Lamellae Structural Evolution of Poly(butylene adipate-ran-terephthalate) Aliphatic-Aromatic Copolyester. Macromolecules 2019, 52, 1334–1347. [Google Scholar] [CrossRef]
- Morales-Gamez, L.; Soto, D.; Franco, L.; Puiggali, J. Brill transition and melt crystallization of nylon 56 An odd-even polyamide with two hydrogen-bonding directions. Polymer 2010, 51, 5788–5798. [Google Scholar] [CrossRef]
- Kang, H.L.; Wang, Z.; Hao, X.M.; Liu, R.G. Thermal induced crystalline transition of bio-based polyamide 56. Polymer 2022, 242, 124540. [Google Scholar] [CrossRef]
- Hu, W.B. The physics of polymer chain-folding. Phys. Rep.-Rev. Sect. Phys. Lett. 2018, 747, 1–50. [Google Scholar] [CrossRef]
- Nie, Y.J.; Gao, H.H.; Yu, M.H.; Hu, Z.M.; Reiter, G.; Hu, W.B. Competition of crystal nucleation to fabricate the oriented semi-crystalline polymers. Polymer 2013, 54, 3402–3407. [Google Scholar] [CrossRef]
- An, M.F.; Zhang, Q.L.; Lin, Y.F.; Wang, D.L.; Chen, W.; Meng, L.P.; Yin, P.C.; Li, L.B. Stretch-Induced Reverse Brill Transition in Polyamide 46. Macromolecules 2020, 53, 11153–11165. [Google Scholar] [CrossRef]
- Cui, X.W.; Li, W.H.; Yan, D.Y. A study of the crystalline transitions of polyamides X 18. Polym. Int. 2004, 53, 2031–2037. [Google Scholar] [CrossRef]
- Jasinska-Walc, L.; Villani, M.; Dudenko, D.; van Asselen, O.; Klop, E.; Rastogi, S.; Hansen, M.R.; Koning, C.E. Local Conformation and Cocrystallization Phenomena in Renewable Diaminoisoidide-Based Polyamides Studied by FT-IR, Solid State NMR, and WAXD. Macromolecules 2012, 45, 2796–2808. [Google Scholar] [CrossRef]
- Yan, D.Y.; Li, Y.J.; Zhu, X.Y. Brill transition in Nylon 10 12 investigated by variable temperature XRD and real time FT-IR. Macromol. Rapid Commun. 2000, 21, 1040–1043. [Google Scholar] [CrossRef]
- Nair, S.S.; Ramesh, C.; Tashiro, K. Crystalline phases in nylon-11: Studies using HTWAXS and HTFTIR. Macromolecules 2006, 39, 2841–2848. [Google Scholar] [CrossRef]
- Deshmukh, Y.S.; Wilsens, C.; Verhoef, R.; Hansen, M.R.; Dudenko, D.; Graf, R.; Klop, E.A.; Rastogi, S. Conformational and Structural Changes with Increasing Methylene Segment Length in Aromatic-Aliphatic Polyamides. Macromolecules 2016, 49, 950–962. [Google Scholar] [CrossRef]
- Puiggali, J.; Franco, L.; Aleman, C.; Subirana, J.A. Crystal structures of nylon 5,6. A model with two hydrogen bond directions for nylons derived from odd diamines. Macromolecules 1998, 31, 8540–8548. [Google Scholar] [CrossRef]
- Olmo, C.; Casas, M.T.; Martinez, J.C.; Franco, L.; Puiggali, J. Thermally Induced Structural Transitions of Nylon 4 9 as a New Example of Even-Odd Polyamides. Polymers 2018, 10, 198. [Google Scholar] [CrossRef]
- Hu, W.B.; Frenkel, D.; Mathot, V.B.F. Intramolecular nucleation model for polymer crystallization. Macromolecules 2003, 36, 8178–8183. [Google Scholar] [CrossRef]
- Al-Hussein, M.; Strobl, G. Strain-controlled tensile deformation behavior of isotactic poly(1-butene) and its ethylene copolymers. Macromolecules 2002, 35, 8515–8520. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.Q.; Zhang, Z.J.; Huang, S.Y.; Chen, Q.; Colby, R.H. Shear-Induced Oriented Crystallization for Isotactic Poly(1-butene) and Its Copolymer with Ethylene. Macromolecules 2020, 53, 3071–3081. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, D.; Liu, Y.; Hu, T.; Fan, S.; Cui, L.; Liao, G.; Xie, Z.; Zhu, X.; Yang, K. Pseudo-Eutectic of Isodimorphism to Design Biaxially-Oriented Bio-Based PA56/512 with High Strength, Toughness and Barrier Performances. Polymers 2024, 16, 1176. https://doi.org/10.3390/polym16081176
Gan D, Liu Y, Hu T, Fan S, Cui L, Liao G, Xie Z, Zhu X, Yang K. Pseudo-Eutectic of Isodimorphism to Design Biaxially-Oriented Bio-Based PA56/512 with High Strength, Toughness and Barrier Performances. Polymers. 2024; 16(8):1176. https://doi.org/10.3390/polym16081176
Chicago/Turabian StyleGan, Diansong, Yuejun Liu, Tianhui Hu, Shuhong Fan, Lingna Cui, Guangkai Liao, Zhenyan Xie, Xiaoyu Zhu, and Kejian Yang. 2024. "Pseudo-Eutectic of Isodimorphism to Design Biaxially-Oriented Bio-Based PA56/512 with High Strength, Toughness and Barrier Performances" Polymers 16, no. 8: 1176. https://doi.org/10.3390/polym16081176