Optimised Degradation of Lignocelluloses by Edible Filamentous Fungi for the Efficient Biorefinery of Sugar Beet Pulp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sugar Beet Pulp
2.2. Fungal Strains
2.3. Fungal Inoculum Preparation
2.4. Hydrolytic Activity Evaluation
2.5. Shake-Flask Experiments
2.6. Enzyme Activity Determination
2.6.1. Cellulase Activity Determination
2.6.2. Endoglucanase Activity Determination
2.6.3. β-Glucosidase Activity Determination
2.7. Chromatographic Quantification of Monosaccharides
2.8. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Selection of Potential Fungal Strains for Sugar Beet Pulp Degradation
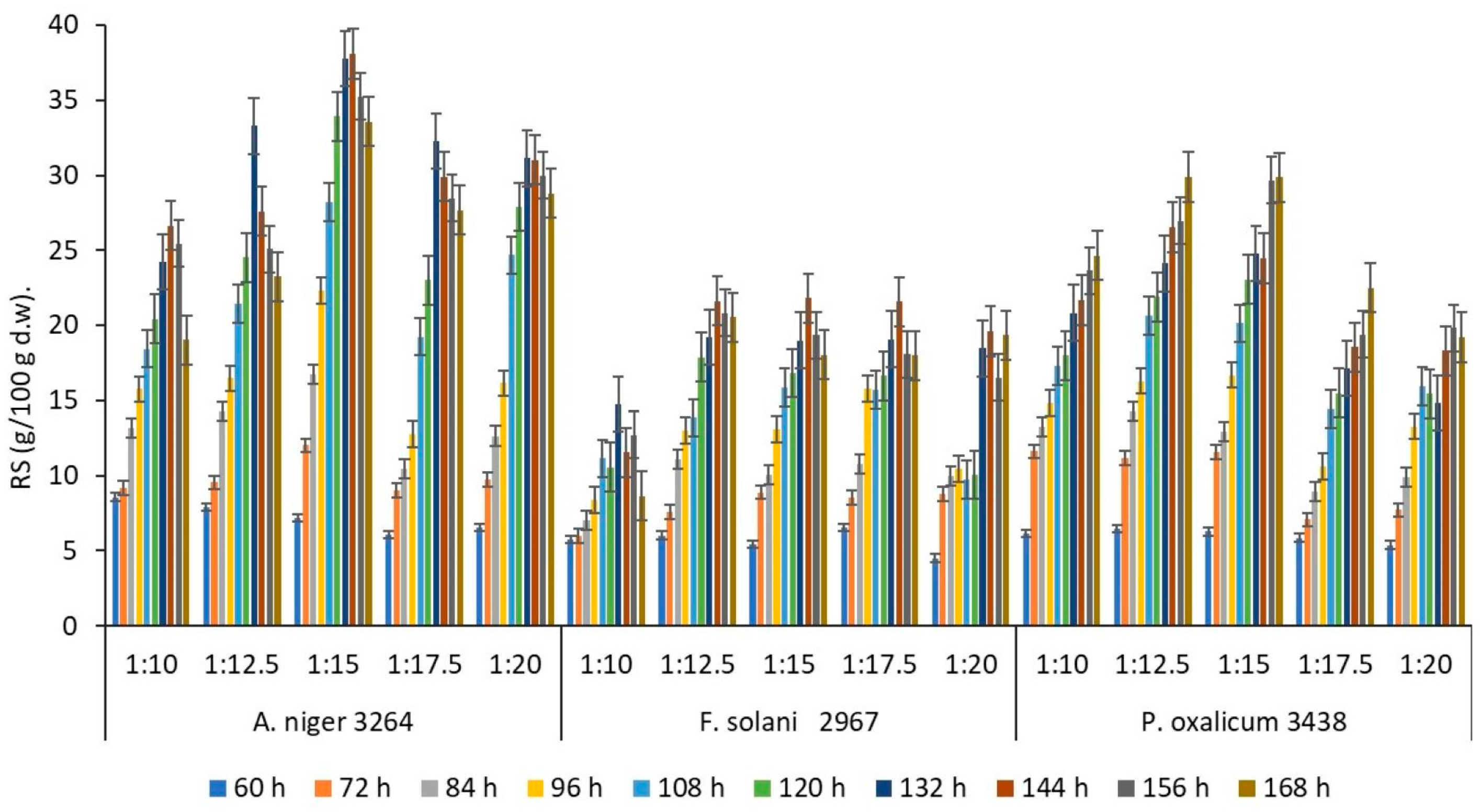
3.2. One-Factor Experiments
3.3. The Optimisation of Degradation Processing and Mathematical Model Analysis
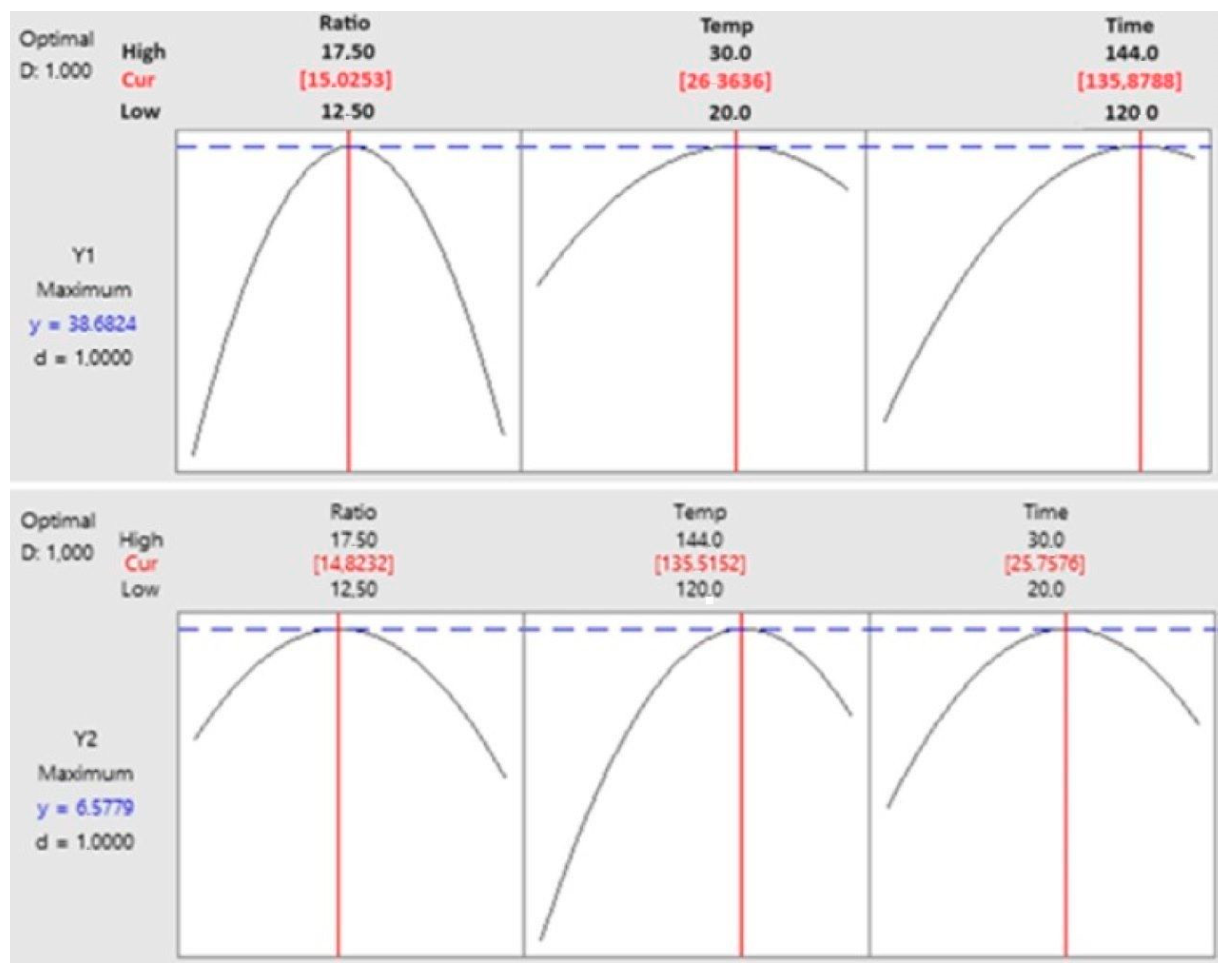
3.4. Optimisation and Prediction of Process Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cárdenas-Fernández, M.; Bawn, M.; Hamley-Bennett, C.; Bharat, P.K.V.; Subrizi, F.; Suhaili, N.; Ward, D.P.; Bourdin, S.; Dalby, P.A.; Hailes, H.C.; et al. An integrated biorefinery concept for conversion of sugar beet pulp into value-added chemicals and pharmaceutical intermediates. Faraday Discuss. 2017, 202, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Muir, B.M.; Anderson, A.R. Development and diversification of sugar beet in Europe. Sugar Tech 2022, 24, 992–1009. [Google Scholar] [CrossRef]
- Dygas, D.; Kręgiel, D.; Berłowska, J. Sugar beet pulp as a biorefinery substrate for designing feed. Molecules 2023, 28, 2064. [Google Scholar] [CrossRef] [PubMed]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Z.; Sharma, M.; Diwan, D.; Tripathi, M.; Whale, E.; Jayakody, L.N.; Moreau, B.; Thakur, V.K.; Tuohy, M.; Gupta, V.K. Valorization of Sugar Beet Pulp to Value-Added Products: A Review. Bioresour. Technol. 2022, 346, 126580. [Google Scholar] [CrossRef]
- Joanna, B.; Michal, B.; Piotr, D.; Agnieszka, W.; Dorota, K.; Izabela, W. Sugar beet pulp as a source of valuable biotechnological products. In Advances in Biotechnology for Food Industry; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: London, UK, 2018; pp. 359–392. [Google Scholar]
- Glaser, S.J.; Abdelaziz, O.Y.; Demoitié, C.; Galbe, M.; Pyo, S.-H.; Hati-Kaul, R. Fractionation of sugar beet pulp polysaccharides into component sugars and pre-feasibility analysis for further valorisation. Biomass. Conv. Bioref. 2022, 14, 3575–3588. [Google Scholar] [CrossRef]
- Leijdekkers, A.G.; Bink, J.P.; Geutjes, S.; Schols, H.A.; Gruppen, H. Enzymatic saccharification of sugar beet pulp for the production of galacturonic acid and arabinose; a study on the impact of the formation of recalcitrant oligosaccharides. Bioresour. Technol. 2013, 12, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Bibra, M.; Samanta, D.; Sharma, N.K.; Singh, G.; Johnson, G.R.; Sani, R.K. Food waste to bioethanol: Opportunities and challenges. Fermentation 2023, 9, 8. [Google Scholar] [CrossRef]
- Berlowska, J.; Cieciura-Wloch, W.; Kalinowska, H.; Kregiel, D.; Borowski, S.; Pawlikowska, E.; Binczarski, M.; Witonska, I. Enzymatic conversion of sugar beet pulp: A comparison of simultaneous saccharification and fermentation and separate hydrolysis and fermentation for lactic acid production. Food Technol. Biotechnol. 2018, 56, 188. [Google Scholar] [CrossRef] [PubMed]
- Cavalaglio, G.; Gelosia, M.; D’Antonio, S.; Nicolini, A.; Pisello, A.L.; Barbanera, M.; Cotana, F. Lignocellulosic ethanol production from the recovery of stranded driftwood residues. Energies 2016, 9, 634. [Google Scholar] [CrossRef]
- Li, C.; Lin, F.; Zhou, L.; Qin, L.; Li, B.; Zhou, Z.; Jin, M.; Chen, Z. Cellulase hyper-production by Trichoderma reesei mutant SEU-7 on lactose. Biotechnol. Biofuels 2017, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Gabelle, J.C.; Jourdier, E.; Licht, R.B.; Ben Chaabane, F.; Henaut, I.; Morchain, J.; Augier, F. Impact of rheology on the mass transfer coefficient during the growth phase of Trichoderma reesei in stirred bioreactors. Chem. Eng. Sci. 2012, 75, 408–417. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Effect of xylan and lignin removal by batch and flowthrough pretreatment on the enzymatic digestibility of corn stover cellulose. Biotechnol. Bioeng. 2004, 86, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Goodell, B. Current understanding of brown-rot fungal biodegradation mechanisms: A review. In Deterioration and Protection of Sustainable Biomaterials; Schultz, T.P., Goodell, B., Nicholas, D.D., Eds.; American Chemical Society: Washington, DC, USA, 2014; pp. 4–21. [Google Scholar]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—A review. Renew. Sust. Energ. Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, H.; Sarkar, B. Effect of substrate and fermentation conditions on pectinase and cellulase production by Aspergillus niger NCIM 548 in submerged (SmF) and solid state fermentation (SSF). Food Sci. Biotechnol. 2011, 20, 1289–1298. [Google Scholar] [CrossRef]
- Marzo, C.; Díaz, A.B.; Caro, I.; Blandino, A. Conversion of exhausted sugar beet pulp into fermentable sugars from a biorefinery approach. Foods 2020, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Aberkane, A.; Cuenca-Estrella, M.; Gomez-Lopez, A.; Petrikkou, E.; Mellado, E.; Monzón, A.; Rodriguez-Tudela, J.L. Comparative evaluation of two different methods of inoculum preparation for antifungal susceptibility testing of filamentous fungi. J. Antimi. Chemother. 2002, 50, 719–722. [Google Scholar] [CrossRef]
- Maki, M.L.; Idrees, A.; Leung, K.T.; Qin, W. Newly isolated and characterized bacteria with great application potential for decomposition of lignocellulosic biomass. J. Mol. Microbiol. Biotechnol. 2012, 22, 156–166. [Google Scholar] [CrossRef]
- Namnuch, N.; Thammasittirong, A.; Thammasittirong, S.N. Lignocellulose hydrolytic enzymes production by Aspergillus flavus KUB2 using submerged fermentation of sugarcane bagasse waste. Mycology 2020, 12, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, J.M.; Werle, L.B.; Foletto, E.L.; Kuhn, R.C.; Jahn, S.L.; Mazutti, M.A. Production of cellulolytic enzymes and application of crude enzymatic extract for saccharification of lignocellulosic biomass. Appl. Biochem. Biotechnol. 2015, 175, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Verchot, L.V.; Borelli, T. Application of para-nitrophenol (pNP) enzyme assays in degraded tropical soils. Soil Biol. Biochem. 2005, 37, 625–633. [Google Scholar] [CrossRef]
- David, W.W.; Stout, T.R. Disc plate method of microbiological antibiotic assay. I. Factors influencing variability and error. Appl. Microbiol. 1971, 22, 659–665. [Google Scholar]
- Akintunde, O.; Chukwudozie, C. Hydrolytic and inhibitory activity of two closely related Bacillus isolates. J. Appl. Environ. Microbiol. 2021, 9, 5–8. [Google Scholar] [CrossRef]
- Vaithanomsat, P.; Songpim, M.; Malapant, T.; Kosugi, A.; Thanapase, W.; Mori, Y. Production of β-glucosidase from a newly isolated Aspergillus species using response surface methodology. Int. J. Microbiol. 2011, 2011, 949252. [Google Scholar] [CrossRef] [PubMed]
- Mrudula, S.; Murugamnal, R. Production of cellulase by Aspergillus niger under submerged and solid state fermentation using Coir waste as a substrate. Braz. J. Microbiol. 2011, 42, 1119–1127. [Google Scholar] [CrossRef]
- Moran-Aguilar, M.G.; Costa-Trigo, I.; Calderón-Santoyo, M.; Domínguez, J.M.; Aguilar-Uscanga, M.G. Production of cellulases and xylanases in solid-state fermentation by different strains of Aspergillus niger using sugarcane bagasse and brewery spent grain. Biochem. Eng. J. 2021, 172, 108060. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, A.; Dutt, D. Co-Cultivation of Penicillium sp. AKB-24 and Aspergillus nidulans AKB-25 as a cost-effective method to produce cellulases for the hydrolysis of pearl millet stover. Fermentation 2016, 2, 12. [Google Scholar] [CrossRef]
- de Oliveira Simões, L.C.; da Silva, R.R.; de Oliveira Nascimento, C.E.; Boscolo, M.; Gomes, E.; da Silva, R. Purification and physicochemical characterization of a novel thermostable xylanase secreted by the fungus Myceliophthora heterothallica F.2.1.4. Appl. Biochem. Biotechnol. 2019, 188, 991–1008. [Google Scholar] [CrossRef] [PubMed]
- Leite, R.S.R.; Alves-Prado, H.F.; Cabral, H.; Pagnocca, F.C.; Gomes, E.; Da-Silva, R. Production and characteristics comparison of crude β-glucosidases produced by microorganisms Thermoascus aurantiacus e Aureobasidium pullulans in agricultural wastes. Enzyme Microb. Technol. 2008, 43, 391–395. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Rajasree, K.P.; Mathew, G.M.; Pandey, A.; Sukumaran, R.K. Highly glucose tolerant β-glucosidase from Aspergillus unguis NII08123 for enhanced hydrolysis of biomass. J. Ind. Microbiol. Biotechnol. 2013, 40, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Knesebeck, M.; Schäfer, D.; Schmitz, K.; Rüllke, M.; Benz, J.P.; Weuster-Botz, D. Enzymatic one-pot hydrolysis of extracted sugar beet press pulp after solid-state fermentation with an engineered Aspergillus niger strain. Fermentation 2023, 9, 582. [Google Scholar] [CrossRef]
- Rezić, T.; Oros, D.; Marković, I.; Kracher, D.; Ludwig, R.; Šantek, B. Integrated hydrolyzation and fermentation of sugar beet pulp to bioethanol. J. Microbiol. Biotechnol. 2013, 23, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Sandri, I.G.; Lorenzoni, C.M.T.; Fontana, R.C.; da Silveira, M.M. Use of pectinases produced by a new strain of Aspergillus niger for the enzymatic treatment of apple and blueberry juice. LWT 2013, 51, 469–475. [Google Scholar] [CrossRef]
- Park, H.-C.; Kim, Y.-J.; Lee, C.-W.; Rho, Y.-T.; Kang, J.W.; Lee, D.-H.; Seong, Y.-J.; Park, Y.-C.; Lee, D.; Kim, S.-G. Production of d-ribose by metabolically engineered Escherichia coli. Process Biochem. 2017, 52, 73–77. [Google Scholar] [CrossRef]
- Metreveli, E.; Khardziani, T.; Elisashvili, V. The carbon source controls the secretion and yield of polysaccharide-hydrolyzing enzymes of basidiomycetes. Biomolecules 2021, 11, 1341. [Google Scholar] [CrossRef]
- Passamani, F.R.F.; Hernandes, T.; Alves Lopes, N.; Carvalho Bastos, S.; Douglas Santiago, W.; Das Graças Cardoso, M.; Batist, L.R. Effect of temperature, water activity, and pH on growth and production of ochratoxin a by Aspergillus niger and Aspergillus carbonarius from Brazilian grapes. J. Food Prot. 2014, 77, 1947–1952. [Google Scholar] [CrossRef]
- Hui, Y.; Berlin Nelson, J.R. Effect of temperature on Fusarium solani and F. tricinctum growth and disease development in soybean. Can. J. Plant Pathol. 2020, 42, 527–537. [Google Scholar]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar]
- de Vries, R.P.; Patyshakuliyeva, A.; Garrigues, S.; Agarwal-Jans, S. The current biotechnological status and potential of plant and algal biomass degrading/modifying enzymes from ascomycete fungi. In Grand Challenges in Fungal Biotechnology; Nevalainen, H., Ed.; Springer: Cham, Switzerland, 2020; pp. 81–120. [Google Scholar]
- van den Brink, J.; de Vries, R.P. Fungal enzyme sets for plant polysaccharide degradation. Appl. Microbiol. Biotechnol. 2011, 91, 1477–1492. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Møller, L.L.; Larsen, T.O.; Kumar, R.; Arnau, J. Safety of the fungal workhorses of industrial biotechnology: Update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei. Appl. Microbiol. Biotechnol. 2018, 102, 9481–9515. [Google Scholar] [CrossRef]
- Cubells, S.G.; Kun, R.S.; Peng, M.; Bauer, D.; Keymanesh, K.; Lipzen, A.; Ng, V.; Grigoriev, I.V.; de Vries, R.P. Unraveling the regulation of sugar beet pulp utilization in the industrially relevant fungus Aspergillus niger. iScience 2022, 25, 104065. [Google Scholar]
- Gönen, Ç.; Akter Önal, N.; Deveci, E.Ü. Optimization of sugar beet pulp pre-treatment with weak and strong acid under pressure and non-pressure conditions via RSM. Biomass Conv. Bioref. 2023, 13, 9213–9226. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Methods 920.152, 942.05, 942.20, 2002.02, 985.29, 991.43, 996.11; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2018. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure (LAP). 2004. Available online: https://api.semanticscholar.org/CorpusID:100361490 (accessed on 5 April 2024).
- Bouaziz, F.; Koubaa, M.; Ben Jeddou, K.; Kallel, F.; Helbert, C.B.; Khelfa, A.; Ghorbel, R.E.; Chaabouni, S.E. Water-soluble polysaccharides and hemicelluloses from almond gum: Functional and prebiotic properties. Int. J. Biol. Macromol. 2016, 93 Pt A, 359–368. [Google Scholar] [CrossRef]

| Components | SBP |
|---|---|
| Protein | 5.37 ± 0.54 |
| Fat | 0.74 ± 0.01 |
| Sugars | 6.98 ± 0.31 |
| SDF | 18.64 ± 1.22 |
| IDF | 58.65 ± 1.14 |
| Lignin * | 3.61 ± 0.35 |
| Ash | 6.01 ± 0.12 |
| TCM | 1.08 × 106 |
| Variable | Symbol | Coding Level | ||
|---|---|---|---|---|
| –1 | 0 | +1 | ||
| Solid/water ratio (s/w) | X1 | 12.5 | 15 | 17.5 |
| Temperature (T, °C) | X2 | 20 | 25 | 30 |
| Time (t, h) | X3 | 120 | 132 | 144 |
| Fungal Strain | Clear Zone Diameter, mm | Colony Diameter, mm | RHA |
|---|---|---|---|
| Botrytis cinerea CCF 2361 | 61.84 ± 2.61 | 53.71 ± 1.89 | 1.15 |
| Aspergillus nidulants CCF 2912 | 31.71 ± 0.93 | 26.34 ± 0.89 | 1.20 |
| Aspergillus niger CCF 3264 | 42.78 ± 1.07 | 33.17 ± 1.07 | 1.29 |
| Fusarium avenaceum CCF 3306 | 71.68 ± 1.84 | 62.81 ± 0.83 | 1.14 |
| Fusarium solani CCF 2967 | 43.20 ± 5.23 | 37.43 ± 4.16 | 1.16 |
| Fusarium oxysporum CCF 1389 | 44.88 ± 2.63 | 39.13 ± 1.94 | 1.15 |
| Fusarium graminearum CCF 1626 | 54.33 ± 3.06 | 47.33 ± 1.08 | 1.15 |
| Penicillium oxalicum CCF 3438 | 29.47 ± 1.53 | 22.67 ± 0.79 | 1.30 |
| Rhizoctonia solani CCF 1360 | 43.17 ± 2.06 | 41.24 ± 1.79 | 1.05 |
| Verticillium spp. CCF 1896 | 44.21 ± 3.17 | 41.07 ± 2.83 | 1.07 |
| Fungi | Cellulase | Endoglucanase | β-Glucosidase |
|---|---|---|---|
| A. niger CCF 3264 | 7.35 ± 0.56 a | 1.72 ± 0.31 a | 0.77 ± 1.36 a |
| B. cinerea CCF 2361 | 5.33 ± 0.41 b | 1.39 ± 0.14 b | 0.62 ± 1.76 b |
| F. solani CCF 2967 | 3.17 ± 0.27 c | 0.94 ± 0.12 c | 0.31 ± 1.95 d |
| P. oxalicum CCF 3438 | 5.31 ± 0.49 b | 0.83 ± 0.08 d | 0.46 ± 0.22 c |
| Fungi | Total RS | Fructose | Glucose | Arabinose | Xylose | Mannose |
|---|---|---|---|---|---|---|
| A. niger CCF 3264 | 25.13 ± 1.34 | 0.927 | 5.704 | 6.572 | 4.806 | 3.897 |
| B. cinerea CCF 2361 | 24.86 ± 0.87 | 1.906 | 4.633 | 5.326 | 5.649 | 4.385 |
| F. solani CCF 2967 | 21.06 ± 0.57 | 1.995 | 4.272 | 4.501 | 3.109 | 3.141 |
| P. oxalicum CCF 3438 | 24.17 ± 0.36 | 1.404 | 4.726 | 5.608 | 5.109 | 4.549 |
| Exp. No. | Process Variables | Y1 (g/100 g d.w.) | Y2 (U/g d.w.) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| X1 (s/w) | X2 (T, °C) | X3 (t, h) | Exp | Predicted | /RE/(%) | Exp | Predicted | /RE/(%) | |
| 1 | 1 | 1 | 1 | 33.18 ± 1.02 | 33.02 | 0.48 | 4.98 | 4.99 | 0.20 |
| 2 | 1 | 1 | −1 | 31.21 ± 0.86 | 31.11 | 0.32 | 4.54 | 4.54 | 0.10 |
| 3 | 1 | −1 | 1 | 27.12 ± 0.32 | 26.77 | 1.26 | 4.30 | 4.31 | 0.21 |
| 4 | 1 | −1 | −1 | 26.63 ± 0.64 | 26.62 | 0.04 | 3.49 | 3.52 | 0.80 |
| 5 | −1 | 1 | 1 | 32.33 ± 0.68 | 32.31 | 0.07 | 5.14 | 5.13 | 0.21 |
| 6 | −1 | 1 | −1 | 30.24 ± 0.37 | 30.35 | 0.37 | 3.85 | 3.86 | 0.49 |
| 7 | −1 | −1 | 1 | 28.15 ± 0.35 | 28.20 | 0.17 | 4.30 | 4.31 | 0.25 |
| 8 | −1 | −1 | −1 | 27.68 ± 1.12 | 28.00 | 1.15 | 3.64 | 3.63 | 0.11 |
| 9 | 1 | 0 | 0 | 32.58 ± 0.87 | 32.59 | 0.01 | 5.81 | 5.87 | 1.10 |
| 10 | 0 | 1 | 0 | 37.86 ± 0.57 | 38.31 | 1.18 | 6.12 | 6.20 | 1.27 |
| 11 | 0 | 0 | 1 | 36.96 ± 0.51 | 36.97 | 0.04 | 6.13 | 6.11 | 0.25 |
| 12 | −1 | 0 | 0 | 33.08 ± 0.33 | 32.92 | 0.47 | 5.91 | 5.99 | 1.40 |
| 13 | 0 | −1 | 0 | 34.26 ± 1.02 | 34.01 | 0.73 | 5.15 | 5.17 | 0.37 |
| 14 | 0 | 0 | −1 | 35.91 ± 0.28 | 35.92 | 0.01 | 5.59 | 5.65 | 1.13 |
| 15 | 0 | 0 | 0 | 38.06 ± 0.86 | 37.91 | 0.40 | 6.54 | 6.55 | 0.08 |
| 16 | 0 | 0 | 0 | 38.04 ± 1.14 | 37.91 | 0.34 | 6.56 | 6.55 | 0.14 |
| 17 | 0 | 0 | 0 | 37.96 ± 0.87 | 37.91 | 0.13 | 6.57 | 6.55 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaizauskaite, Z.; Zvirdauskiene, R.; Svazas, M.; Basinskiene, L.; Zadeike, D. Optimised Degradation of Lignocelluloses by Edible Filamentous Fungi for the Efficient Biorefinery of Sugar Beet Pulp. Polymers 2024, 16, 1178. https://doi.org/10.3390/polym16091178
Gaizauskaite Z, Zvirdauskiene R, Svazas M, Basinskiene L, Zadeike D. Optimised Degradation of Lignocelluloses by Edible Filamentous Fungi for the Efficient Biorefinery of Sugar Beet Pulp. Polymers. 2024; 16(9):1178. https://doi.org/10.3390/polym16091178
Chicago/Turabian StyleGaizauskaite, Zydrune, Renata Zvirdauskiene, Mantas Svazas, Loreta Basinskiene, and Daiva Zadeike. 2024. "Optimised Degradation of Lignocelluloses by Edible Filamentous Fungi for the Efficient Biorefinery of Sugar Beet Pulp" Polymers 16, no. 9: 1178. https://doi.org/10.3390/polym16091178