Effect of Simulated Dental Pulpal Pressure Using Fetal Bovine Serum for the Bonding Performance of Contemporary Adhesive to Dentin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Teeth Collection and Specimen Preparation
2.2. Bonding Procedures
2.3. Microtensile Bond Strength (μTBS) Test
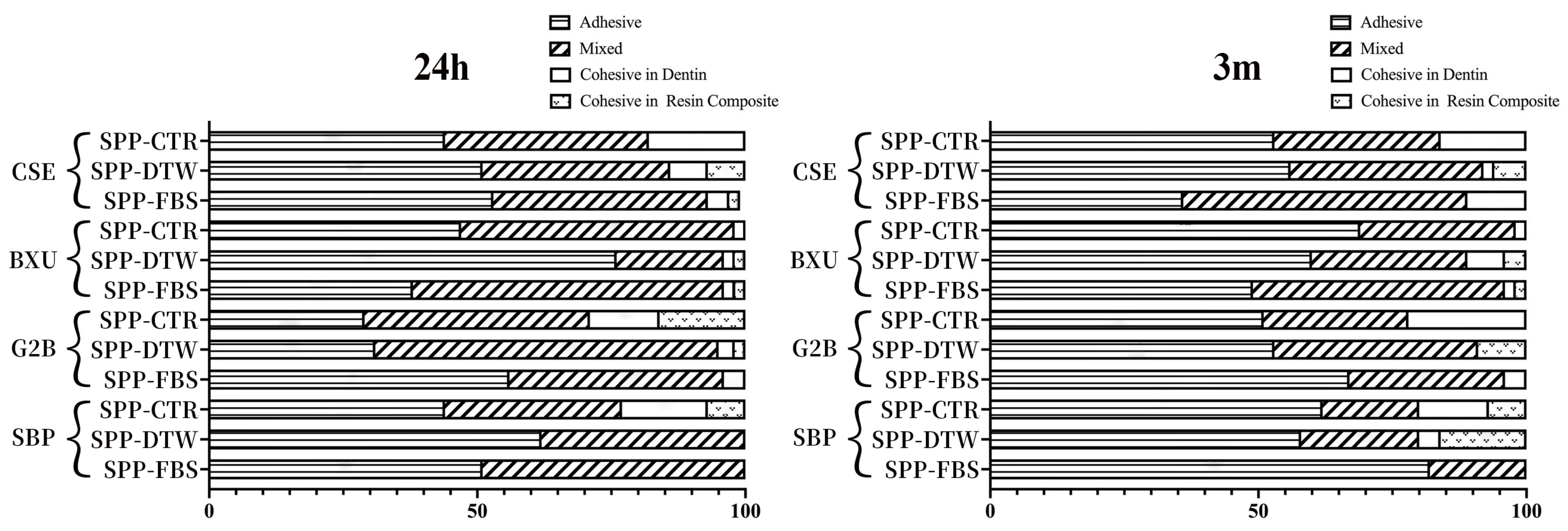
2.4. Fracture Mode Analysis
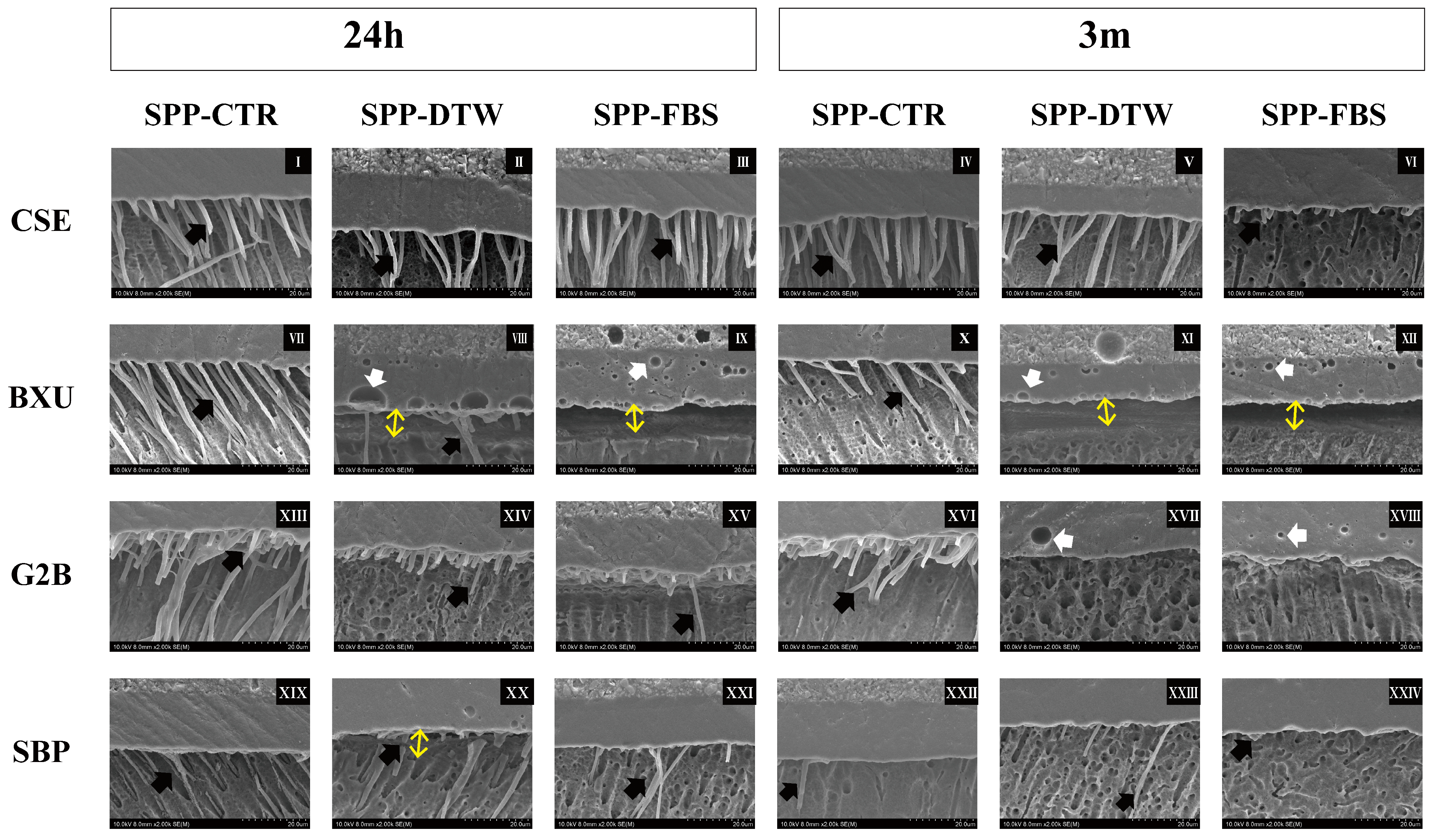
2.5. Observation of the Resin-Dentin Interface
2.6. Degree of Conversion Analysis
2.7. Statistical Analysis
3. Results
3.1. μTBS Test
3.2. Failure Mode Observation
3.3. Interface Observation
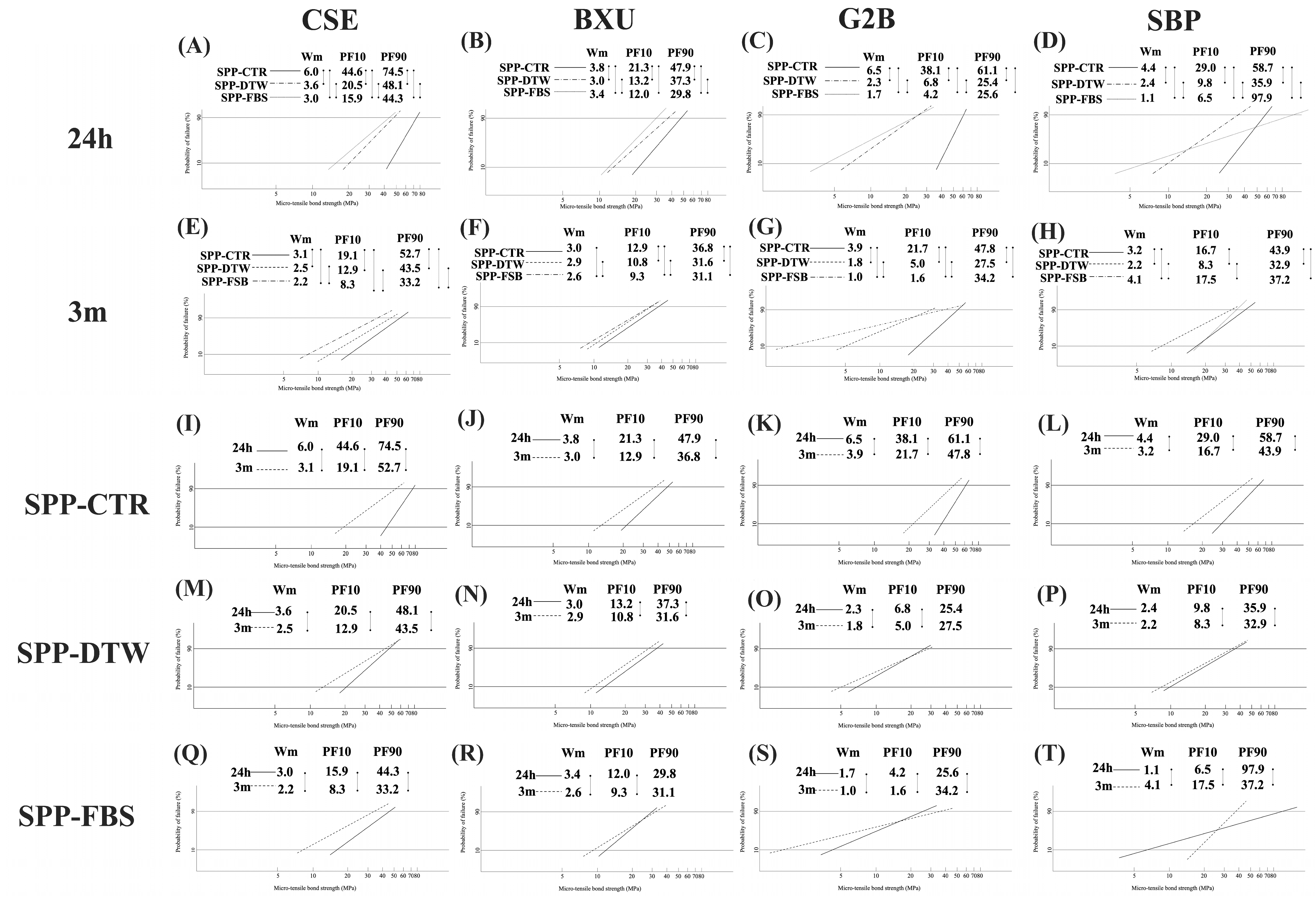
3.4. Bonding Reliability and Durability
3.5. DC
4. Discussion
5. Conclusions
- –
- The studied factors, (1) different adhesives, (2) pulpal pressure conditions, and (3) storage time, affected the bond strength of adhesives to dentin, therefore rejecting the related null hypotheses.
- –
- Different adhesive systems produced distinct bond strength to dentin under the proposed experimental conditions.
- –
- Clearfil SE Bond 2 achieved the highest bond strength at 24 h of storage when no pulpal pressure was simulated. G2-Bond produced the lowest bond strength after 3 months of storage when pulp pressure was simulated, regardless of the solution used.
- –
- Overall, the simulated pulp pressure and the 3 months of storage decreased the bond strength of the adhesive systems to dentin.
- –
- The effect of simulating pulpal pressure with different fluids depended on the adhesive. Interestingly, the fetal bovine serum increased the bond strength reliability of Scotchbond Universal Plus after 3 months of storage.
6. Clinical Significance
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Perdigão, J.; Araujo, E.; Ramos, R.Q.; Gomes, G.; Pizzolotto, L. Adhesive Dentistry: Current concepts and clinical considerations. J. Esthet. Rest. Dent. 2021, 33, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s Pioneering Acid-Etch Technique to Self-Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar] [PubMed]
- Ciucchi, B.; Bouillaguet, S.; Holz, J.; Pashley, D. Dentinal fluid dynamics in human teeth, in vivo. J. Endod. 1995, 21, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Chapter 8 Dentin-pulp complex. In Ten Cate’s Oral Histology, 9th ed.; Nanci, A., Ed.; Elsevier: St. Louis, MO, USA, 2018; pp. 157–192. [Google Scholar]
- Maita, E.; Simpson, M.D.; Tao, L.; Pashley, D.H. Fluid and protein flux across the pulpo dentine complex of the dog in vivo. Arch. Oral Biol. 1991, 36, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Pucci, C.R.; Gu, L.-S.; Zeng, C.; Gou, Y.-P.; Tay, F.R.; Niu, L.-N. Susceptibility of contemporary single-bottle self-etch dentine adhesives to intrinsic water permeation. J. Dent. 2017, 66, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J. Current perspectives on dental adhesion: (1) dentin adhesion—Not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, E.H.; El-Deeb, H.A.; Yousry, M.M. Influence of different intrapulpal pressure simulation liquids on the microtensile bond strength of adhesive systems to dentin. J. Adhes. Dent. 2013, 15, 519–526. [Google Scholar] [PubMed]
- Gonçalves, L.L.; Da Silva, T.M.; Prakki, A.; Barcellos, D.C.; Caneppele, T.M.F.; De Oliveira, H.P.M.; Gonçalves, S.E.P. Universal adhesive: The effect of different simulated pulpal pressure fluids and bonding modes to dentin. Odontology 2022, 110, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Harnirattisai, C.; Luangaram, C.; Kuphasuk, W.; Senwangose, P. The influence of a local anesthetic containing vasoconstrictor on microtensile bond strengths of two adhesive systems to human dentin in situ. J. Adhes. Dent. 2010, 12, 11–18. [Google Scholar]
- Cadenaro, M.; Josic, U.; Mravic, T.; Mazzitelli, C.; Marchesi, G.; Mancuso, E.; Breschi, L.; Mazzoni, A. Progress in dental adhesive materials. J. Dent. Res. 2023, 102, 254–262. [Google Scholar] [CrossRef]
- Rego, H.M.; Alves, T.S.; Bresciani, E.; Niu, L.-N.; Tay, F.R.; Pucci, C.R. Can long-term dentine bonding create in real life be forecasted by parameters established in the laboratory? Sci. Rep. 2016, 6, 37799. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, K.; Nakajima, M.; Yamauti, M.; Aksornmuang, J.; Ikeda, M.; Foxton, R.M.; Pashley, D.H.; Tagami, J. Effect of simulated pulpal pressure on all-in-one adhesive bond strengths to dentine. J. Dent. 2007, 35, 207–213. [Google Scholar] [CrossRef]
- Nikaido, T.; Burrow, M.F.; Tagami, J.; Takatsu, T. Effect of pulpal pressure on adhesion of resin composite to dentin: Bovine serum versus saline. Quintessence Int. 1995, 26, 221–226. [Google Scholar] [PubMed]
- Gernhardt, C.R.; Bekes, K.; Fecher, K.; Schaller, H.-G. The influence of human plasma used for in vitro dentin perfusion on microtensile bond strength of 5 self-conditioning dentin adhesives. Quintessence Int. 2006, 37, 429–435. [Google Scholar] [PubMed]
- Carvalho, R.M.; Manso, A.P.; Geraldeli, S.; Tay, F.R.; Pashley, D.H. Durability of bonds and clinical success of adhesive restorations. Dent. Mater. 2012, 28, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Frankenberger, R. The value and remaining need of bond-strength testing. J. Adhes. Dent. 2020, 22, 123–124. [Google Scholar] [PubMed]
- Armstrong, S.; Breschi, L.; Özcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (μTBS) approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Bacelar-Sa, R.; Sauro, S.; Abuna, G.; Vitti, R.P.; Nikaido, T.; Tagami, J.; Ambrosano, G.M.B.; Giannini, M. Adhesion evaluation of dentin sealing, micropermeability, and bond strength of current HEMA_free adhesives to dentin. J. Adhes. Dent. 2017, 19, 357–364. [Google Scholar] [PubMed]
- Tang, C.; Ahmed, M.H.; Yao, C.; Mercelis, B.; Yoshihara, K.; Peumans, M.; Van Meerbeek, B. Bonding performance of experimental HEMA_free two-step universal adhesives to low C-factor flat dentin. Dent. Mater. 2023, 39, 603–615. [Google Scholar] [CrossRef]
- Bolaños-Carmona, V.; Benavides-Reyes, C.; González-López, S.; González-Rodríguez, P.; Álvarez-Lloret, P. Influence of Spectroscopic Techniques on the Estimation of the Degree of Conversion of Bulk-fill Composites. Oper. Dent. 2020, 45, 92–103. [Google Scholar] [CrossRef]
- Nakazawa, M.; Maeno, M.; Komoto, M.; Nara, Y. Appropriate Immediate Dentin Sealing to Improve the Bonding of CAD/CAM Ceramic Crown Restorations. Polymers 2022, 14, 4541. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.M.; De Munck, J.; Pongprueksa, P.; Van Ende, A.; Van Meerbeek, B. Correlative analysis of cement-dentin interfaces using an interfacial fracture toughness and micro-tensile bond strength approach. Dent. Mater. 2016, 32, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbach, J.; Isolan, C.P.; Münchow, E.A. Is the presence of 10-MDP associated to higher bonding performance for self-etching adhesive systems? A meta-analysis of in vitro studies. Dent. Mater. 2021, 37, 1463–1485. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, S.; Theis-Mahon, N.; Perdigão, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Papadogiannis, D.; Dimitriadi, M.; Zafiropoulou, M.; Gaintantzopoulou, M.D.; Eliades, G. Universal Adhesives: Setting Characteristics and Reactivity with Dentin. Materials 2019, 12, 1720. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef] [PubMed]
- ISO/TS 11405:2003; Dental Materials—Testing Adhesion to Tooth Structure. ISO: Geneva, Switzerland, 2003.
- Quinn, J.B.; Quinn, G.D. A practical and systematic review of Weibull statistics for reporting strengths of dental materials. Dent. Mater. 2010, 26, 135–147. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Luehrs, A.K.; Poitevin, A.; Van Ende, A.; Van Meerbeek, B. Fracture toughness versus micro-tensile bond strength testing of adhesive-dentin interfaces. Dent. Mater. 2013, 29, 635–644. [Google Scholar] [CrossRef]
- Hurtado, A.; Fuentes, V.; Cura, M.; Tamayo, A.; Ceballos, L. Long-term in vitro adhesive properties of two universal adhesives to dentin. Materials 2023, 16, 3458. [Google Scholar] [CrossRef]
- Tichy, A.; Hosaka, K.; Abdou, A.; Nakajima, M.; Tagami, J. Degree of conversion contributes to dentin bonding durability of contemporary universal adhesives. Oper. Dent. 2020, 45, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Balkaya, H.; Demirbuga, S. Evaluation of six different one-step universal adhesive systems in terms of dentin bond strength, adhesive interface characterization, surface tension, contact angle, degree of conversion and solvent evaporation after immediate and delayed use. J. Esthet. Restor. Dent. 2022, 35, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.F.M.; Siqueira, F.S.F.; Nuñez, A.; Nonato, R.F.; Cavalcanti, K.G.B.A.; Soares, C.J.; Reis, A.; Loguercio, A.L. Influence of irradiation and exposure times on the mechanical and adhesive properties of universal adhesives with dentin. Oper. Dent. 2022, 47, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Wendlinger, M.; Nuñez, A.; Moreira, P.H.A.; Carneiro, T.S.; Cochinski, G.D.; Siqueira, F.S.F.; Cardenas, A.F.M.; Loguercio, A.D. Effect of the absence of HEMA on the bonding properties of adhesive systems containing 10-MDP: An in vitro study. Oper. Dent. 2023, 48, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.H.; De Munck, J.; Van Landuyt, K.; Peumans, M.; Yoshihara, K.; Van Meerbeek, B. Do universal adhesives benefit from an extra bonding layer? J. Adhes. Dent. 2019, 21, 117–132. [Google Scholar] [PubMed]
- Ermis, R.B.; Ugurlu, M.; Ahmed, M.H.; Van Meerbeek, B. Universal adhesives benefit from an extra hydrophobic adhesive layer when light cured beforehand. J. Adhes. Dent. 2019, 21, 179–188. [Google Scholar] [PubMed]
- Ahmed, M.H.; Yao, C.; Van Landuyt, K.; Peumans, M.; Van Meerbeek, B. Extra bonding layer compensates universal adhesive’s thin film thickness. J. Adhes. Dent. 2020, 22, 483–501. [Google Scholar]
- Fuentes, M.V.; PerdigNao, J.; Baracco, B.; Giraldéz, I.; Ceballos, L. Effect of an additional bonding resin on the 5-year performance of a universal adhesive: A randomized clinical trial. Clin. Oral. Investig. 2023, 27, 837–848. [Google Scholar] [CrossRef]

| Adhesives; Manufacturers; Abbreviations | pH | Composition * | Application Procedures |
|---|---|---|---|
| Beautibond Xtreme Universal; Shofu INC., Kyoto, Japan; BXU | 2.4 | Acetone, water, Bis-GMA, carboxylic acid monomer, TEGDMA, organophosphate monomer, acid-resistant silane coupling agent. | 1. Apply the adhesive and leave for 10 s. 2. Gently air-blowing for 3 s and blow strongly for 7 s. 3. Light-cure for 10 s. |
| Clearfil Megabond 2; Kuraray Noritake Dental Co., Tokyo, Japan; CSE | 2.0 | Primer:10-MDP.HEMA, hydrophilic aliphatic, dimethacrylate, dl-CQ, water. Bond: 10-MDP, Bis-GMA, HEMA, dI-CQ, hydrophobic aliphatic dimethacrylate. | 1. Apply the primer and leave for 20 s. 2. Gentle air-blowing > 5 s. 3. Apply the bond. 4. Gentle air-blowing to make the film uniform. 5. Light-cure for 10 s. |
| G2-Bond Universal; GC Dental Corp., Tokyo, Japan; G2B | 1.5 | Primer: 4-MET, 10-MDP, 10-MDTP, dimethacrylate monomer, acetone, water, initiators, fillers. Bond: dimethacrylate monomer, Bis-GMA, filler, photoinitiator. | 1. Apply the primer and leave for 10 s. 2. Dry with moderate air-blow for 5 s. 3. Apply the bond. 4. Gentle air-blowing to make the film uniform. 5. Light-cure for 5 s. |
| Scotchbond Universal Plus; 3M Oral Care, Seefeld, Germany; SBP | 2.7 | 10-MDP, Vitrebond TM, Co-polymer, HEMA, dimethacrylate resins, filler, initiators, ethanol, water. | 1. Apply the adhesives and leave for 20 s. 2. Gently air-blowing > 5 s until it does not move. 3. Light-cure for 10 s. |
| Simulated Pulpal Pressure | |||
|---|---|---|---|
| Control | Distilled Water | Fetal Bovine Serum | |
| 24h | |||
| BXU | 34.80 (4.45) D | 25.14 (6.77) E,F | 20.94 (4.51) F,G |
| CSE | 60.25 (6.57) A | 34.35 (3.63) D | 29.87 (3.08) D,E |
| G2B | 50.18 (3.97) B | 15.80 (4.57) G,H | 13.83 (5.62) H |
| SBP | 44.20 (6.27) C | 22.44 (4.94) F,G | 35.21 (8.70) D |
| 3m | |||
| BXU | 24.75 (3.00) c,d | 21.01 (2.75) d,e | 19.94 (2.49) e |
| CSE | 35.71 (4.04) a | 27.83 (3.30) b,c | 20.15 (1.34) e |
| G2B | 33.02 (7.64) a | 15.33 (3.93) f | 11.71 (5.90) f |
| SBP | 30.28 (2.23) b | 20.07 (1.53) e | 27.49 (2.84) b,c |
| Adhesive Systems | Intensity/Amplitude |
|---|---|
| BXU | 93.665 (3.313) A,B |
| CSE | 95.689 (1.588) A,B |
| G2B | 90.127 (1.436) B |
| SBP | 96.007 (1.284) A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Maeno, M.; Cifuentes-Jimenez, C.C.; Komoto, M.; Liu, Y.; Nara, Y.; Sano, H.; Alvarez-Lloret, P.; Yamauti, M.; Tomokiyo, A. Effect of Simulated Dental Pulpal Pressure Using Fetal Bovine Serum for the Bonding Performance of Contemporary Adhesive to Dentin. Polymers 2024, 16, 1219. https://doi.org/10.3390/polym16091219
Li Y, Maeno M, Cifuentes-Jimenez CC, Komoto M, Liu Y, Nara Y, Sano H, Alvarez-Lloret P, Yamauti M, Tomokiyo A. Effect of Simulated Dental Pulpal Pressure Using Fetal Bovine Serum for the Bonding Performance of Contemporary Adhesive to Dentin. Polymers. 2024; 16(9):1219. https://doi.org/10.3390/polym16091219
Chicago/Turabian StyleLi, Yitong, Masahiko Maeno, Carolina Cecilia Cifuentes-Jimenez, Mei Komoto, Yunqing Liu, Yoichiro Nara, Hidehiko Sano, Pedro Alvarez-Lloret, Monica Yamauti, and Atsushi Tomokiyo. 2024. "Effect of Simulated Dental Pulpal Pressure Using Fetal Bovine Serum for the Bonding Performance of Contemporary Adhesive to Dentin" Polymers 16, no. 9: 1219. https://doi.org/10.3390/polym16091219





