Synthetic Polymer Scaffolds for Stem Cell Transplantation in Retinal Tissue Engineering
Abstract
: Age-related macular degeneration and retinitis pigmentosa are two leading causes of irreversible blindness characterized by photoreceptor loss. Cell transplantation may be one of the most promising approaches of retinal repair. However, several problems hinder the success of retinal regeneration, including cell delivery and survival, limited cell integration and incomplete cell differentiation. Recent studies show that polymer scaffolds can address these three problems. This article reviews the current literature on synthetic polymer scaffolds used for stem cell transplantation, especially retinal progenitor cells. The advantages and disadvantages of different polymer scaffolds, the role of different surface modifications on cell attachment and differentiation, and controlled drug delivery are discussed. The development of material and surface modification techniques is vital in making cell transplantation a clinical success.1. Introduction
Photoreceptor loss causes irreversible blindness in many retinal degenerative diseases. As the inner retinal circuitry remains intact, transplantation of cells to the degenerative retina is one of the most promising approaches of retinal repair. However, a number of problems need to be solved, such as more precise cell delivery and enhanced cell survival, robust cell integration and controlled cell differentiation, before the success of cell replacement can be realized. Recent studies show that the seeding of cells onto polymer scaffolds can address these three problems.
2. Cell Transplantation
2.1. Cell Delivery and Survival
Cell delivery and survival is a formidable challenge. To achieve functional retinal regeneration, an efficient and reliable means of cell delivery is required. Bolus injection of cell suspension is the typical method. However, this technique results in disorganized or incorrectly localized grafts. Bolus injection also contributes to poor cell survival due to shearing forces during injection and reflux of cells from the injection site [1]. Our experience, as well as that of many others, suggests the survival of grafted cells is quite low by bolus injection, with less than 1% of the grafted cells surviving 2–4 weeks after transplantation [2,3]. In contrast, our work delivering cells to the retina via a degradable poly(L-lactic acid)/poly(lactic-co-glycolic acid) (PLLA/PLGA) scaffold demonstrated a decrease in the number of cells lost to reflux and/or shearing forces by nearly 50%. Four weeks after transplantation, the scaffold improved overall survival by 10-fold over cell suspension grafts, corresponding to a survival rate of 78% [1]. This suggests the use of polymer scaffolds can solve both cell delivery and survival problems, resulting in an improved graft outcome.
2.2. Cell Integration
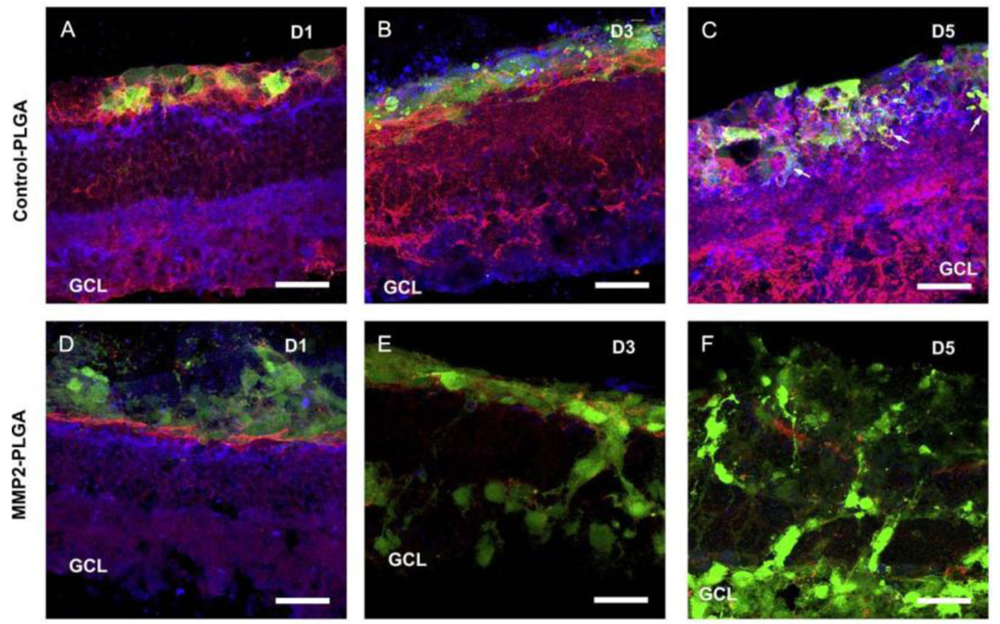
The limited integration of grafted cells is another problem. This is in large partly due to the barrier formed by inhibitory extracellular matrix (ECM) and cell adhesion molecules associated with reactive gliosis after degeneration or injury [4-6]. A previous study used adult mice deficient in glial fibrillary acidic protein and vimentin as the host, and demonstrated that the grafted cells integrated robustly into the host retina with distinct neuronal identity and appropriate neuronal projections [7]. This suggests removal of the barrier produced by reactive glial cells in the degenerative host retina is important in encouraging cell integration. In an attempt to increase cell integration, different drugs with the ability to disrupt the outer limiting membrane or degrade ECM molecules or their hyaluronan binding targets have been tried, such as DL-alpha-aminoadipic acid, chondroitinase ABC and MMP2 [8-10]. The transplantation of different cell types has also been investigated. Gage et al. demonstrated that adult rat hippocampus-derived neural progenitor cells (NPCs) could integrate to a high degree in most layers of the retina when injected into the eyes of neonatal rats or mature rats with active retinal degeneration [11,12]. The high migratory potential of these cells could be attributed to the expression of matrix metalloproteinase-2 (MMP2) [10]. We isolated retinal progenitor cells (RPCs) from postnatal mammalian neuroretina and showed they also possessed integrative abilities similar to NPCs [2]. Transplantation of RPCs stimulated the host retina to increase MMP2 secretion, partly from activated glial cells. In turn, increased MMP2 expression induced degradation of the inhibitory glial scar related molecules, such as CD44 and neurocan, deposited at the outer limits of the degenerative retina. This resulted in enhanced host donor integration following retinal transplantation [13]. This study provided the first identification of an MMP2-dependent mechanism in cell integration. As the endogenous MMP2 was not enough to achieve sufficient cell integration in vivo, we then transplanted a biodegradable MMP2-PLGA scaffold, in conjunction with RPCs, to the subretinal space of Rho–/– mice and demonstrated enhancement of cell integration with evident degradation of CD44 and neurocan [14] (Figure 1). Similarly, when RPCs were co-transplanted with biodegradable MMP2-PLGA microspheres, an increased number of cells were integrated with the degenerative host retina [15]. Maclaren et al. used photoreceptor precursors from the developing retina at the peak of rod genesis and showed enhanced cell integration, suggesting the post-mitotic developmental donor stage may facilitate cell integration [3]. Our work with induced pluripotent stem cells (iPS) derived-photoreceptors demonstrated significant integration of grafted cells with host retina [16], suggesting photoreceptor-committed cells may also promote cell integration.
2.3. Cell Differentiation
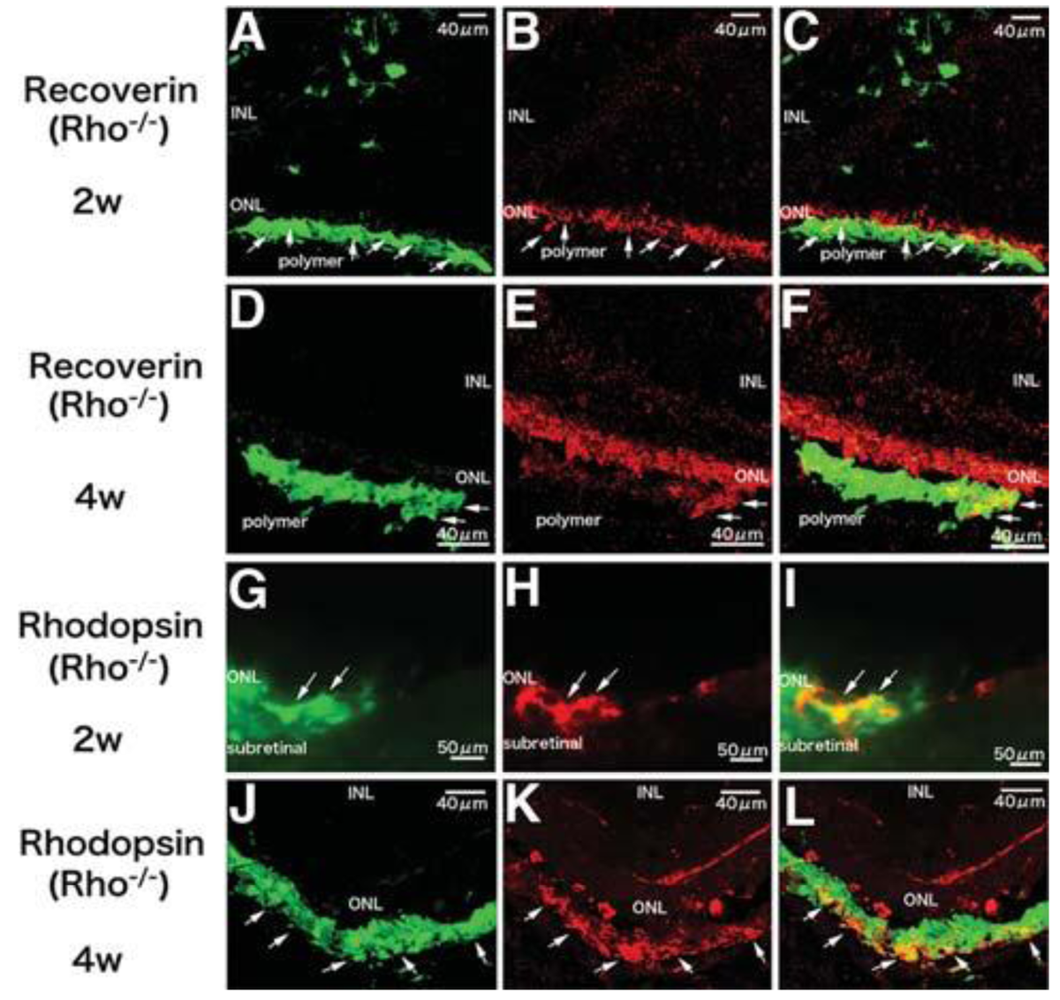
A third problem is controlling the differentiation of grafted cells to a specified cell type, which is an essential requirement for functional cell replacement. Despite high integration and morphological evidence of differentiation, NPCs cannot express retinal specific markers [12], which may in part be attributed to cell intrinsic factors. Photoreceptor precursors derived only from immature post-mitotic rod precursors and not from proliferating progenitor cells could differentiate into mature rod photoreceptors, form synaptic connections and improve visual function [3]. We transplanted iPS-derived photoreceptors into Rho–/– mice and demonstrated that integrated cells in the degenerative retina could express photoreceptor specific markers recoverin, rhodopsin and opsin, form synaptic connections with the inner retina and improve visual function [16]. RPCs were also able to differentiate into mature photoreceptors, express photoreceptor markers recoverin, rhodopsin and opsin, as well as exhibit photoreceptor morphological similarities [2]. Moreover, recipient mice showed improved visual function as determined by light-mediated behavior over a 25-week period when only a few photoreceptors left. However, no rhodopsin positive cells could be found in the Rho–/– mice model whether these cells were transplanted alone or co-transplanted with MMP2 microspheres [15]. This may be related to the complete absence of rhodopsin expression in Rho–/– hosts. Polymer scaffolds have been demonstrated to promote differentiation of RPCs in vitro prior to transplantation. When RPCs cultured on polymer scaffolds for one week were transplanted, they were able to express rhodopsin even in Rho–/– mice [1,13] (Figure 2). These results suggest post-mitotic photoreceptor precursors and photoreceptor-committed cells (differentiated from iPS, RPCs or other cells), may facilitate differentiation into mature photoreceptors regardless of host settings. Limited source is a problem for photoreceptor precursors. In vitro expansion of proliferating donor cells can overcome this problem. Using polymer scaffolds to direct stem cells towards photoreceptor-committed cells may facilitate the desired outcome while simultaneously inhibiting undesirable proliferation.
3. Polymer Scaffolds
Tissue engineering can be defined as using a polymer scaffold with the appropriate architecture to guide the repair and restoration of function to damaged tissues or organs [17]. The field was largely developed in order to address the shortage of tissues and organs available for transplantation [18]. Previous studies have shown that cells grown on a polymer scaffold were able to reconstruct complex tissues correctly, including bone, skin, cartilage and blood vessels [19-25]. In the past 20 years, retinal transplantation has been investigated. Various dissociated cells and tissue microaggregates were transplanted via subretinal and intravitreal injection and showed disorganized or incorrectly localized grafts [26-29]. Studies of retinal pigment epithelial (RPE) cells showed the importance of cellular continuity and polarity in the success of transplantation [30-32]. Following this, biodegradable polymer scaffolds were used to obtain organized sheets of RPE with distinct apical/basal characteristics [33]. A number of polymers, both natural and synthetic, have been widely investigated for use in retinal tissue engineering. Despite the advantage of closely resembling native ECM, concerns exist regarding the use of natural polymers, such as limitation of availability, purity of animal-derived polymers, transmission of disease, serious immune response as well as the consistency of quality, mechanical properties and degradation rates [34-41]. Conversely, the composition and corresponding properties of synthetic polymers are easily controlled. Biodegradable synthetic polymers used in the eye provide temporary scaffolding and then dissolve as the tissue forms, avoiding long-term foreign body responses and retinal detachment [34,41]. A number of biodegradable polymers, including PLGA, poly(glycerol-sebacate) (PGS), and polycaprolactone (PCL) have been applied in retinal engineering.
3.1. PLGA
The most common synthetic biodegradable polymers are poly(-lactic acid) (PLA), poly(glycolic acid) (PGA), and their copolymer, PLGA. These polymers are degraded by hydrolysis of the ester linkage into lactic and glycolic acids, which are subsequently metabolized by the body. By controlling the crystallinity, molecular weight, and the ratio of lactic to glycolic acid subunits, the degradation time of PLGA can be tailored over a wide range [42]. PLGA has been approved by the FDA for uses in drug delivery, diagnostics and other applications of clinical science research, including cardiovascular disease, cancer, vaccine and tissue engineering [43-45]. Furthermore, PLGA has been shown to exhibit a high degree of biocompatibility in the eye [34]. We have shown that PLGA/PLLA scaffolds improved the survival of RPCs in different retinal degenerative models, promoted differentiation of RPCs and provided physical guidance, resulting in a more normal anatomical organization [1,46]. RPC/PLGA scaffold constructs showed at least a 16-fold and 10-fold increase in cell delivery and survival respectively, thereby promoting cellular integration. RPCs delivered via the PLGA scaffolds could express photoreceptor markers recoverin and rhodopsin [1]. These results suggest PLGA scaffolds facilitate the survival, integration and differentiation of the grafted cells. Despite the aforementioned benefits, PLGA scaffolds have extremely limited flexibility, even when blended with PLLA. Their thickness (150–250 μm) is substantially greater than the photoreceptor layer of the rodent retina (∼30 μm), thus resulting in damage to the host retina and presenting a substantial barrier to functional photoreceptor replacement in rodent models [1,46]. In addition to retinal detachment, other complications have also been reported, such as inflammation, fibrosis and foreign body response [47,48] (Table 1).
3.2. Poly(methyl methacrylate) (PMMA)
In order to reduce the incidence of trauma, ultra-thin PMMA scaffolds (6 μm) were introduced. Due to their thinness, these scaffolds increased the ease of delivery and reduced the risk of trauma, thereby enhancing potential integration with the host. The grafted RPCs were able to express at least three markers of mature retinal cells. Moreover, no foreign body responses were seen four weeks after transplantation [49]. However, PMMA cannot be degraded and remains in the subretinal space permanently or until removed. We suggest that this may interfere with retinal reattachment and increase the risk of scarring and inflammatory response (Table 1).
3.3. PGS
A biodegradable and elastomeric PGS scaffold of intermediate thickness (45 μm) was then introduced [50]. Compared to PLGA, PGS has several advantages: (1) PGS has improved biocompatibility, including less inflammation and fibrosis and no foreign body giant cell response [48,50]. (2) PGS has an ideal degradation profile. PGS has been shown to be degraded by surface erosion, which results in preservation of geometry, no detectable swelling, and slow loss of mechanical strength relative to mass, whereas PLGA underwent bulk degradation accompanied by extensive deformation, swelling, and faster loss of mechanical strength than mass. PLGA lost > 98% of its mechanical strength after 7 days which was approximately the cell seeding time prior to transplantation, whereas PGS lost only about 8% over the same time despite a greater loss in mass. Significant loss of mechanical strength increased fragility of the scaffolds and difficulty of transplantation. Moreover, PGS is a rapidly degrading polymer with an in vivo degradation time of about 4–8 weeks [51]. (3) PGS is an elastic and soft material with an elastic modulus of 1.66 ± 0.23 MPa and a maximum strain at failure of 113 ± 22%, whereas PLLA/PLGA is a brittle material with an elastic modulus of 9.0 ± 1.7 MPa and a maximum strain at failure of only about 9% [46,52]. As retinal tissue has an elastic modulus of 0.1 MPa and a maximum strain at failure of about 83% [53], PGS possesses improved mechanical properties that are more similar to those of retinal tissue. The elastomeric property of PGS allows the polymer scaffolds to be scrolled and transplanted via syringe injection, thereby protecting cells from shearing forces and reducing trauma to retinal tissue (Table 1). We have shown that PGS scaffolds promoted RPC survival, differentiation and glutamate induced calcium influxes. By using PGS scaffolds as a cell delivery vehicle, 13% and 7% of grafted cells migrated into the C57BL/6 and Rho–/– mouse retinal explants at one week. 1.5% and 0.8% of grafted cells migrated into the C57BL/6 and Rho–/ – mouse retina four weeks post-transplantation [54].
3.4. PCL
Another potential scaffold material is PCL, which has also been utilized extensively for the delivery of drugs or proteins [55-57]. To date, the PCL scaffolds are the thinnest biodegradable substrates used in retinal tissue engineering (5 μm) [58]. They are fifty times thinner than the PLGA scaffolds and nine times thinner than the PGS scaffolds. They can be placed into the subretinal space with minimal physical distortion. Moreover, PCL is highly permeable, allowing for the passage of physiologically significant molecules. However, it takes about 2–3 years to degrade completely. PCL degradation occurs gradually from its surfaces, the same as PGS, with no pathologic increase in local acidity [58,59] (Table 1). We showed that structured PCL scaffolds increased the expression of mature photoreceptor and bipolar markers and that the PCL-delivered RPCs migrated into both normal and degenerative retina [60].
4. Surface Modification
4.1. Surface Chemistry
In order to achieve functional retinal regeneration, controlled cell differentiation is required. Studies of photoreceptor precursors and iPS suggest photoreceptor-committed cells may facilitate differentiation into mature photoreceptors after transplantation [3,16]. An ideal polymer scaffold should not only deliver an appropriate number of healthy cells to the retina, but also promote cell differentiation towards photoreceptor lineage prior to transplantation in order to improve the yield of desired cellular phenotypes and decrease the probability of undesired mitotic activity post-transplantation. All the above mentioned polymer scaffolds have demonstrated the ability to promote differentiation of RPCs and other cell types [61-65]. To achieve this, cells must attach to the polymer scaffolds first. This can be more difficult for some cell types, such as RPCs, which often form non-adherent neurospheres and do not readily adhere to a growth substrate. Generally, an extracellular matrix protein, such as laminin [2], is used to modify the surface for cell attachment. Laminin is known to influence differentiation of progenitor cells toward mature retinal phenotypes [66,67]. Other proteins, including fibronectin, collagen and poly-L-lysine, are also used. Significantly longer outgrowth of neurites occurred on laminin coated fibers compared to fibronectin coated fibers [68]. Collagen-functionalized nanofibers improved cell adhesion and migration compared to non-functionalized nanofibers [69]. Polymer scaffolds made from PLGA [1,46], PMMA [49] and PGS [52,54] required protein modification of the surface with either laminin or a combination of laminin and poly-L-lysine to achieve RPC attachment. Additionally, PLLA/PLGA scaffolds needed a concentrated cell solution in order to achieve appropriate levels of cell attachment [46].
4.2. Surface Topography
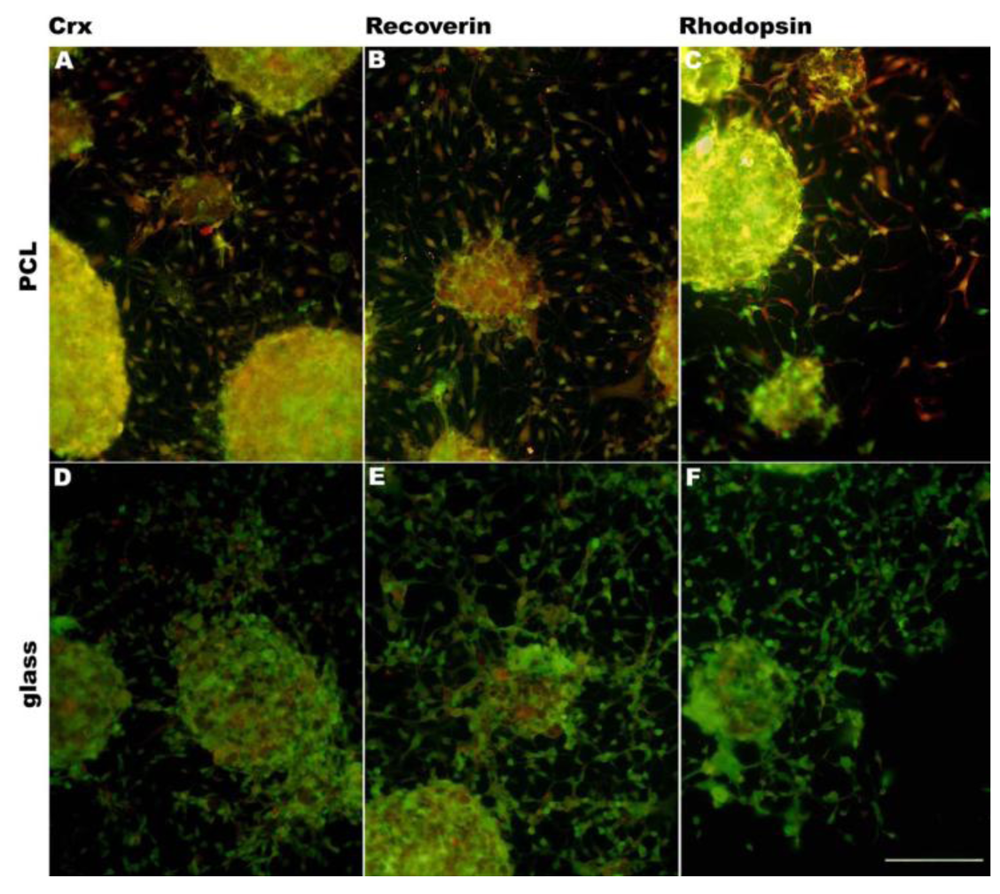
In addition to changing surface chemistry, polymer surface topography and architecture can also be altered. Microscale and nanoscale topographies have been found to induce reorientation and differentiation of multiple cell types, including neural progenitor cells [62], astroglial cells [70], smooth muscle cells [71] and mesenchymal stem cells [72]. Furthermore, surface topography can direct cell morphologic adaptations and subsequent changes in protein expression levels [62,73-76]. Surface topographic cues similar to endogenous ECM can direct cell morphogenesis via cell-surface contact [60,75]. It is suggested that the influence of surface topography on differentiation is via cell–cell surface receptors which act as mechanochemical transducers, activating signal transduction pathways and ultimately modulating gene expression and protein expression [76]. In the intact retina, the orientation of photoreceptors and retinal bipolar cells is radial with respect to the curvature of the globe. To approximate this highly ordered physical characteristics of the retina, a variety of techniques have been attempted. RPCs attached to the PLGA porous scaffolds created by phase-inversion casting and solid-liquid phase separation [77] demonstrated down-regulation of immature markers and up-regulation of markers of differentiation. These cells also exhibited morphologies consistent with photoreceptors, including a high degree of polarization of the cells and high survival [46]. PMMA scaffolds microfabricated [78] to provide precisely defined pores demonstrated enhanced RPC adherence during transplantation and allowed for greater process outgrowth and cell migration into the host retinal layers. Integrated cells expressed the mature neuronal marker neurofilament-200, the glial marker glial fibrillary acidic protein and the retinal-specific marker recoverin [49]. Utilizing microfabrication processes and soft lithography (or creation of a polydimethylsiloxane (PDMS) inverse replica) in combination with polymer molding, porous scaffolds can be structured precisely, simply, rapidly, and at low-cost without the further use of specialized microfabrication equipment. Scaffolds of varying porosity and pore structure can be quickly and easily produced by this technique. PGS scaffolds fabricated using this method demonstrated increased differentiation of RPCs towards mature phenotypes as evidenced by changes in mRNA, protein levels, and enhanced sensitivity to glutamate. Transplanting composites into the subretinal space of C57BL/6 and Rho–/– mice resulted in long-term RPC survival, and integrated cells exhibiting mature retinal markers [52,53]. RPCs grown on PCL scaffolds molded from a PDMS inverse replica exhibited an increase in gene expression for the photoreceptor markers recoverin and rhodopsin [61]. PCL scaffolds fabricated by template synthesis [58] (or inverse replication) from a nanoporous anodized aluminum oxide substrate comprised a surface topography of vertically oriented nanowires which facilitated RPC adhesion, growth, long-time survival and differentiation [60]. Recently, we have investigated a PCL scaffold templated from nanostructured silicon wafers. RPCs cultured on these scaffolds had a tendency to differentiate towards photoreceptors, expressing photoreceptor markers crx, recoverin and rhodopsin (Figure 3).
5. Drug Delivery
Most synthetic polymers utilized in scaffold fabrication are also well-suited for use as drug delivery systems [14,15,18,44,79,80]. Therapeutic drugs could feasibly be integrated into the polymer scaffolds during fabrication and processing. The release kinetics are dependent on a number of factors, including drug hydrophilicity, loading concentration and distribution, as well as the polymer properties, such as degradation rate and surface area [34]. The MMP2-PLGA microspheres have a continuously controlled delivery of active MMP2 up to 30 days [15]. The PCL implant can elute steroid for a period of at least 4 weeks [79].
Different drug mechanisms have been investigated for retinal tissue engineering. A variety of growth factors and neurotrophins, such as retinoic acid, taurine, ciliary neurotrophic factor (CNTF) [81] have been implicated in the differentiation of photoreceptors. Aside from directing donor cell response, drugs to modulate the host environment may also be necessary in order to promote integration of the transplanted constructs. Studies of MMP2 showed the importance of MMP2 in encouraging cell integration by degradation of inhibitory ECM and adhesion molecules, such as CD44 and neurocan, at the outer surface of degenerative retina [13,82]. A PLGA scaffold was then created by two-phase electrospinning to produce a highly porous interwoven mat of PLGA fibers ranging from 100 to 5,000 nm in diameter interlaced with picoliter domains of active-MMP2. Compared to control polymers, the controlled release of MMP2 from the polymer scaffold was able to enhance RPC integration and repopulation of the retinal outer nuclear layer while preserving retinal architecture and stem cell function [14]. PLGA microspheres containing MMP2 developed by a water-oil-water solvent evaporation technique showed enhancement of cell integration coincident with degradation of CD44 and neurocan. Over the course of the study, there was no discernible detrimental influence on the host retina [15]. Therefore, polymers used as a drug delivery system can provide sustained, controlled, and localized drug delivery to the posterior segment after transplantation. Although the subretinal space is an immuno-privileged site, the state is disrupted after retinal degeneration [83]. Restoration of immune privilege through delivery of immunosuppressive or anti-inflammatory factors may be an important strategy for modulating the host environment and promoting graft-host integration [84,85]. In the future, polymer scaffolds may serve as a platform to incorporate drugs with the aim of enhancing cell integration, directing cell differentiation and/or promoting synaptic connections.
6. Conclusions
Retinal tissue engineering is a multidisciplinary field. To achieve successful cell transplantation, several hurdles must be overcome. Polymer scaffolds can deliver cells to the correct site, improve cell survival, enhance cell integration and direct cell differentiation, thereby providing a promising platform for cell transplantation. Different polymers have distinct advantages and disadvantages. An ideal polymer scaffold should have the following properties: biocompatible, degradable by surface, thin, strong yet flexible and easy for drug loading. Surface modification facilitates cell attachment and differentiation, but reliable and optimal architecture has not been determined. Seeding cells on polymer scaffolds with or without drug loading to induce changes towards a more differentiated phenotype prior to transplantation represents a future direction. Elucidating the interactions between cells and polymer scaffolds with development of materials and surface modification techniques may lead to the success of cell transplantation.
| citation | thickness | fabrication method | surface chemistry | surface topography | advantages | disadvantages | research levels | results | |
|---|---|---|---|---|---|---|---|---|---|
| PLGA/ PLLA | Lavik et al. [45] | 150 μm | phase-inversion casting and solid–liquid phase separation | uniform pore structure and high degree of porosity with diameters of approximately 100-200 μm, oriented normal to the plane of scaffolds | (1) degradable; (2) bicompatible; (3) better for drug and protein loading | (1) thickest; (2) no flexibility; (3) inflammation, fibrosis and foreign body response; (4) bulk degradation resulting in a non- uniform release profile | in vitro cell/polymer constructs | down-regulation of immature markers and up-regulation of markers of differentiation | |
| in vitro explant (3-week-old rd1 mice) | exhibiting morphologies consistent with photoreceptors, including a high degree of polarization of the cells | ||||||||
| in vivo subretinal transplantation (adult SD rat) | increased RPC survival | ||||||||
| Tomita et al. [1] | 150 μm | phase-inversion casting and solid-liquid phase separation | laminin | largely uniform pore structure with diameters of approximately 35-50 μm, oriented normal to the plane of scaffolds | in vitro cell/polymer constructs | remaining undifferentiated except a subset expressing early markers of more mature neuron or glia | |||
| in vivo subretinal transplantation (4-8-week-old C57BL/6 and Rho−/− mice) | (1) at least 16-fold and 10-fold increase in cell delivery and survival; (2) grafted cells migrating into the host retina and expressing mature markers, including recoverin and rhodopsin | ||||||||
| Tucker et al. [13]* | two-phase electrospinning | laminin | a highly porous interwoven mat of PLGA fibers ranging from 100-5,000 nm in diameter | in vitro explant (adult rd1 and Rho−/− mice) | (1) significant degradation of CD44 and neurocan without disruption of retinal architecture; (2) enhancement of RPC integration into the host retina; (3) grafted cells in the outer nuclear layer adopting photoreceptor morphology and expressing mature photoreceptor markers recoverin and rhodopsin | ||||
| in vivo subretinal transplantation (adult Rho−/− mice) | |||||||||
| PMMA | Tao et al. [48] | 6 μm | photolithography and reactive ion etching | laminin and poly-L lysine | pores with diameters of approximately 11 μm and an interpore distance of 63 μm | (1) thin; (2) no foreign body response | (1) non-degradable | in vivo subretinal transplantation (adult C57BL/6 mice) | (1) porous scaffolds demonstrating enhanced RPC adherence and allowing for greater process outgrowth and cell migration; (2)integrated cells expressing at least three mature markers, including recoverin |
| PGS | Neeley et al. [51] | 45 μm | soft lithography and polymer casting | laminin | 50 μm diameter pores spaced 175 μm apart, center to center | (1) improved biocompatibility; (2) superiority of degradation; (3) elastic | (1) thick; (2) difficult to fabricate drugs or proteins | in vitro cell/polymer constructs | strongly adherence to the scaffolds and expresing a mixture of immature and mature markers |
| Stephen et al. [53] | 45 μm | soft lithography and polymer casting | laminin | 50 μm diameter pores spaced 175 μm apart | in vitro cell/polymer constructs | RPCs adherent to scaffolds differentiating to mature phenotypes and enhancing sensitivity to glutamate | |||
| in vitro explant (adult C57BL/6 and Rho−/− mice) | long-term survival and expression of mature markers, including crx and rhodopsin | ||||||||
| in vivo subretinal transplantation (adult C57BL/6 and Rho−/− mice) | |||||||||
| PCL | Stephen et al. [59] | 5 μm | hot melt template synthesis | laminin | nanowires with an average diameter of 150-200 nm and an interwire distance of 20 nm | (1) thinnest; (2) permeable; (3) easy to fabricate drugs or proteins | (1) long degradation time | in vitro cell/polymer constructs | down-regulation of several early progenitor markers and increased expression of mature bipolar and photoreceptor markers |
| in vitro explant (adult C57BL/6 and Rho−/− mice) | cells migrating into the host retina and expressing mature markers including recoverin and PKC | ||||||||
| in vivo subretinal transplantation (adult C57BL/6 and Rho−/− mice) | (1) developing an apparent cell polarity with early photoreceptor-like morphology; (2) expressing photoreceptor marker recoverin in cells migrating into the outer nuclear layer | ||||||||
| Steedman et al. [60] | 5μm | soft lithography and polymer casting | 25 μm diameter microwells spaced 25 μm apart | in vitro cell/polymer constructs | (1) increased cell attachement and organization;(2) higer gene expression for photoreceptor markers recoverin and rhodopsin |
*PLGA scaffolds used in [13] were loaded with active MMP2. Cells used in these researches were RPCs.
Acknowledgments
The authors acknowledge the Foundation Fighting Blindness for support.
References
- Tomita, M.; Lavik, E.; Klassen, H.; Zahir, T.; Langer, R.; Young, M.J. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem Cells 2005, 23, 1579–1588. [Google Scholar]
- Klassen, H.J.; Ng, T.F.; Kurimoto, Y.; Kirov, I.; Shatos, M.; Coffey, P.; Young, M.J. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest. Ophthalmol. Vis. Sci. 2004, 45, 4167–4173. [Google Scholar]
- MacLaren, R.E.; Pearson, R.A.; MacNeil, A.; Douglas, R.H.; Salt, T.E.; Akimoto, M.; Swaroop, A.; Sowden, J.C.; Ali, R.R. Retinal repair by transplantation of photoreceptor precursors. Nature 2006, 444, 203–207. [Google Scholar]
- Zhang, Y.; Caffe, A.R.; Azadi, S.; van Veen, T.; Ehinger, B.; Perez, M.T. Neuronal integration in an abutting-retinas culture system. Invest. Ophthalmol. Vis. Sci. 2003, 44, 4936–4946. [Google Scholar]
- Inatani, M.; Tanihara, H.; Oohira, A.; Honjo, M.; Kido, N.; Honda, Y. Upregulated expression of neurocan, a nervous tissue specific proteoglycan, in transient retinal ischemia. Invest. Ophthalmol. Vis. Sci. 2000, 41, 2748–2754. [Google Scholar]
- Krishnamoorthy, R.; Agarwal, N.; Chaitin, M.H. Upregulation of CD44 expression in the retina during the rds degeneration. Brain Res. Mol. Brain Res. 2000, 77, 125–130. [Google Scholar]
- Kinouchi, R.; Takeda, M.; Yang, L.; Wilhelmsson, U.; Lundkvist, A.; Pekny, M.; Chen, D.F. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat. Neurosci. 2003, 6, 863–868. [Google Scholar]
- West, E.L.; Pearson, R.A.; Tschernutter, M.; Sowden, J.C.; Maclaren, R.E.; Ali, R.R. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp. Eye. Res. 2008, 86, 601–611. [Google Scholar]
- Suzuki, T.; Akimoto, M.; Imai, H.; Ueda, Y.; Mandai, M.; Yoshimura, N.; Swaroop, A.; Takahashi, M. Chondroitinase ABC treatment enhances synaptogenesis between transplant and host neurons in model of retinal degeneration. Cell Transplant. 2007, 16, 493–503. [Google Scholar]
- Suzuki, T.; Mandai, M.; Akimoto, M.; Yoshimura, N.; Takahashi, M. The simultaneous treatment of MMP-2 stimulants in retinal transplantation enhances grafted cell migration into the host retina. Stem Cells 2006, 24, 2406–2411. [Google Scholar]
- Takahashi, M.; Palmer, T.D.; Takahashi, J.; Gage, F.H. Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Mol. Cell Neurosci. 1998, 12, 340–348. [Google Scholar]
- Young, M.J.; Ray, J.; Whiteley, S.J.; Klassen, H.; Gage, F.H. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol. Cell Neurosci. 2000, 16, 197–205. [Google Scholar]
- Zhang, Y.; Klassen, H.J.; Tucker, B.A.; Perez, M.T.; Young, M.J. CNS progenitor cells promote a permissive environment for neurite outgrowth via a matrix metalloproteinase-2-dependent mechanism. J. Neurosci. 2007, 27, 4499–4506. [Google Scholar]
- Tucker, B.A.; Redenti, S.M.; Jiang, C.; Swift, J.S.; Klassen, H.J.; Smith, M.E.; Wnek, G.E.; Young, M.J. The use of progenitor cell/biodegradable MMP2-PLGA polymer constructs to enhance cellular integration and retinal repopulation. Biomaterials 2010, 31, 9–19. [Google Scholar]
- Yao, J.; Tucker, B.A.; Zhang, X.; Checa-Casalengua, P.; Herrero-Vanrell, R.; Young, M.J. Robust cell integration from co-transplantation of biodegradable MMP2-PLGA microspheres with retinal progenitor cells. Biomaterials 2011, 32, 1041–1050. [Google Scholar]
- Tucker, B.A.; Park, I.H.; Klassen, H.J.; Redenti, S.M.; Jiang, C.; Qi, S.D.; Daley, G.Q.; Young, M.J. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in retinal degenerative mice. In Abstracts of ARVO Annual Meeting, Proceedings of ARVO Annual Meeting, Fort Lauderdale, FL, USA, 3–6 May 2010.
- Hynes, S.R.; Lavik, E.B. A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 763–778. [Google Scholar]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar]
- Pomahac, B.; Svensjo, T.; Yao, F.; Brown, H.; Eriksson, E. Tissue engineering of skin. Crit. Rev. Oral Biol. Med. 1998, 9, 333–344. [Google Scholar]
- Temenoff, J.S.; Mikos, A.G. Review: Tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar]
- Niklason, L.E.; Gao, J.; Abbott, W.M.; Hirschi, K.K.; Houser, S.; Marini, R.; Langer, R. Functional arteries grown in vitro. Science 1999, 284, 489–493. [Google Scholar]
- Mooney, D.J.; Sano, K.; Kaufmann, P.M.; Majahod, K.; Schloo, B.; Vacanti, J.P.; Langer, R. Long-term engraftment of hepatocytes transplanted on biodegradable polymer sponges. J. Biomed. Mater. Res. 1997, 37, 413–420. [Google Scholar]
- Hadlock, T.; Elisseeff, J.; Langer, R.; Vacanti, J.; Cheney, M. A tissue-engineered conduit for peripheral nerve repair. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 1081–1086. [Google Scholar]
- Atala, A. Tissue engineering for bladder substitution. World J. Urol. 2000, 18, 364–370. [Google Scholar]
- Silverman, M.S.; Hughes, S.E. Transplantation of photoreceptors to light-damaged retina. Invest. Ophthalmol. Vis. Sci. 1989, 30, 1684–1690. [Google Scholar]
- Gouras, P.; Lopez, R.; Kjeldbye, H.; Sullivan, B.; Brittis, M. Transplantation of retinal pigment epithelium prevents photoreceptor degeneration in the RCS rat. Prog. Clin. Biol. Res. 1989, 314, 659–671. [Google Scholar]
- Gouras, P.; Du, J.; Kjeldbye, H.; Yamaoto, S.; Zack, D.J. Reconstruction of degenerate rd mouse retina by transplantation of transgenic photoreceptors. Invest. Ophthalmol. Vis. Sci. 1992, 33, 2579–2586. [Google Scholar]
- Litchfield, T.M.; Whiteley, S.J.; Lund, R.D. Transplantation of retinal pigment epithelial, photoreceptor and other cells as treatment for retinal degeneration. Exp. Eye Res. 1997, 64, 655–666. [Google Scholar]
- Lund, R.D.; Coffey, P.J.; Sauve, Y.; Lawrence, J.M. Intraretinal transplantation to prevent photoreceptor degeneration. Ophthalmic. Res. 1997, 29, 305–319. [Google Scholar]
- Castillo, B.V., Jr.; Little, C.W.; del Cerro, C.; del Cerro, M. An improved method of isolating fetal human retinal pigment epithelium. Curr. Eye Res. 1995, 14, 677–683. [Google Scholar]
- Little, C.W.; Cox, C.; Wyatt, J.; del Cerro, C.; del Cerro, M. Correlates of photoreceptor rescue by transplantation of human fetal RPE in the RCS rat. Exp. Neurol. 1998, 149, 151–160. [Google Scholar]
- Giordano, G.G.; Thomson, R.C.; Ishaug, S.L.; Mikos, A.G.; Cumber, S.; Garcia, C.A.; Lahiri-Munir, D. Retinal pigment epithelium cells cultured on synthetic biodegradable polymers. J. Biomed. Mater. Res. 1997, 34, 87–93. [Google Scholar]
- Colthurst, M.J.; Williams, R.L.; Hiscott, P.S.; Grierson, I. Biomaterials used in the posterior segment of the eye. Biomaterials 2000, 21, 649–665. [Google Scholar]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug. Deliv. Rev. 2007, 59, 207–233. [Google Scholar]
- Karwatowski, W.S.; Jeffries, T.E.; Duance, V.C.; Albon, J.; Bailey, A.J.; Easty, D.L. Preparation of Bruch's membrane and analysis of the age-related changes in the structural collagens. Br. J. Ophthalmol. 1995, 79, 944–952. [Google Scholar]
- Sheridan, C.; Williams, R.; Grierson, I. Basement membranes and artificial substrates in cell transplantation. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 68–75. [Google Scholar]
- Veis, A.; Cohen, J. Reversible transformation of gelatin to the collagen structure. Nature 1960, 186, 720–721. [Google Scholar]
- Del Priore, L.V.; Tezel, T.H.; Kaplan, H.J. Survival of allogeneic porcine retinal pigment epithelial sheets after subretinal transplantation. Invest. Ophthalmol. Vis. Sci. 2004, 45, 985–992. [Google Scholar]
- Blomback, B. Fibrinogen and fibrin-proteins with complex roles in hemostasis and thrombosis. Thromb. Res. 1996, 83, 1–75. [Google Scholar]
- Lavik, E.; Langer, R. Tissue engineering: current state and perspectives. Appl. Microbiol. Biotechnol. 2004, 65, 1–8. [Google Scholar]
- Gunatillake, P.; Mayadunne, R.; Adhikari, R. Recent developments in biodegradable synthetic polymers. Biotechnol. Annu. Rev. 2006, 12, 301–347. [Google Scholar]
- Lü, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar]
- Langer, R. Biomaterials in drug delivery and tissue engineering: One laboratory's experience. Acc. Chem. Res. 2000, 33, 94–101. [Google Scholar]
- Lavik, E.; Teng, Y.D.; Snyder, E.; Langer, R. Seeding neural stem cells on scaffolds of PGA, PLA, and their copolymers. Methods Mol. Biol. 2002, 198, 89–97. [Google Scholar]
- Lavik, E.B.; Klassen, H.; Warfvinge, K.; Langer, R.; Young, M.J. Fabrication of degradable polymer scaffolds to direct the integration and differentiation of retinal progenitors. Biomaterials 2005, 26, 3187–3196. [Google Scholar]
- Warfvinge, K.; Kiilgaard, J.F.; Lavik, E.B.; Scherfig, E.; Langer, R.; Klassen, H.J.; Young, M.J. Retinal progenitor cell xenografts to the pig retina—Morphologic integration and cytochemical differentiation. Arch. Ophthalmol. 2005, 123, 1385–1393. [Google Scholar]
- Sundback, C.A.; Shyu, J.Y.; Wang, Y.; Faquin, W.C.; Langer, R.S.; Vacanti, J.P.; Hadlock, T.A. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials 2005, 26, 5454–5464. [Google Scholar]
- Tao, S.; Young, C.; Redenti, S.; Zhang, Y.; Klassen, H.; Desai, T.; Young, M.J. Survival, migration and differentiation of retinal progenitor cells transplanted on micro-machined poly(methyl methacrylate) scaffolds to the subretinal space. Lab Chip 2007, 7, 695–701. [Google Scholar]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar]
- Wang, Y.; Kim, Y.M.; Langer, R. In vivo degradation characteristics of poly(glycerol sebacate). J. Biomed. Mater. Res. A 2003, 66, 192–197. [Google Scholar]
- Neeley, W.L.; Redenti, S.; Klassen, H.; Tao, S.; Desai, T.; Young, M.J.; Langer, R. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials 2008, 29, 418–426. [Google Scholar]
- Wollensak, G.; Spoerl, E. Biomechanical characteristics of retina. Retina 2004, 24, 967–970. [Google Scholar]
- Redenti, S.; Neeley, W.L.; Rompani, S.; Saigal, S.; Yang, J.; Klassen, H.; Langer, R.; Young, M.J. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials 2009, 30, 3405–3414. [Google Scholar]
- Huang, Q.; Goh, J.C.; Hutmacher, D.W.; Lee, E.H. In vivo mesenchymal cell recruitment by a scaffold loaded with transforming growth factor beta 1 and the potential for in situ chondrogenesis. Tissue Eng. 2002, 8, 469–482. [Google Scholar]
- Giavaresi, G.; Tschon, M.; Borsari, V.; Daly, J.H.; Liggat, J.J.; Fini, M.; Bonazzi, V.; Nicolini, A.; Carpi, A.; Morra, M.; et al. New polymers for drug delivery systems in orthopaedics: In vivo biocompatibility evaluation. Biomed. Pharmacother. 2004, 58, 411–417. [Google Scholar]
- Beeley, N.R.; Rossi, J.V.; Mello-Filho, P.A.; Mahmoud, M.I.; Fujii, G.Y.; de Juan, E., Jr.; Varner, S.E. Fabrication, implantation, elution, and retrieval of a steroid-loaded polycaprolactone subretinal implant. J. Biomed. Mater. Res. A 2005, 73, 437–444. [Google Scholar]
- Tao, S.L.; Desai, T.A. Aligned arrays of biodegradable poly(epsilon-caprolactone) nanowires and nanofibers by template synthesis. Nano Lett. 2007, 7, 1463–1468. [Google Scholar]
- Grayson, A.C.; Voskerician, G.; Lynn, A.; Anderson, J.M.; Cima, M.J.; Langer, R. Differential degradation rates in vivo and in vitro of biocompatible poly(lactic acid) and poly(glycolic acid) homo- and co-polymers for a polymeric drug-delivery microchip. J. Biomater. Sci. Polym. Ed. 2004, 15, 1281–1304. [Google Scholar]
- Redenti, S.; Tao, S.; Yang, J.; Gu, P.; Klassen, H.; Saigal, S.; Desai, T.; Young, M.J. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly(e-caprolactone) nanowire scaffold. J. Ocul. Biol. Dis. Infor. 2008, 1, 19–29. [Google Scholar]
- Steedman, M.R.; Tao, S.L.; Klassen, H.; Desai, T.A. Enhanced differentiation of retinal progenitor cells using microfabricated topographical cues. Biomed. Microdevices 2010, 12, 363–369. [Google Scholar]
- Recknor, J.B.; Sakaguchi, D.S.; Mallapragada, S.K. Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials 2006, 27, 4098–4108. [Google Scholar]
- Endres, M.; Hutmacher, D.W.; Salgado, A.J.; Kaps, C.; Ringe, J.; Reis, R.L.; Sittinger, M.; Brandwood, A.; Schantz, J.T. Osteogenic induction of human bone marrow-derived mesenchymal progenitor cells in novel synthetic polymer-hydrogel matrices. Tissue Eng. 2003, 9, 689–702. [Google Scholar]
- Marletta, G.; Ciapetti, G.; Satriano, C.; Perut, F.; Salerno, M.; Baldini, N. Improved osteogenic differentiation of human marrow stromal cells cultured on ion-induced chemically structured poly-epsilon-caprolactone. Biomaterials 2007, 28, 1132–1140. [Google Scholar]
- Garcia-Giralt, N.; Izquierdo, R.; Nogués, X.; Perez-Olmedilla, M.; Benito, P.; Gómez-Ribelles, J.L.; Checa, M.A.; Suay, J.; Caceres, E.; Monllau, J.C. A porous PCL scaffold promotes the human chondrocytes redifferentiation and hyaline-specific extracellular matrix protein synthesis. J. Biomed. Mater. Res. A 2008, 85, 1082–1089. [Google Scholar]
- Hunter, D.D.; Brunken, W.J. Beta 2 laminins modulate neuronal phenotype in the rat retina. Mol. Cell Neurosci. 1997, 10, 7–15. [Google Scholar]
- Libby, R.; Hunter, D.; Brunken, W. Developmental expression of laminin beta2 in rat retina: Further support for a role in rod morphogenesis. Invest. Ophthalmol. Vis. Sci. 1996, 37, 1651–1661. [Google Scholar]
- Wen, X.; Tresco, P.A. Effect of filament diameter and extracellular matrix molecule precoating on neurite outgrowth and Schwann cell behavior on multifilament entubulation bridging device in vitro. J. Biomed. Mater. Res. A 2006, 76, 626–637. [Google Scholar]
- Gerardo-Nava, J.; Fuhrmann, T.; Klinkhammer, K.; Seiler, N.; Mey, J.; Klee, D.; Möller, M.; Dalton, P.D.; Brook, G.A. Human neural cell interactions with orientated electrospun nanofibers in vitro. Nanomedicine (Lond) 2009, 4, 11–30. [Google Scholar]
- Recknor, J.B.; Recknor, J.C.; Sakaguchi, D.S.; Mallapragada, S.K. Oriented astroglial cell growth on micropatterned polystyrene substrates. Biomaterials 2004, 25, 2753–2767. [Google Scholar]
- Yim, E.K.; Reano, R.M.; Pang, S.W.; Yee, A.F.; Chen, C.S.; Leong, K.W. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials 2005, 26, 5405–5413. [Google Scholar]
- Yim, E.K.; Pang, S.W.; Leong, K.W. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp. Cell Res. 2007, 313, 1820–1829. [Google Scholar]
- Andersson, A.S.; Backhed, F.; von Euler, A.; Richter-Dahlfors, A.; Sutherland, D.; Kasemo, B. Nanoscale features influence epithelial cell morphology and cytokine production. Biomaterials 2003, 24, 3427–3436. [Google Scholar]
- Leng, T.; Wu, P.; Mehenti, N.Z.; Bent, S.F.; Marmor, M.F.; Blumenkranz, M.S.; Fisherman, H.A. Directed retinal nerve cell growth for use in a retinal prosthesis interface. Invest. Ophthalmol. Vis. Sci. 2004, 45, 4132–4137. [Google Scholar]
- Foley, J.D.; Grunwald, E.W.; Nealey, P.F.; Murphy, C.J. Cooperative modulation of neuritogenesis by PC12 cells by topography and nerve growth factor. Biomaterials 2005, 26, 3639–3644. [Google Scholar]
- Flemming, R.G.; Murphy, C.J.; Abrams, G.A.; Goodman, S.L.; Nealey, P.F. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573–588. [Google Scholar]
- Schurgens, C.; Maquet, V.; Grandfils, C.; Jerome, R.; Teyssie, P. Polylactide macroporous biodegradable implants for cell transplantation. II. Preparation of polylactide foams by liquid-liquid phase separation. J. Biomed. Mater. Res. 1996, 30, 449–461. [Google Scholar]
- Tao, S.L.; Lubeley, M.W.; Desai, T.A. Bioadhesive poly(methyl methacrylate) microdevices for controlled drug delivery. J. Control. Release 2003, 8, 215–228. [Google Scholar]
- Beeley, N.R.; Rossi, J.V.; Mello-Filho, P.A.; Mahmoud, M.I.; Fujii, G.Y.; de Juan, E., Jr.; Varner, S.E. Fabrication, implantation, elution, and retrieval of a steroid-loaded polycaprolactone subretinal implant. J. biomed. Mater. Res. A 2005, 73, 437–444. [Google Scholar]
- Sun, Z.J.; Chen, C.; Sun, M.Z.; Ai, C.H.; Lu, X.L.; Zheng, Y.F.; Yang, B.F.; Dong, D.L. The application of poly(glycerol-sebacate) as biodegradable drug carrier. Biomaterials 2009, 30, 5209–5214. [Google Scholar]
- Cepko, C.L.; Austin, C.P.; Yang, X.; Alexiades, M.; Ezzeddine, D. Cell fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. USA 1996, 93, 589–595. [Google Scholar]
- Tucker, B.; Klassen, H.; Yang, L.; Chen, D.F.; Young, M.J. Elevated MMP Expression in the MRL mouse retina creates a permissive environment for retinal regeneration. Invest. Ophthalmol. Vis. Sci. 2008, 49, 1686–1695. [Google Scholar]
- Forrester, J.V.; Xu, H.; Kuffová, L.; Dick, A.D.; McMenamin, P.G. Dendritic cell physiology and function in the eye. Immunol. Rev. 2010, 234, 282–304. [Google Scholar]
- Singhal, S.; Lawrence, J.M.; Bhatia, B.; Ellis, J.S.; Kwan, A.S.; Macneil, A.; Luthert, P.J.; Fawcett, J.W.; Perez, M.T.; Khaw, P.T.; Limb, G.A. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Müller stem cells into degenerating retina. Stem Cells 2008, 26, 1074–1082. [Google Scholar]
- Singhal, S.; Lawrence, J.M.; Salt, T.E.; Khaw, P.T.; Limb, G.A. Triamcinolone attenuates macrophage/microglia accumulation associated with NMDA-induced RGC death and facilitates survival of Müller stem cell grafts. Exp. Eye Res. 2010, 90, 308–315. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yao, J.; Tao, S.L.; Young, M.J. Synthetic Polymer Scaffolds for Stem Cell Transplantation in Retinal Tissue Engineering. Polymers 2011, 3, 899-914. https://doi.org/10.3390/polym3020899
Yao J, Tao SL, Young MJ. Synthetic Polymer Scaffolds for Stem Cell Transplantation in Retinal Tissue Engineering. Polymers. 2011; 3(2):899-914. https://doi.org/10.3390/polym3020899
Chicago/Turabian StyleYao, Jing, Sarah L. Tao, and Michael J. Young. 2011. "Synthetic Polymer Scaffolds for Stem Cell Transplantation in Retinal Tissue Engineering" Polymers 3, no. 2: 899-914. https://doi.org/10.3390/polym3020899




