Supramolecular Assemblies from Poly(styrene)-block-poly(4-vinylpyridine) Diblock Copolymers Mixed with 6-Hydroxy-2-naphthoic Acid
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
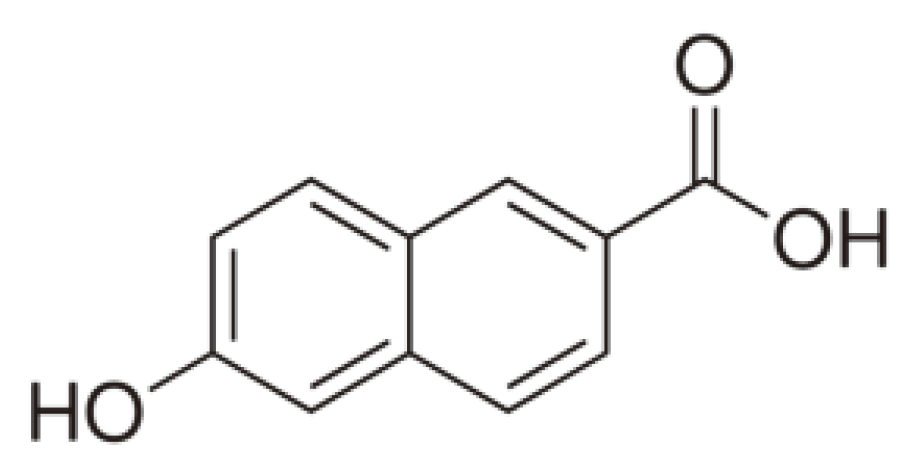
2.2. Preparation of Samples
2.3. Dynamic Light Scattering (DLS) Measurements
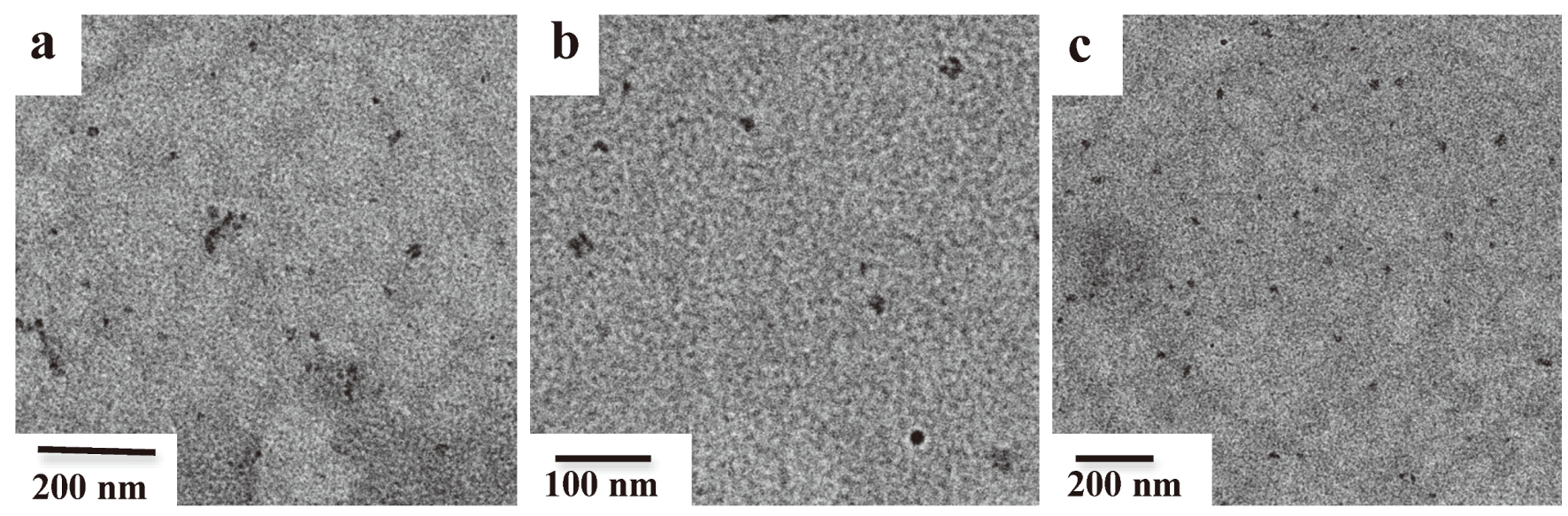
2.4. Transmission Electron Microscopy (TEM) Measurements
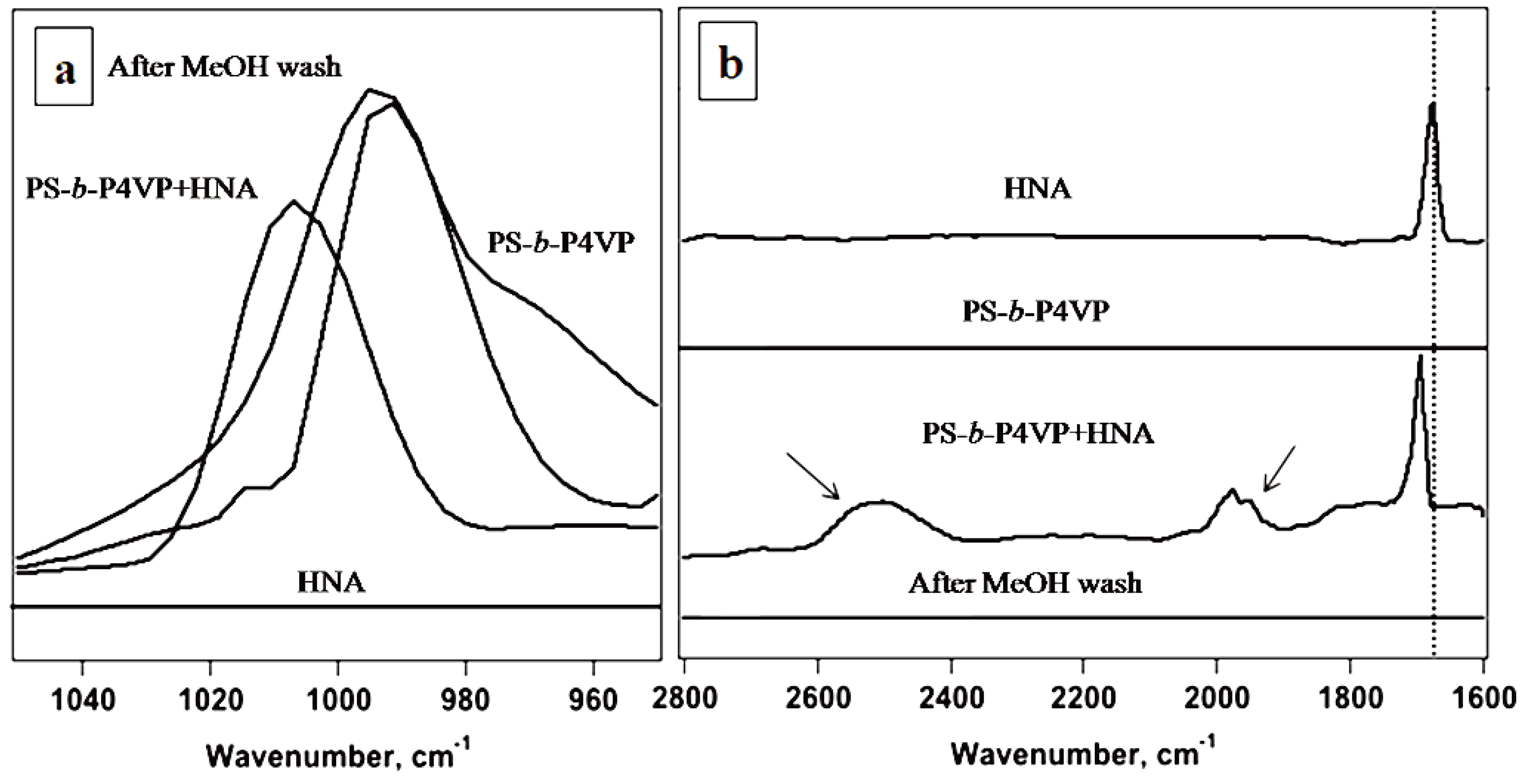
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Measurements
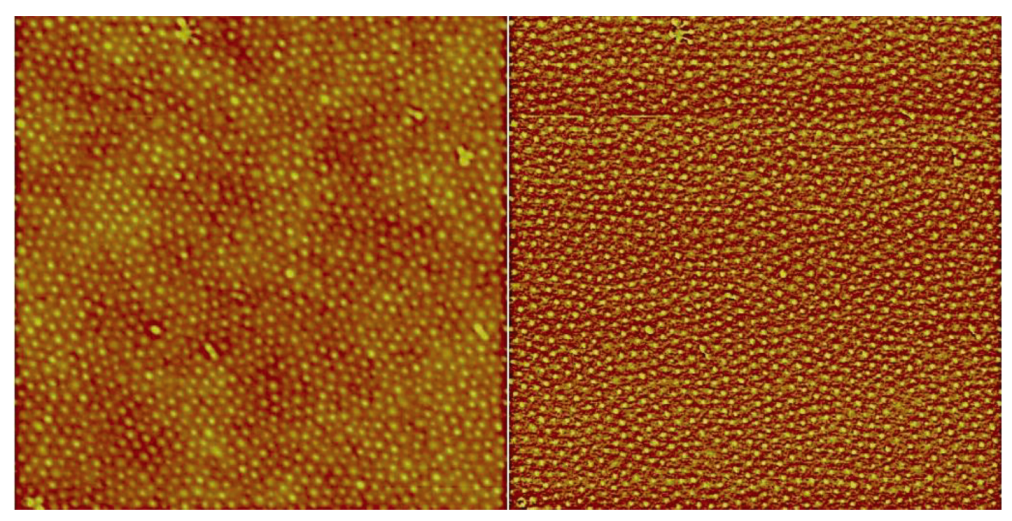
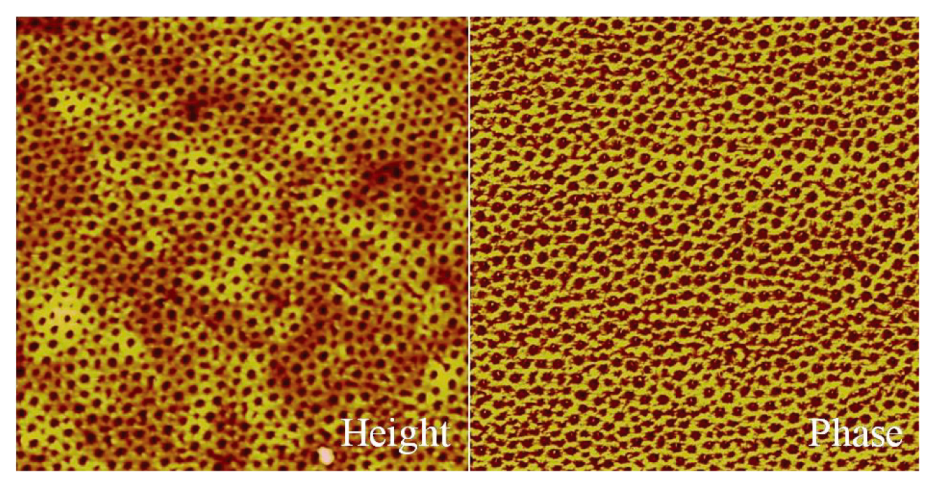
2.6. Atomic Force Microscopy (AFM) Measurements
3. Results and Discussion
3.1. Micellar Structures in 1,4-Dioxane
3.2. Spectroscopic Investigations on Hydrogen-Bond Formation between PS-b-P4VP and HNA
3.3. Formation of Thin Films from PS-b-P4VP/HNA Mixtures


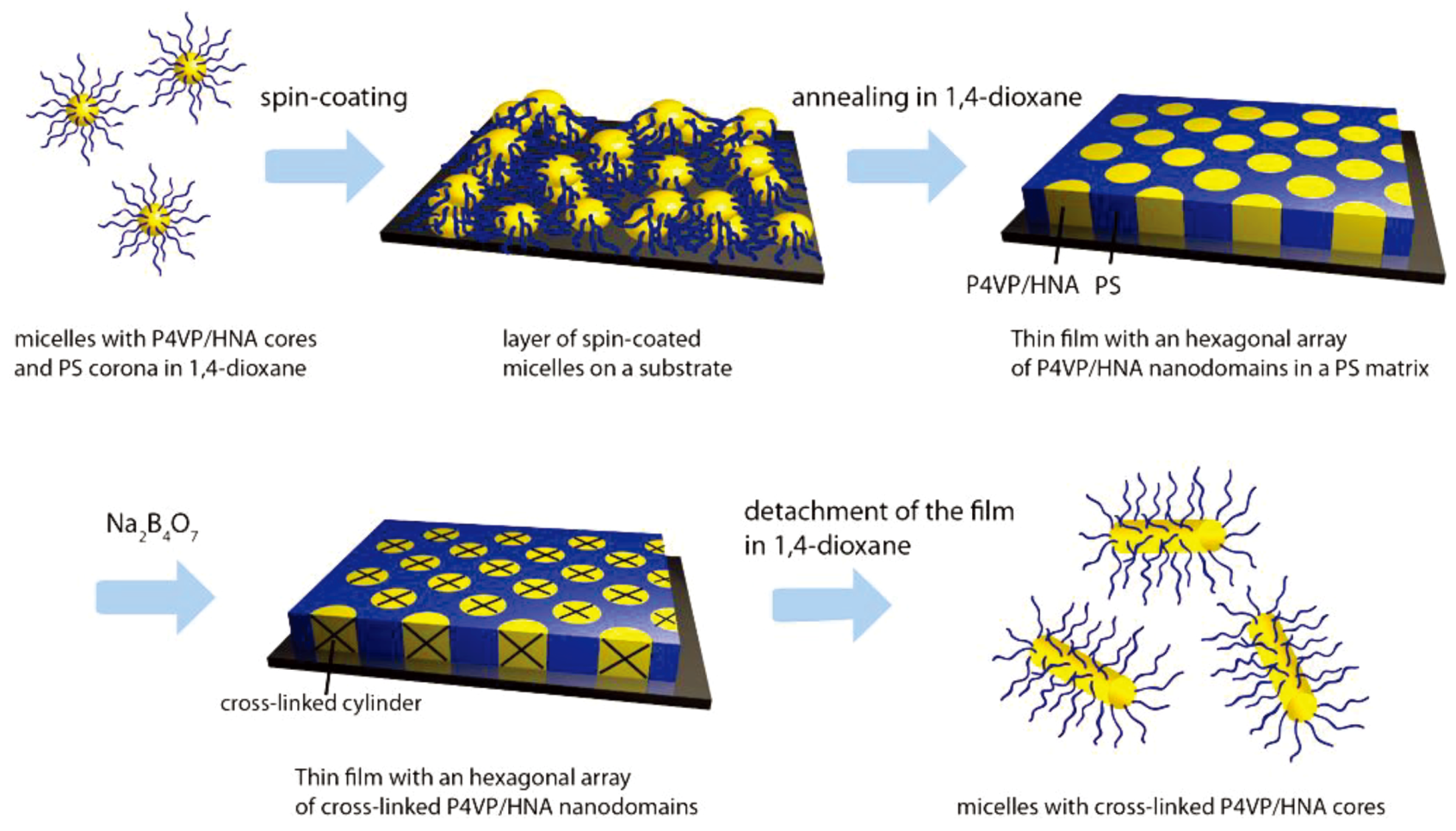
4. Conclusions
Conflict of interest
Acknowledgements
References
- Hamley, I.W. The Physics of Block Copolymers; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Bates, F.S.; Fredrickson, G.H. Block copolymers-designer soft materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Krausch, G.; Magerle, R. Nanostructured thin films via self-assembly of block copolymers. Adv. Mater. 2002, 14, 1579–1583. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block copolymer thermodynamics: Theory and experiments. Annu. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Thurn-Albrecht, T.; Schotter, J.; Kastle, G.A.; Emley, N.; Shibauchi, T.; Krusin-Elbaum, L.; Guarini, K.; Black, C.T.; Tuominen, M.T.; Russell, T.P. Ultrahigh-density nanowire arrays grown in self-assembled diblock copolymer templates. Science 2000, 290, 2126–2129. [Google Scholar] [CrossRef]
- Ramanathan, M.; Nettleton, E.; Darling, S.B. Simple orientational control over cylindrical organic-inorganic block copolymer domains for etch mask applications. Thin Solid Films 2009, 517, 4474–4478. [Google Scholar] [CrossRef]
- Knoll, A.; Horvat, A.; Lyakhova, K.S.; Krausch, G.; Sevink, G.J.A.; Zvelindovsky, A.V.; Magerle, R. Phase behavior in thin films of cylinder-forming block copolymers. Phys. Rev. Lett. 2002, 89, 35501–35504. [Google Scholar]
- Park, M.; Harrison, C.; Chaikin, P.M.; Register, R.A.; Adamson, D.H. Block copolymer lithography: Periodic arrays of ~1011 holes in 1 square centimeter. Science 1997, 276, 1401–1404. [Google Scholar] [CrossRef]
- Haberkorn, N.; Lechmann, M.C.; Sohn, B.H.; Char, K.; Gutmann, J.S.; Theato, P. Templated organic and hybrid materials for optoelectronic applications. Macromol. Rapid Commun. 2009, 30, 1146–1166. [Google Scholar] [CrossRef]
- Lo, K.H.; Chen, M.C.; Ho, R.M.; Sung, H.W. Pore-filling nanoporous templates from degradable block copolymers for nanoscale drug delivery. ACS Nano 2009, 3, 2660–2666. [Google Scholar] [CrossRef]
- Kuila, B.K.; Gowd, E.B.; Stamm, M. Supramolecular assembly of poly(styrene)-b-poly(4-vinylpyridine) and 1-pyrenebutyric acid in thin film and their use for nanofabrication. Macromolecules 2010, 43, 7713–7721. [Google Scholar] [CrossRef]
- Kuila, B.K.; Nandan, B.; Bohme, M.; Janke, A.; Stamm, M. Vertically orientedarrays of polyaniline nanorods and their super electrochemical properties. Chem. Commun. 2009, 5749–5751. [Google Scholar]
- Kuila, B.K.; Stamm, M. Fabrication of oriented polyaniline nanostructures using block copolymer nanotemplates and their optical, electrochemical and electric properties. J. Mater. Chem. 2010, 20, 6086–6094. [Google Scholar] [CrossRef]
- Gohy, J.F.; Mores, S.; Varshney, S.K.; Jerome, R. Self-organization of water-soluble complexes of a poly(2-vinylpyridinium)-block-poly(ethylene oxide) diblock with fluorinated anionic surfactants. Macromolecules 2003, 36, 2579–2581. [Google Scholar]
- Ruokolainen, J.; Saariaho, M.; Ikkala, O.; ten Brinke, G.; Thomas, E.L.; Torkkeli, M.; Serimaa, R. Supramolecular routes to hierarchical structures: Comb-coil diblock copolymers organized with two length scales. Macromolecules 1999, 32, 1152–1158. [Google Scholar]
- Ikkala, O.; ten Brinke, G. Hierarchical self-assembly in polymeric complexes: Towards functional materials. Chem. Commun. 2004, 2131–2137. [Google Scholar] [CrossRef]
- Tokarev, I.; Krenek, R.; Burkov, Y.; Schmeisser, D.; Sidorenko, A.; Minko, S.; Stamm, M. Microphase separation in thin films of poly(styrene-block-4-vinylpyridine) copolymer–2-(4′-hydroxybenzeneazo) benzoic acid assembly. Macromolecules 2005, 38, 507–516. [Google Scholar] [CrossRef]
- Sidorenko, A.; Tokarev, I.; Minko, S.; Stamm, M. Ordered reactive nanomembranes/nanotemplates from thin films of block copolymer supramolecular assembly. J. Am. Chem. Soc. 2003, 125, 12211–12216. [Google Scholar] [CrossRef]
- Laforgue, A.; Bazuin, C.G.; Prud’homme, R.E. A study of the supramolecular approach in controlling diblock copolymer nanopatterning and nanoporosity on surfaces. Macromolecules 2006, 39, 6473–6482. [Google Scholar] [CrossRef]
- Tang, C.; Hur, S.; Stahl, B.C.; Sivanandan, K.; Dimitriou, M.; Pressly, E.; Fredrickson, G.H.; Kramer, E.J.; Hawker, C.J. Thin film morphology of block copolymer blends with tunable supramolecular interactions for lithographic applications. Macromolecules 2010, 43, 2880–2889. [Google Scholar] [CrossRef]
- Tung, S.H.; Kalarickal, N.C.; Mays, J.W.; Xu, T. Hierarchical assemblies of block-copolymer-based supramolecules in thin films. Macromolecules 2008, 41, 6453–6462. [Google Scholar] [CrossRef]
- Ruokolainen, J.; Tanner, J.; ten Brinke, G.; Ikkala, O.; Torkelli, M.; Serimaa, R. Poly(4-vinyl pyridine)/zinc dodecyl benzene sulfonate mesomorphic state due to coordination complexation. Macromolecules 1995, 28, 7779–7784. [Google Scholar] [CrossRef]
- Wood, K.C.; Little, S.R.; Langer, R.; Hammond, P.T. A family of hierarchically self-assembling linear-dendritic hybrid polymers for highly efficient targeted gene delivery. Angew. Chem. Int. Ed. 2005, 44, 6704–6708. [Google Scholar]
- Faul, C.F.; Antonietti, M. Ionic self-assembly: Facile synthesis of supramolecular materials. Adv. Mater. 2003, 15, 673–683. [Google Scholar] [CrossRef]
- Thunemann, A.F. Polyelectrolyte–surfactant complexes (synthesis, structure and materials aspects). Prog. Polym. Sci. 2002, 27, 1473–1572. [Google Scholar] [CrossRef]
- Van Ekenstein, G.A.; Polushkin, E.; Nijland, H.; Ikkala, O.; ten Brinke, G. Shear alignment at two length scales: Comb-shaped supramolecules self-organized as cylinders-within-lamellar hierarchy. Macromolecules 2003, 36, 3684–3688. [Google Scholar] [CrossRef]
- Kosonen, H.; Ruokolainen, J.; Knaapila, M.; Torkkeli, M.; Serimaa, R.; Bras, W.; Monkman, A.P.; ten Brinke, G.; Ikkala, O. Self-organized nanostructures of conducting polyaniline and hydrogen bonded amphiphiles. Synth. Met. 2001, 121, 1277–1278. [Google Scholar] [CrossRef]
- Xuan, Y.; Peng, J.; Cui, L.; Wang, H.F.; Li, B.Y.; Han, Y.C. Morphology development of ultrathin symmetric diblock copolymer film via solvent vapor treatment. Macromolecules 2004, 37, 7301–7307. [Google Scholar] [CrossRef]
- Elbs, H.; Drummer, C.; Abetz, V.; Krausch, G. Thin film morphologies of ABC triblock copolymers prepared from solution. Macromolecules 2002, 35, 5570–5577. [Google Scholar] [CrossRef]
- Bosworth, J.K.; Paik, M.Y.; Ruiz, R.; Schwartz, E.L.; Huang, J.; Ko, A.W.; Smilgies, D.M.; Black, C.T.; Ober, C.K. Control of self-assembly of lithographically patternable block copolymer films. ACS Nano 2008, 2, 1396–1402. [Google Scholar]
- Huang, W.; Chen, P.Y.; Tung, S.H. Effects of annealing solvents on the morphology of block copolymer-based supramolecular thin films. Macromolecules 2012, 45, 1562–1569. [Google Scholar] [CrossRef]
- Van Zoelen, W.; Asumaa, T.; Ruokolainen, J.; Ikkala, O.; ten Brinke, G. Phase behavior of solvent vapor annealed thin films of PS-b-P4VP(PDP) supramolecules. Macromolecules 2008, 41, 3199–3208. [Google Scholar] [CrossRef]
- Van Zoelen, W.; Bondzic, S.; Landaluce, T.F.; Brondijk, J.; Loos, K.; Schouten, A.J.; Rudolph, P.; ten Brinke, G. Nanostructured polystyrene-block-poly(4-vinyl pyridine)(pentadecylphenol) thin films as templates for polypyrrole synthesis. Polymer 2009, 50, 3617–3625. [Google Scholar] [CrossRef]
- Seifarth, O.; Krenek, R.; Tokarev, I.; Burkov, Y.; Sidorenko, A.; Minko, S.; Stamm, M.; Schmeisser, D. Metallic nickel nanorod arrays embedded into ordered block copolymer templates. Thin Solid Films 2007, 515, 6552–6556. [Google Scholar] [CrossRef]
- Bohme, M.; Kuila, B.; Schlorb, H.; Nandan, B.; Stamm, M. Thin films of block copolymer supramolecular assemblies: Microphase separation and nanofabrication. Phys. Status Solidi B 2010, 247, 2458–2469. [Google Scholar] [CrossRef]
- Nandan, B.; Vyas, M.K.; Bohme, M.; Stamm, M. Composition-dependent morphological transitions and pathways in switching of fine structure in thin films of block copolymer supramolecular assemblies. Macromolecules 2010, 43, 2463–2473. [Google Scholar] [CrossRef]
- Roland, S.; Gaspard, D.; Prud’homme, R.E.; Bazuin, C.G. Morphology evolution in slowly dipcoated supramolecular PS-b-P4VP thin films. Macromolecules 2012, 45, 5463–5476. [Google Scholar]
- Roland, S.; Prud’homme, R.E.; Bazuin, C.G. Morphology, thickness, and composition evolution in supramolecular block copolymer films over a wide range of dip-coating rates. ACS Macro Lett. 2012, 1, 973–976. [Google Scholar] [CrossRef]
- Roland, S.; Pellerin, C.; Bazuin, C.G.; Prud’homme, R.E. Evolution of small molecule content and morphology with dip-coating rate in supramolecular PS-P4VP thin films. Macromolecules 2012, 45, 7964–7972. [Google Scholar] [CrossRef]
- Brandys, F.A.; Bazuin, C.G. Mixtures of an acid-functionalized mesogen with poly(4-vinylpyridine). Chem. Mater. 1996, 8, 83–92. [Google Scholar] [CrossRef]
- Lee, J.Y.; Painter, P.C.; Coleman, M. Hydrogen bonding in polymer blends. 4. Blends involving polymers containing methacrylic acid and vinylpyridine groups. Macromolecules 1988, 21, 954–960. [Google Scholar] [CrossRef]
- Kato, T.; Kihara, H.; Uryu, T.; Fujishima, A.; Fréchet, J. Molecular self-assembly of liquid crystalline side-chain polymers through intermolecular hydrogen bonding—Polymeric complexes built from a polyacrylate and stilbazoles. Macromolecules 1992, 25, 6836–6841. [Google Scholar] [CrossRef]
- Kim, S.H.; Misner, M.J.; Xu, T.; Kimura, M.; Russell, T.P. Highly oriented and ordered arrays from block copolymers via solvent evaporation. Adv. Mater. 2004, 16, 226–231. [Google Scholar] [CrossRef]
- Ruokolainen, J.; ten Brinke, G.; Ikkala, O. Supramolecular polymeric materials with hierarchical structure-within-structure morphologies. Adv. Mater. 1999, 11, 777–780. [Google Scholar] [CrossRef]
- Lee, S.M.; Chung, W.Y.; Kim, J.K.; Suh, D.H. A novel fluorescence temperature sensor based on a surfactant-free PVA/borax/2-naphthol hydrogel network system. J. Appl. Polym. Sci. 2004, 93, 2114–2118. [Google Scholar] [CrossRef]
- Wu, S.; Bubeck, C. Macro- and microphase separation in block copolymer supramolecular assemblies induced by solvent annealing. Macromolecules 2013, 46, 3512–3518. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bharatiya, B.; Schumers, J.-M.; Poggi, E.; Gohy, J.-F. Supramolecular Assemblies from Poly(styrene)-block-poly(4-vinylpyridine) Diblock Copolymers Mixed with 6-Hydroxy-2-naphthoic Acid. Polymers 2013, 5, 679-695. https://doi.org/10.3390/polym5020679
Bharatiya B, Schumers J-M, Poggi E, Gohy J-F. Supramolecular Assemblies from Poly(styrene)-block-poly(4-vinylpyridine) Diblock Copolymers Mixed with 6-Hydroxy-2-naphthoic Acid. Polymers. 2013; 5(2):679-695. https://doi.org/10.3390/polym5020679
Chicago/Turabian StyleBharatiya, Bhavesh, Jean-Marc Schumers, Elio Poggi, and Jean-François Gohy. 2013. "Supramolecular Assemblies from Poly(styrene)-block-poly(4-vinylpyridine) Diblock Copolymers Mixed with 6-Hydroxy-2-naphthoic Acid" Polymers 5, no. 2: 679-695. https://doi.org/10.3390/polym5020679
APA StyleBharatiya, B., Schumers, J.-M., Poggi, E., & Gohy, J.-F. (2013). Supramolecular Assemblies from Poly(styrene)-block-poly(4-vinylpyridine) Diblock Copolymers Mixed with 6-Hydroxy-2-naphthoic Acid. Polymers, 5(2), 679-695. https://doi.org/10.3390/polym5020679




