Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
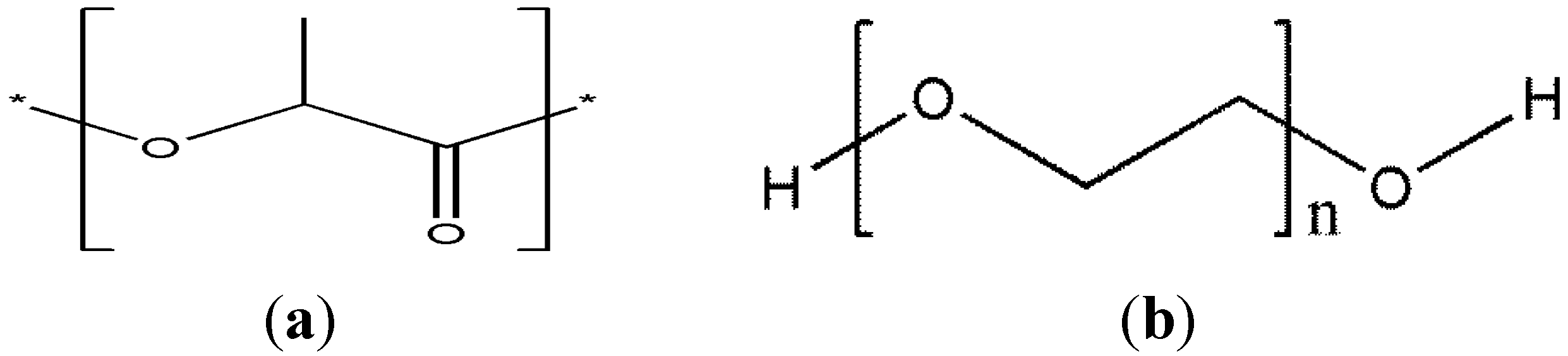
2.2. Preparation of PLA/PEG/xGnP Nanocomposites
2.3. Characterizations
2.3.1. Tensile Properties Measurement
2.3.2. X-ray Diffraction (XRD)
2.3.3. Fourier Transform Infrared (FTIR)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
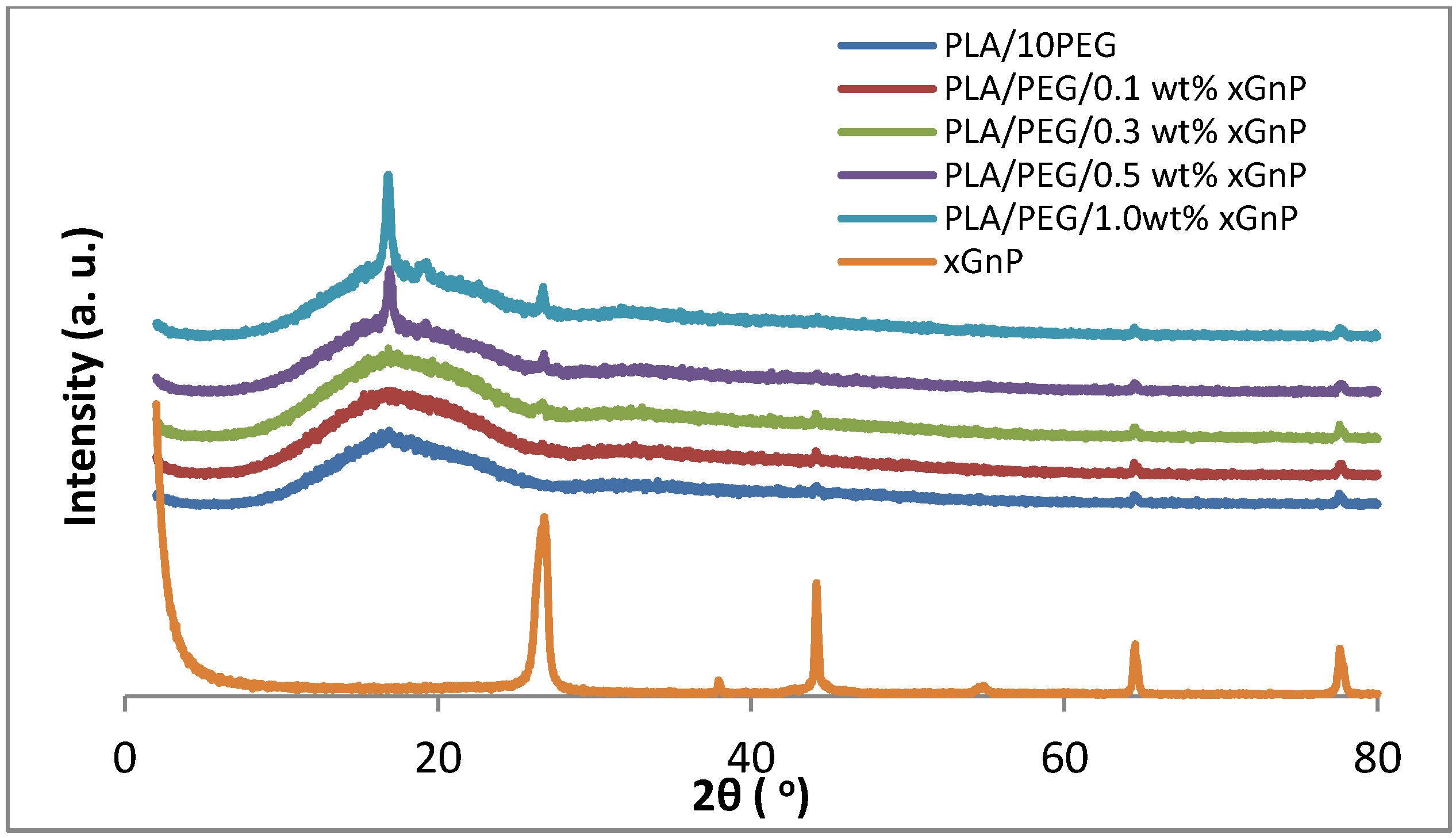
3.1. X-ray Diffraction
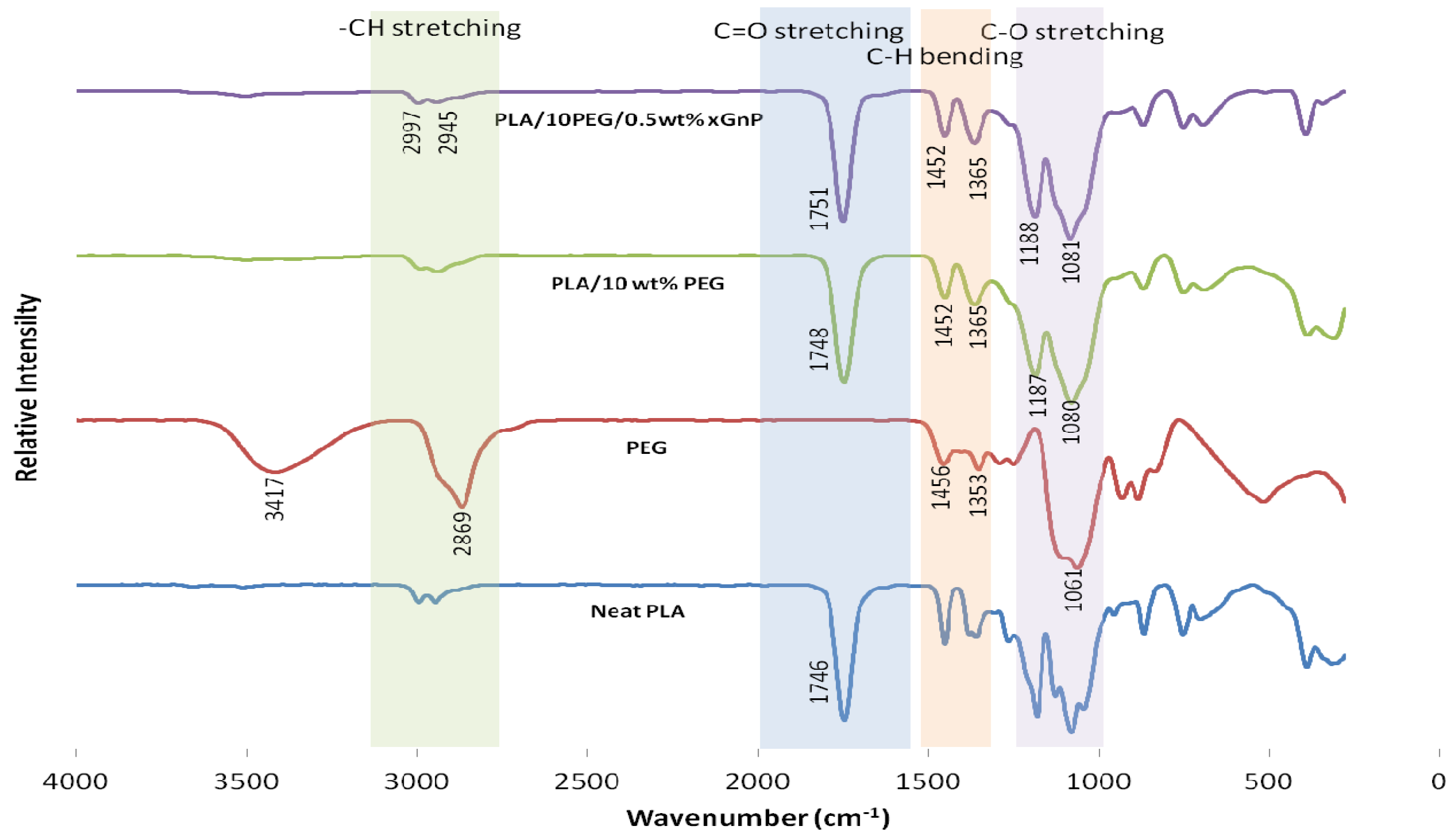
3.2. Fourier-Transform Infrared
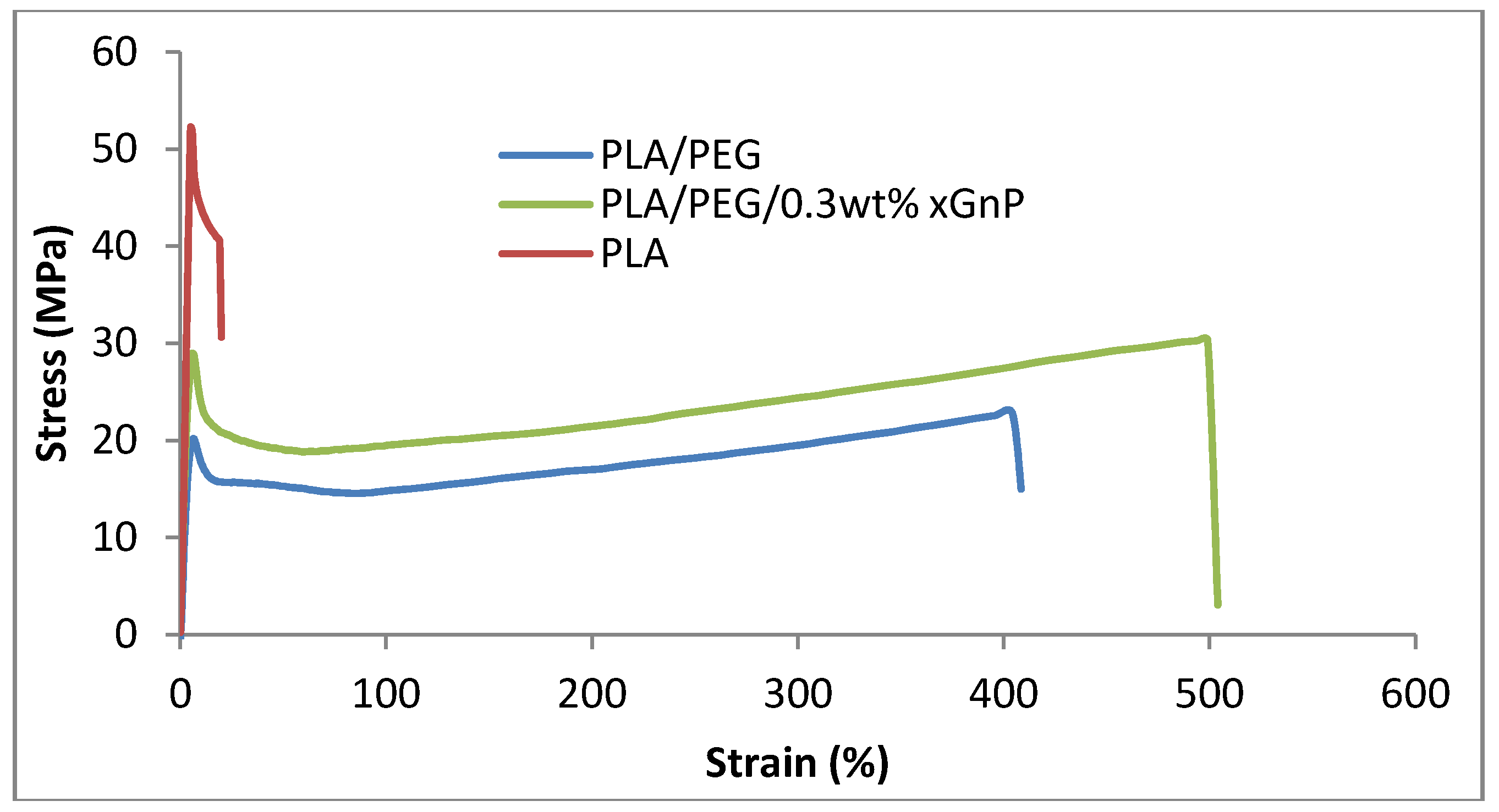
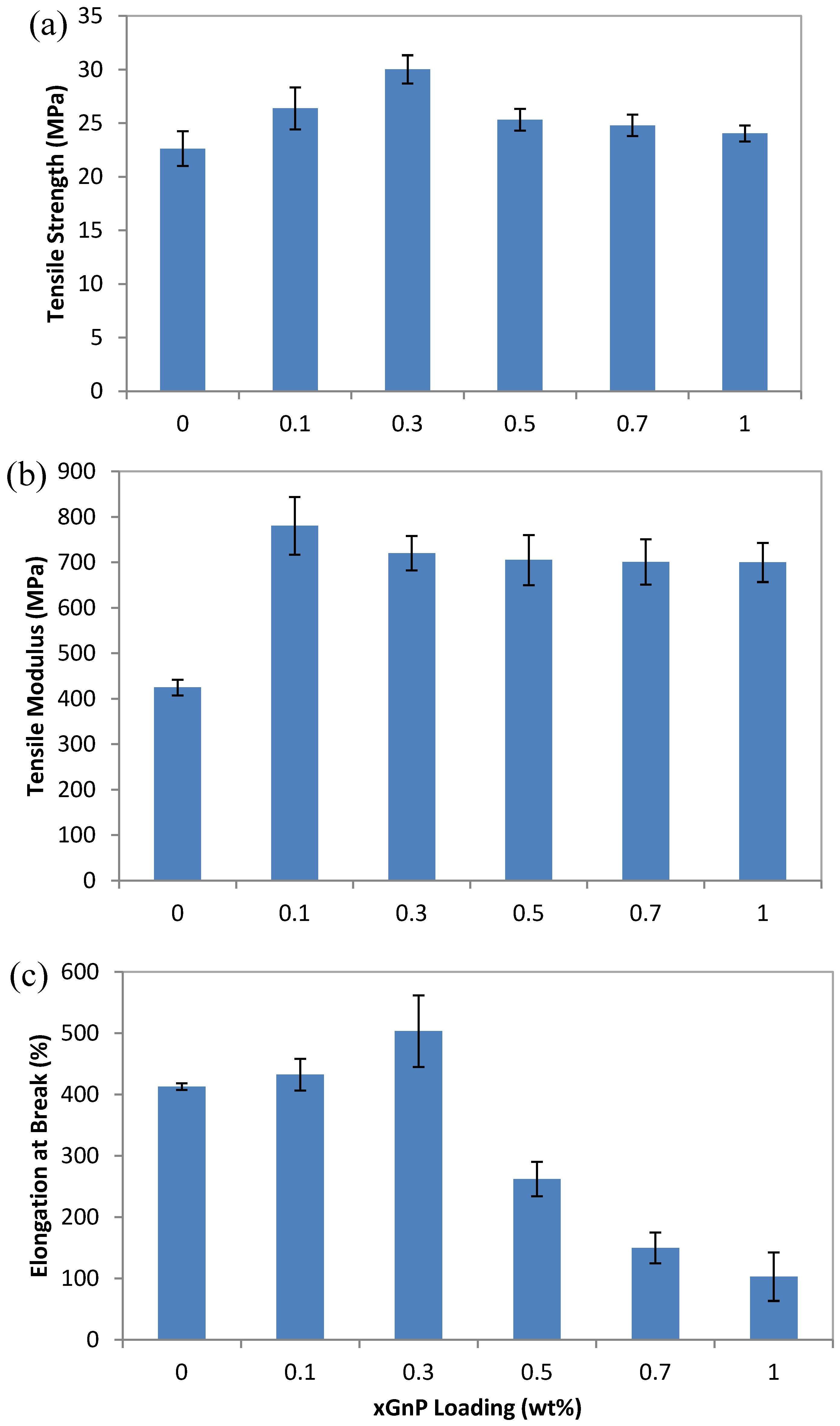
3.3. Mechanical Properties
3.4 Thermal Properties

 is melting enthalpy of the 100% PLA (93.6 J/g). As shown in Table 1, the crystallinity of PLA/PEG nanocomposites is increased after adding the xGnP, which corresponds well with the XRD results. However, the change of PLA crystallinity is so slight that it cannot induce a significant impact on the mechanical properties of the composites. Therefore, the significant reinforcement of the strength and modulus for PLA/PEG nanocomposites can be mostly attributed to the homogeneous dispersion of xGnP in the polymer matrix and strong interactions between both components [21].
is melting enthalpy of the 100% PLA (93.6 J/g). As shown in Table 1, the crystallinity of PLA/PEG nanocomposites is increased after adding the xGnP, which corresponds well with the XRD results. However, the change of PLA crystallinity is so slight that it cannot induce a significant impact on the mechanical properties of the composites. Therefore, the significant reinforcement of the strength and modulus for PLA/PEG nanocomposites can be mostly attributed to the homogeneous dispersion of xGnP in the polymer matrix and strong interactions between both components [21].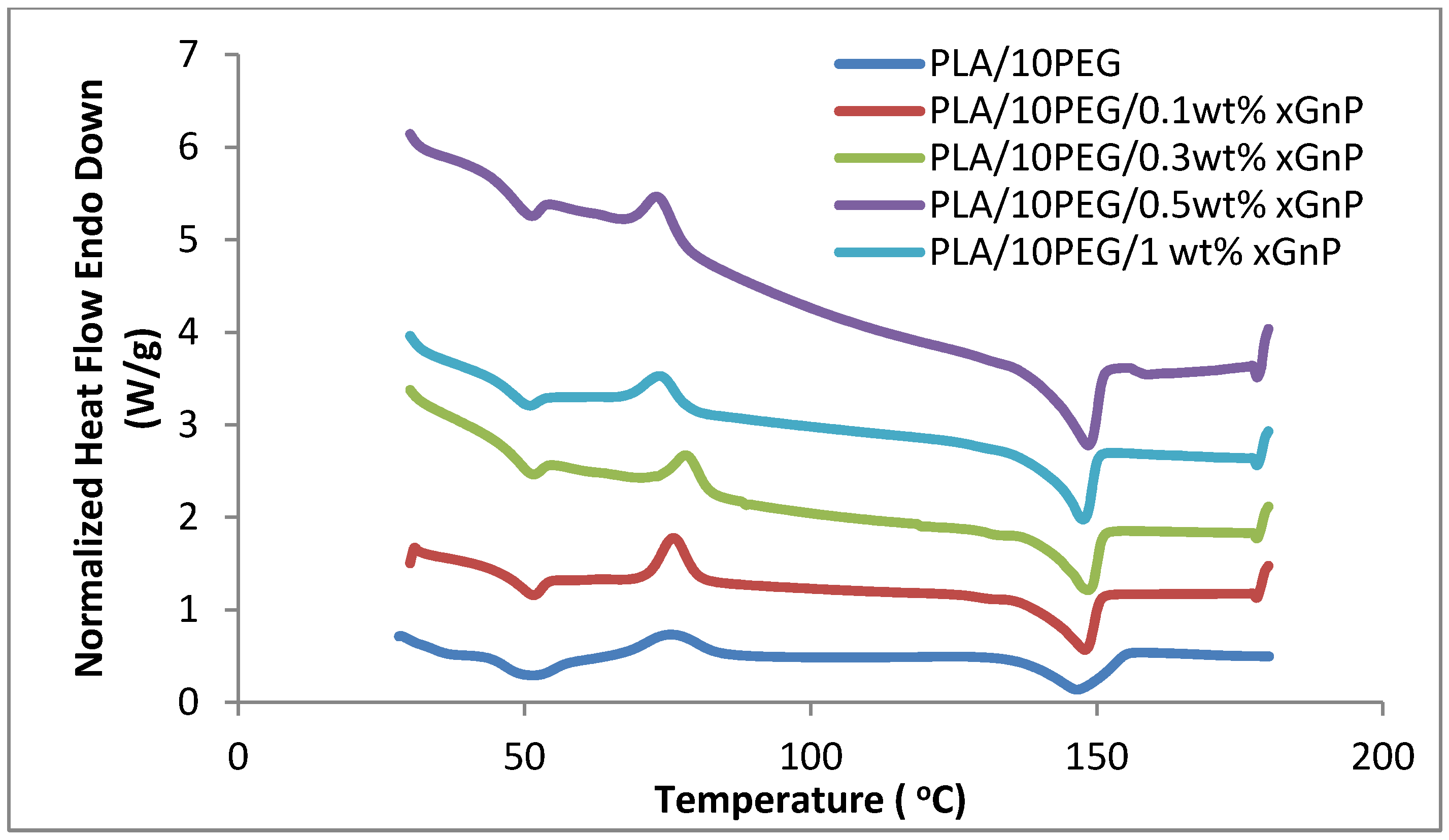
| Samples | Tg (°C) | Tcc (°C) | Tm (°C) | Xc (%) |
|---|---|---|---|---|
| PLA/10PEG | 51.63 | 74.30 | 146.48 | 49.43 |
| PLA/10 PEG/0.1 wt% xGnP | 51.26 | 75.98 | 147.82 | 50.95 |
| PLA/10 PEG/0.3 wt% xGnP | 51.26 | 78.26 | 148.25 | 51.77 |
| PLA/10 PEG/0.5 wt% xGnP | 50.79 | 73.29 | 148.27 | 53.91 |
| PLA/10 PEG/1 wt% xGnP | 50.45 | 73.80 | 147.30 | 54.61 |
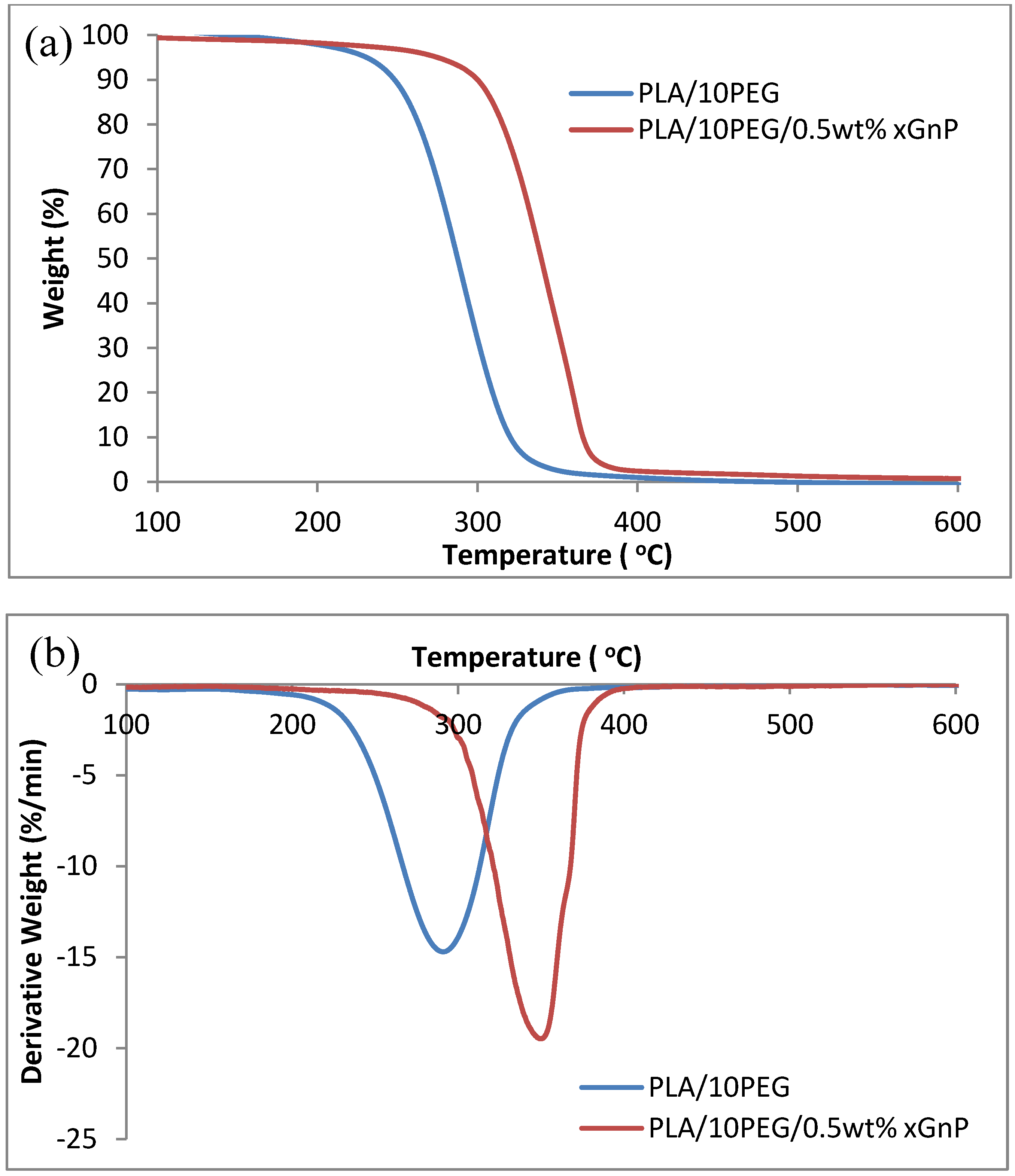
| Samples | Tonset (°C) | Tmax (°C) | T50 (°C) |
|---|---|---|---|
| PLA/PEG | 194.5 | 291.0 | 285.7 |
| PLA/PEG/0.5 wt% xGnP | 250.4 | 344.0 | 339.8 |
3.5 Morphology
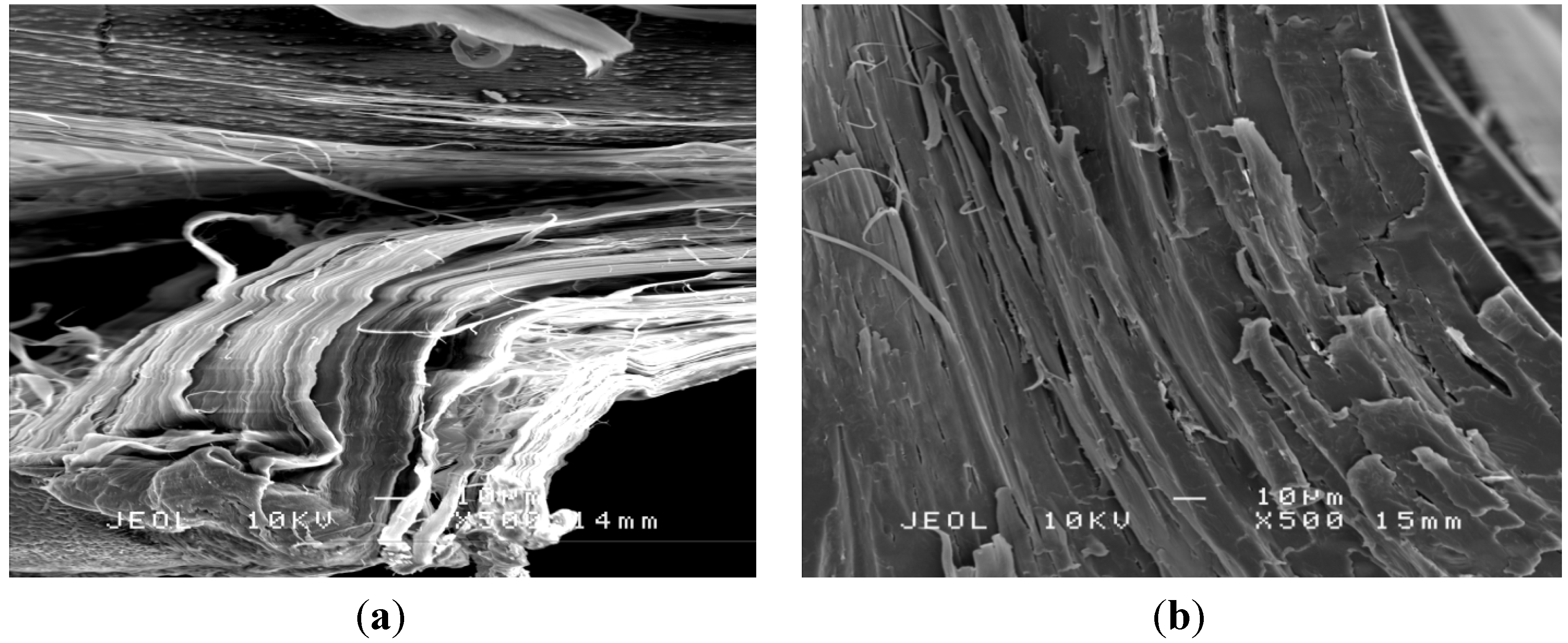
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kuila, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Martínez, G.; Ellis, G. Recent advances in the covalent modification of graphene with polymers. Macromol. Rapid Commun. 2011, 32, 1771–1789. [Google Scholar] [CrossRef]
- Chiang, M.-F.; Wu, T.-M. Synthesis and characterization of biodegradable poly(l-lactide)/layered double hydroxide nanocomposites. Compos. Sci. Technol. 2010, 70, 110–115. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. Mechanical properties and morphological characterization of exfoliated graphite-polypropylene nanocomposites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1675–1682. [Google Scholar] [CrossRef]
- Fukushima, H. Graphite nanoreinforcements in polymer nanocomposites. Ph.D. Thesis, Michigan State University, Michigan, MI, USA, 2003. [Google Scholar]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. A new compounding method for exfoliated graphite-polypropylene nanocomposites with enhanced flexural properties and lower percolation threshold. Compos. Sci. Technol. 2007, 67, 2045–2051. [Google Scholar] [CrossRef]
- Miloaga, D.G.; Hosein, H.-A.A.; Misra, M.; Drzal, L.T. Crystallization of poly(3-hydroxybutyrate) by exfoliated graphite nanoplatelets. J. Appl. Polym. Sci. 2007, 106, 2548–2558. [Google Scholar] [CrossRef]
- Pinto, A.M.; Cabral, J.; Tanaka, D.A.P.; Mendes, A.M.; Magalhães, F.D. Effect of incorporation of graphene oxide and graphene nanoplatelets on mechanical and gas permeability properties of poly(lactic acid) films. Polym. Int. 2013, 62, 33–40. [Google Scholar] [CrossRef]
- Gumus, S.; Ozkoc, G.; Aytac, A. Plasticized and unplasticized PLA/organoclay nanocomposites: Short- and long-term thermal properties, morphology, and nonisothermal crystallization behavior. J. Appl. Polym. Sci. 2011, 123, 2837–2848. [Google Scholar] [CrossRef]
- Pluta, M.; Paul, M.-A.; Alexandre, M.; Dubois, P. Plasticized polylactide/clay nanocomposites. I. The role of filler content and its surface organo-modification on the physico-chemical properties. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 299–311. [Google Scholar] [CrossRef]
- Sungsanit, K.; Kao, N.; Bhattacharya, S.N. Properties of linear poly(lactic acid)/polyethylene glycol blends. Polym. Eng. Sci. 2012, 52, 108–116. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, Y.S.; Topolkaraev, V.; Hiltner, A.; Baer, E. Crystallization and phase separation in blends of high stereoregular poly(lactide) with poly(ethylene glycol). Polymer 2003, 44, 5681–5689. [Google Scholar] [CrossRef]
- Gui, Z.; Xu, Y.; Gao, Y.; Lu, C.; Cheng, S. Novel polyethylene glycol-based polyester-toughened polylactide. Mater. Lett. 2012, 71, 63–65. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly(L-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Wan Yunus, W.M.Z.; Hussein, M.Z. Plasticized poly(lactic acid) with low molecular weight poly(ethylene glycol): Mechanical, thermal, and morphology properties. J. Appl. Polym. Sci. 2013, 130, 4576–4580. [Google Scholar]
- Hu, Y.; Hu, Y.S.; Topolkaraev, V.; Hiltner, A.; Baer, E. Aging of poly(lactide)/poly(ethylene glycol) blends. Part 2. Poly(lactide) with high stereoregularity. Polymer 2003, 44, 5711–5720. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Tensile Properties of Plastics. ASTM D638-02a; ASTM International: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Zhao, X.; Zhang, Q.; Chen, D.; Lu, P. Enhanced mechanical properties of graphene-based poly(vinyl alcohol) composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar] [CrossRef]
- Xu, Y.; Hong, W.; Bai, H.; Li, C.; Shi, G. Strong and ductile poly(vinyl alcohol)/graphene oxide composite films with a layered structure. Carbon 2009, 47, 3538–3543. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, Z. Crystallization kinetics and morphology of biodegradable poly(l-lactic acid)/graphene oxide nanocomposites: Influences of graphene oxide loading and crystallization temperature. Thermochim. Acta 2012, 527, 40–46. [Google Scholar] [CrossRef]
- Xu, H.-S.; Dai, X.J.; Lamb, P.R.; Li, Z.-M. Poly(L-lactide) crystallization induced by multiwall carbon nanotubes at very low loading. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 2341–2352. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2014, 6, 93-104. https://doi.org/10.3390/polym6010093
Chieng BW, Ibrahim NA, Yunus WMZW, Hussein MZ. Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers. 2014; 6(1):93-104. https://doi.org/10.3390/polym6010093
Chicago/Turabian StyleChieng, Buong Woei, Nor Azowa Ibrahim, Wan Md Zin Wan Yunus, and Mohd Zobir Hussein. 2014. "Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets" Polymers 6, no. 1: 93-104. https://doi.org/10.3390/polym6010093





