Synthesis of a Terpene-Based New Chiral Inducer and Preparation of an Asymmetric Polymer
Abstract
:1. Introduction
2. Experimental Section
2.1. Instruments
2.2. Materials
3. Results and Discussion
3.1. Synthesis and Twisting Power of a Chiral Inducer


| HTP a (μm−1) | MHTP b (μm−1·mol−1·kg) | βM c (μm−1) |
|---|---|---|
| 4.07 | 1.56 | 5.93 |
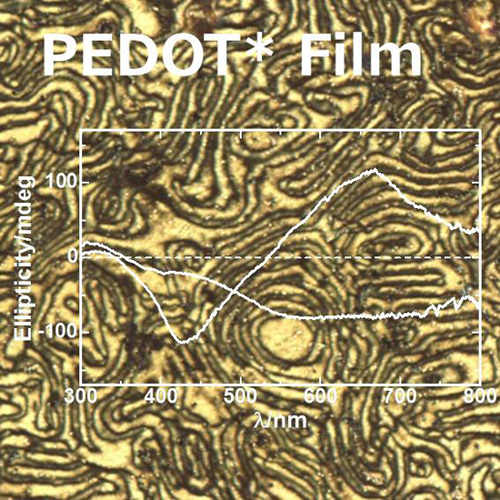
3.2. Synthesis and Properties of an Optically Active PEDOT* Film
| Material | Role | Chemical Structure | Quantity (mg, μmol) |
|---|---|---|---|
| 4-Cyano-4'-hexyl biphenyl (6CB) | Matrix | 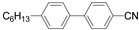 | 98.55, 374.19 |
| Chiral inducer | Induction of chirality | 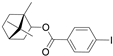 | 3.11, 8.08 |
| terEDOT | Monomer | 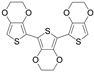 | 1.95, 4.62 |
| Tetrabutyl ammonium perchlorate (TBAP) | Supporting salt | 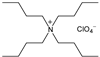 | 0.19, 0.54 |


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Resta, C.; di Pietro, S.; Majerić Elenkov, M.; Hamersak, Z.; Pescitelli, G.; di Bari, L. Consequences of chirality on the aggregation behavior of poly[2-methoxy-5-(2'-ethylhexyloxy)-p-phenylenevinylene] (MEH-PPV). Macromolecules 2014, 47, 4847–4850. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Whitesides, G.M. Dynamic aggregation of chiral spinners. Science 2002, 296, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, Y.; Fujiki, M.; Nakano, Y. Limonene Magic: Noncovalent molecular chirality transfer leading to ambidextrous circularly polarized luminescent π-conjugated polymers. New J. Chem. 2010, 34, 637–647. [Google Scholar] [CrossRef]
- Khatri, C.A.; Pavlova, Y.; Green, M.M.; Morawetz, H. Chiral solvation as a means to quantitatively characterize preferential solvation of a helical polymer in mixed solvents. J. Am. Chem. Soc. 1997, 119, 6991–6995. [Google Scholar] [CrossRef]
- Wang, Y.; Sakamoto, T.; Nakano, T. Molecular chirality induction to an achiral π-conjugated polymer by circularly polarized light. Chem. Commun. 2012, 48, 1871–1873. [Google Scholar] [CrossRef]
- Savoini, M.; Biagioni, P.; Meskers, S.C.J.; Duo, L.; Hecht, B.; Finazzi, M. Spontaneous formation of left- and right-handed cholesterically ordered domains in an enantiopure chiral polyfluorene film. J. Phys. Chem. Lett. 2011, 2, 1359–1362. [Google Scholar] [CrossRef]
- Shockravi, A.; Javadi, A.; Abouzari-Lotf, E. Binaphthyl-based macromolecules: A review. RSC Adv. 2013, 3, 6717–6746. [Google Scholar] [CrossRef]
- Watanabe, K.; Akagi, K. Helically assembled π-conjugated polymers with circularly polarized luminescence. Sci. Technol. Adv. Mater. 2014, 15. [Google Scholar] [CrossRef]
- Matsushita, S.; Yan, B.; Yamamoto, S.; Jeong, Y.S.; Akagi, K. Helical carbon and graphite films prepared from helical poly(3,4-ethylenedioxythiophene) films synthesized by electrochemical polymerization in chiral nematic liquid crystals. Angew. Chem. Int. Ed. 2014, 53, 1659–1663. [Google Scholar] [CrossRef]
- Khiew, P.S.; Radiman, S.; Huang, N.M.; Kan, C.S.; Ahmad, M.S. In situ polymerization of conducting polyaniline in bicontinuous cubic phase of lyotropic liquid crystal. Colloids Surf. A Physicochem. Eng. Asp. 2004, 247, 35–40. [Google Scholar] [CrossRef]
- Araya, K.; Mukoh, A.; Narahara, T.; Shirakawa, H. Synthesis of highly-oriented polyacetylene film in a liquid crystal solvent. Chem. Lett. 1986, 14, 199–206. [Google Scholar]
- Yang, J.; Kimb, D.H.; Hendricksb, J.L.; Leachb, M.; Northeya, R.; Martin, D.C. Ordered surfactant-templated poly(3,4-ethylenedioxythiophene) (PEDOT) conducting polymer on microfabricated neural probes. Acta Biomater. 2005, 1, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, S.; Jeong, Y.S.; Akagi, K. Electrochromism-driven linearly and circularly polarised dichroism of poly(3,4-ethylenedioxythiophene) derivatives with chirality and liquid crystallinity. Chem. Commun. 2013, 49, 1883–1890. [Google Scholar] [CrossRef]
- Kawabata, K.; Goto, H. Periodic structure in a fluorene-based polymer prepared by electrochemical polymerization. Chem. Lett. 2009, 38, 706–707. [Google Scholar] [CrossRef]
- Goto, H. Asymmetric polymerisation in liquid crystals and resultant electro-chiroptical effect: Structure organizing polymerisation and chiral charge carrier “Chiralion”. IOP Conf. Ser. Mater. Sci. Eng. 2014, 54. [Google Scholar] [CrossRef]
- Goto, H. Vortex fibril structure and chiroptical electrochromic effect of optically active poly(3,4-ethylenedioxythiophene) (PEDOT*) prepared by chiral transcription electrochemical polymerisation in cholesteric liquid crystal. J. Mater. Chem. 2009, 19, 4914–4921. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Goto, H.; Iimura, S.; Asano, T.; Akagi, K. Chiral BEDOT-based copolymer prepared by chemical and electrochemical polymerization. Curr. Appl. Phys. 2006, 6, 960–963. [Google Scholar] [CrossRef]
- Goto, H. Doping-dedoping-driven optic effect of π-conjugated polymers prepared in cholesteric-liquid-crystal electrolytes. Phys. Rev. Lett. 2007, 98. [Google Scholar] [CrossRef] [PubMed]
- Goto, H. Cholesteric liquid crystal inductive asymmetric polymerization: Synthesis of chiral polythiophene derivatives from achiral monomers in a cholesteric liquid crystal. Macromolecules 2007, 40, 1377–1385. [Google Scholar] [CrossRef]
- Iseki, T.; Kawabata, K.; Kawashima, H.; Goto, H. Catalysis direction selective asymmetric polymerization in chiral liquid crystal medium. Polymer 2014, 55, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Iseki, T.; Goto, H. Synthesis of 1,1'-binaphthyl derivatives by Miyaura–Suzuki cross-coupling in cholesteric liquid crystal phase. Monatshefte für Chem. Chem. Mon. 2014, 145, 1145–1149. [Google Scholar] [CrossRef]
- Yoneyama, H.; Tsujimoto, A.; Goto, H. Preparation of optically active pyridine-based conducting polymer films using a liquid crystal electrolyte containing a cholesterol derivative. Macromolecules 2007, 40, 5279–5283. [Google Scholar] [CrossRef]
- Kawabata, K.; Takeguchi, M.; Goto, H. Optical activity of heteroaromatic conjugated polymer films prepared by asymmetric electrochemical polymerization in cholesteric liquid crystals: Structural function for chiral induction. Macromolecules 2013, 46, 2078–2091. [Google Scholar] [CrossRef]
- Earl, D.J.; Wilson, M.R. Predictions of molecular chirality and helical twisting powers: A theoretical study. J. Chem. Phys. 2003, 119, 10280–10288. [Google Scholar] [CrossRef] [Green Version]
- Devlin, F.J.; Stephens, P.J.; Besse, P. Conformational rigidification via derivatization facilitates the determination of absolute configuration using chiroptical spectroscopy: A case study of the chiral alcohol endo-borneol. J. Org. Chem. 2005, 70, 2980–2993. [Google Scholar] [CrossRef] [PubMed]
- Superchi, S.; Donnoli, M.I.; Proni, G.; Spada, G.P.; Rosini, C. Induction of cholesteric mesophases by simple cyclic derivatives of p,p'-disubstituted 1,2-diphenylethane-1,2-diols: Importance of shape and polarizability effects. J. Org. Chem. 1999, 64, 4762–4767. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Harlow, R.L. Cyano–Halogen Interactions and their role in the crystal structures of the 4-halobenzonitriles. J. Am. Chem. Soc. 1989, 111, 6757–6764. [Google Scholar] [CrossRef]
- Fourmigué, M.; Batail, P. Activation of hydrogen- and halogen-bonding interactions in tetrathiafulvalene-based crystalline molecular conductors. Chem. Rev. 2004, 104, 5379–5418. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, J.M.; Blanchard, P.; Brisset, H.; Akoudad, S.; Roncali, J. Proquinoid acceptors as building blocks for the design of efficient π-conjugated fluorophores with high electron affinity. Chem. Commun. 2000, 2000, 939–940. [Google Scholar] [CrossRef]
- Kuribayashi, M.; Hori, K. Crystal structures of 4-cyano-4'-hexylbiphenyl (6CB) and 4-cyano-4'-heptylbiphenyl (7CB) in relation to odd-even effect. Liq. Cryst. 1999, 26, 809–815. [Google Scholar] [CrossRef]
- Frommer, J. Liquid crystals—As seen by the scanning tunnelling and force microscopes. Liq. Cryst. Today 1993, 3, 1–12. [Google Scholar] [CrossRef]
- Pieraccini, S.; Donnoli, M.I.; Ferrarini, A.; Gottarelli, G.; Licini, G.; Rosini, C.; Superchi, S.; Spada, G.P. A correlation between the absolute configuration of alkyl aryl sulfoxides and their helical twisting powers in nematic liquid crystals. J. Org. Chem. 2003, 68, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.B.; Kamien, R.D.; Lubensky, T.C. Microscopic origin of cholesteric pitch. Phys. Rev. Lett. 1997, 78. [Google Scholar] [CrossRef]
- Todd, S.M.; Alberta Ferrarini, A.; Moro, G.J. Molecular modelling of chiral nematics. Phys. Chem. Chem. Phys. 2001, 3, 5535–5541. [Google Scholar] [CrossRef]
- Eelkema, R.; Feringa, B.L. Amplification of chirality in liquid crystal. Org. Biomol. Chem. 2006, 4, 3729–3745. [Google Scholar] [CrossRef] [PubMed]
- Grenier, C.R.G.; George, S.J.; Joncheray, T.J.; Meijer, E.W.; Reynolds, J.R. Chiral ethylhexyl substituents for optically active aggregates of π-conjugated polymers. J. Am. Chem. Soc. 2007, 129, 10694–10699. [Google Scholar] [CrossRef] [PubMed]
- Amabilino, D.B.; Gomar-Nadal, E.; Veciana, J.; Rovira, C. Chiral teleinduction in the formation of a macromolecular multistate chiroptical redox switch. Adv. Mater. 2005, 17, 2095–2098. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumura, A.; Yang, F.; Goto, H. Synthesis of a Terpene-Based New Chiral Inducer and Preparation of an Asymmetric Polymer. Polymers 2015, 7, 147-155. https://doi.org/10.3390/polym7010147
Matsumura A, Yang F, Goto H. Synthesis of a Terpene-Based New Chiral Inducer and Preparation of an Asymmetric Polymer. Polymers. 2015; 7(1):147-155. https://doi.org/10.3390/polym7010147
Chicago/Turabian StyleMatsumura, Atsushi, Fan Yang, and Hiromasa Goto. 2015. "Synthesis of a Terpene-Based New Chiral Inducer and Preparation of an Asymmetric Polymer" Polymers 7, no. 1: 147-155. https://doi.org/10.3390/polym7010147