Thermal Properties of Methyl Ester-Containing Poly(2-oxazoline)s
Abstract
:1. Introduction

2. Experimental Section
2.1. Materials
2.2. Instrumentation
2.3. Polymer Synthesis
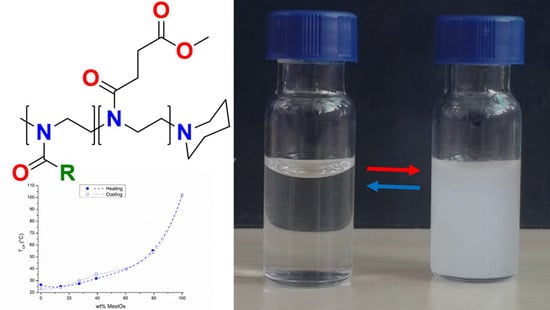
3. Results and Discussion

| Monomer 1 | Monomer 2 | Feed ratio (M1:M2) | Composition a (M1:M2) | SEC b | TCP (°C) c | Tg d (°C) | TGA (°C) e | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mn (103 g/mol) | Ð | Heating | Cooling | 5% | 50% | |||||
| MestOx | - | 100:0 | 100:0 | 19.4 | 1.10 | 101 | 102 | 39 | 258 | 353 |
| C3MestOx | - | 100:0 | 100:0 | 24.1 | 1.12 | 26 | 26 | −1 | 325 | 360 |
| EtOx * | - | 100:0 | 100:0 | 14.0* | 1.19 * | 91 * | 91 * | 54 * | - | - |
| nPropOx | - | 100:0 | 100:0 | 16.6 | 1.10 | 27 | 23 | 35 | 312 | 406 |
| EtOx | MestOx | 90:10 | 91:9 | 18.9 | 1.14 | 93 | 96 | 53 | 322 | 403 |
| EtOx | MestOx | 80:20 | 82:18 | 16.9 | 1.14 | 99 | 102 | 45 | 293 | 389 |
| EtOx | MestOx | 70:30 | 70:30 | 17.4 | 1.13 | 103 | 106 | 44 | 310 | 376 |
| EtOx | MestOx | 50:50 | 49:51 | 19.3 | 1.14 | # | # | 39 | 306 | 365 |
| EtOx | MestOx | 30:70 | 29:71 | 22.8 | 1.16 | 102 | 104 | 38 | 296 | 359 |
| nPropOx | MestOx | 90:10 | 90:10 | 21.0 | 1.12 | 25 | 24 | 30 | 327 | 393 |
| nPropOx | MestOx | 80:20 | 79:21 | 15.5 | 1.12 | 27 | 30 | 30 | 306 | 385 |
| nPropOx | MestOx | 70:30 | 68:32 | 16.3 | 1.13 | 32 | 36 | 30 | 303 | 377 |
| nPropOx | MestOx | 50:50 | 48:52 | 20.6 | 1.15 | 40 | 40 | 32 | 310 | 367 |
| nPropOx | MestOx | 30:70 | 27:73 | 29.3 | 1.19 | 55 | 54 | 35.0 | 309 | 367 |
| EtOx | C3MestOx | 90:10 | 90:10 | 21.1 | 1.13 | 89 | 89 | 43 | 334 | 399 |
| EtOx | C3MestOx | 80:20 | 82:18 | 21.8 | 1.14 | 78 | 79 | 39 | 313 | 382 |
| EtOx | C3MestOx | 70:30 | 70:30 | 21.6 | 1.14 | 71 | 78 | 27 | 316 | 377 |
| EtOx | C3MestOx | 50:50 | 50:50 | 22.7 | 1.14 | 56 | 56 | 17 | 322 | 369 |
| EtOx | C3MestOx | 30:70 | 30:70 | 24.1 | 1.14 | 42 | 43 | -2 | 320 | 363 |
| nPropOx | C3MestOx | 90:10 | 90:10 | 19.2 | 1.21 | 24 | 25 | 28 | 321 | 399 |
| nPropOx | C3MestOx | 80:20 | 79:21 | 20.7 | 1.15 | 25 | 32 | 18 | 328 | 385 |
| nPropOx | C3MestOx | 70:30 | 68:32 | 21.0 | 1.15 | 25 | 31 | 15 | 332 | 379 |
| nPropOx | C3MestOx | 50:50 | 48:52 | 16.7 | 1.21 | 26 | 27 | 12 | 311 | 368 |
| nPropOx | C3MestOx | 30:70 | 28:72 | 18.8 | 1.16 | 25 | 30 | 5 | 304 | 361 |




4. Conclusions
Supplementary Information
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Vancoillie, G.; Frank, D.; Hoogenboom, R. Thermoresponsive poly(oligo ethylene glycol acrylates). Prog. Polym. Sci. 2014, 39, 1074–1095. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Schild, H.G. Poly(n-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Lutz, J.-F. Thermo-switchable materials prepared using the oegma-platform. Adv. Mater. 2011, 23, 2237–2243. [Google Scholar] [CrossRef]
- Kouwer, P.H.J.; Koepf, M.; Le Sage, V.A.A.; Jaspers, M.; van Buul, A.M.; Eksteen-Akeroyd, Z.H.; Woltinge, T.; Schwartz, E.; Kitto, H.J.; Hoogenboom, R.; et al. Responsive biomimetic networks from polyisocyanopeptide hydrogels. Nature 2013, 493, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Koepf, M.; Kitto, H.J.; Schwartz, E.; Kouwer, P.H.J.; Nolte, R.J.M.; Rowan, A.E. Preparation and characterization of non-linear poly(ethylene glycol) analogs from oligo(ethylene glycol) functionalized polyisocyanopeptides. Eur. Polym. J. 2013, 49, 1510–1522. [Google Scholar] [CrossRef]
- Hu, G.; Li, W.; Hu, Y.; Xu, A.; Yan, J.; Liu, L.; Zhang, X.; Liu, K.; Zhang, A. Water-soluble chiral polyisocyanides showing thermoresponsive behavior. Macromolecules 2013, 46, 1124–1132. [Google Scholar] [CrossRef]
- Bloksma, M.M.; Paulus, R.M.; van Kuringen, H.P.C.; van der Woerdt, F.; Lambermont-Thijs, H.M.L.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive poly(2-oxazine)s. Macromol. Rapid Commun. 2012, 33, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Jordan, R. Modulation of the lower critical solution temperature of 2-alkyl-2-oxazoline copolymers. Colloid. Polym. Sci. 2008, 286, 395–402. [Google Scholar] [CrossRef]
- Diehl, C.; Schlaad, H. Thermo-responsive polyoxazolines with widely tuneable lcst. Macromol. Biosci. 2009, 9, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Kataoka, K. Precise control of lower critical solution temperature of thermosensitive poly(2-isopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline as a hydrophilic comonomer. Macromolecules 2006, 39, 6622–6630. [Google Scholar] [CrossRef]
- Park, J.-S.; Kataoka, K. Comprehensive and accurate control of thermosensitivity of poly(2-alkyl-2-oxazoline)s via well-defined gradient or random copolymerization. Macromolecules 2007, 40, 3599–3609. [Google Scholar] [CrossRef]
- Hoogenboom, R. Poly(2-oxazoline)s: A polymer class with numerous potential applications. Angew. Chem. Int. Ed. 2009, 48, 7978–7994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Luxenhofer, R.; Jordan, R. Thermoresponsive poly(2-oxazoline) molecular brushes by living ionic polymerization: Modulation of the cloud point by random and block copolymer pendant chains. Macromol. Chem. Phys. 2012, 213, 1963–1969. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Huber, S.; Hytry, J.; Tong, J.; Kabanov, A.V.; Jordan, R. Chiral and water-soluble poly(2-oxazoline)s. J. Polym. Sci. Polym. Chem. 2013, 51, 732–738. [Google Scholar] [CrossRef]
- Sambe, L.; de La Rosa, V.R.; Belal, K.; Stoffelbach, F.; Lyskawa, J.; Delattre, F.; Bria, M.; Cooke, G.; Hoogenboom, R.; Woisel, P. Programmable polymer-based supramolecular temperature sensor with a memory function. Angew. Chem. Int. Ed. 2014, 53, 5044–5048. [Google Scholar]
- Kanazawa, H. Temperature-responsive polymers for liquid-phase separations. Anal. Bioanal. Chem. 2004, 378, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Gaertner, F.C.; Luxenhofer, R.; Blechert, B.; Jordan, R.; Essler, M. Synthesis, biodistribution and excretion of radiolabeled poly(2-alkyl-2-oxazoline)s. J. Control. Release 2007, 119, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanov, A.V.; Jordan, R. Poly(2-oxazoline)s as polymer therapeutics. Macromol. Rapid Commun. 2012, 33, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, O.; Monnery, B.D.; Filippov, S.K.; Hoogenboom, R.; Hruby, M. Poly(2-oxazoline)s—Are they more advantageous for biomedical applications than other polymers? Macromol. Rapid Commun. 2012, 33, 1648–1662. [Google Scholar] [CrossRef] [PubMed]
- Zalipsky, S.; Hansen, C.B.; Oaks, J.M.; Allen, T.M. Evaluation of blood clearance rates and biodistribution of poly(2-oxazoline)-grafted liposomes. J. Pharm. Sci. 1996, 85, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Pasut, G.; Via, L.D.; Fijten, M.W.M.; Schubert, U.S.; Hoogenboom, R.; Veronese, F.M. Synthesis and characterization of poly(2-ethyl 2-oxazoline)-conjugates with proteins and drugs: Suitable alternatives to peg-conjugates? J. Control. Release 2008, 125, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Luxenhofer, R.; Sahay, G.; Schulz, A.; Alakhova, D.; Bronich, T.K.; Jordan, R.; Kabanov, A.V. Structure–property relationship in cytotoxicity and cell uptake of poly(2-oxazoline) amphiphiles. J. Control. Release 2011, 153, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Bloksma, M.M.; Weber, C.; Perevyazko, I.Y.; Kuse, A.; Baumgärtel, A.; Vollrath, A.; Hoogenboom, R.; Schubert, U.S. Poly(2-cyclopropyl-2-oxazoline): From rate acceleration by cyclopropyl to thermoresponsive properties. Macromolecules 2011, 44, 4057–4064. [Google Scholar] [CrossRef] [Green Version]
- Glassner, M.; Lava, K.; de la Rosa, V.R.; Hoogenboom, R. Tuning the lcst of poly(2-cyclopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline. J. Polym. Sci. Polym. Chem. 2014, 52, 3118–3122. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Thijs, H.M.L.; Jochems, M.J.H.C.; van Lankvelt, B.M.; Fijten, M.W.M.; Schubert, U.S. Tuning the lcst of poly(2-oxazoline)s by varying composition and molecular weight: Alternatives to poly(n-isopropylacrylamide)? Chem. Commun. 2008, 44, 5758–5760. [Google Scholar] [CrossRef] [PubMed]
- Guillerm, B.; Monge, S.; Lapinte, V.; Robin, J.J. How to modulate the chemical structure of polyoxazolines by appropriate functionalization. Macromol. Rapid Commun. 2012, 33, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Volet, G.; Lav, T.X.; Babinot, J.; Amiel, C. Click-chemistry: An alternative way to functionalize poly(2-methyl-2-oxazoline). Macromol. Chem. Phys. 2011, 212, 118–124. [Google Scholar] [CrossRef]
- Rossegger, E.; Schenk, V.; Wiesbrock, F. Design strategies for functionalized poly(2-oxazoline)s and derived materials. Polymers 2013, 5, 956–1011. [Google Scholar]
- Fijten, M.W.M.; Haensch, C.; van Lankvelt, B.M.; Hoogenboom, R.; Schubert, U.S. Clickable poly(2-oxazoline)s as versatile building blocks. Macromol. Chem. Phys. 2008, 209, 1887–1895. [Google Scholar] [CrossRef]
- Levy, A.; Litt, M. Polymerization of cyclic iminoethers. V. 1,3-oxazolines with hydroxy- acetoxy- and carboxymethyl-alkyl groups in 2 position and their polymers. J. Polym. Sci. Polym. Chem. 1968, 6, 1883–1894. [Google Scholar] [CrossRef]
- Taubmann, C.; Luxenhofer, R.; Cesana, S.; Jordan, R. First aldehyde-functionalized poly(2-oxazoline)s for chemoselective ligation. Macromol. Biosci. 2005, 5, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Gress, A.; Volkel, A.; Schlaad, H. Thio-click modification of poly [2-(3-butenyl)-2-oxazoline]. Macromolecules 2007, 40, 7928–7933. [Google Scholar] [CrossRef]
- Kelly, A.M.; Hecke, A.; Wirnsberger, B.; Wiesbrock, F. Synthesis of poly(2-oxazoline)-based hydrogels with tailor-made swelling degrees capable of stimuli-triggered compound release. Macromol. Rapid Commun. 2011, 32, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Bouten, P.J.M.; Hertsen, D.; Vergaelen, M.; Monnery, B.D.; Boerman, M.A.; Goossens, H.; Catak, S.; van Hest, J.C.M.; van Speybroeck, V.; Hoogenboom, R. Accelerated living cationic ring-opening polymerization of a methyl ester functionalized 2-oxazoline monomer. Polym. Chem. 2015, 6, 514–518. [Google Scholar] [CrossRef]
- Bouten, P.J.M.; Hertsen, D.; Vergaelen, M.; Monnery, B.M.; Catak, S.; van Hest, J.C.M.; van Speybroeck, V.; Hoogenboom, R. Synthesis of poly(2-oxazoline)s with side chain methyl ester functionalities: Detailed understanding of copolymerization behavior of methyl ester containing monomers with 2-alkyl-2-oxazolines. J. Polym. Sci. Polym. Chem. 2015. accepted. [Google Scholar] [CrossRef]
- Hoogenboom, R. Polyoxazoline polymers and methods for their preparation conjugates of these polymers and medical uses thereof. WO 2013/103297 A1, 11 July 2013. [Google Scholar]
- Mees, M.; Hoogenboom, R. Functional poly(2-oxazoline)s by direct amidation of methyl ester side chains. Macromolecules 2015, 48, 3531–3538. [Google Scholar] [CrossRef] [Green Version]
- Grogna, M.; Cloots, R.; Luxen, A.; Jerome, C.; Desreux, J.F.; Detrembleur, C. Design and synthesis of novel DOTA(Gd3+)-polymer conjugates as potential mri contrast agents. J. Mater. Chem. 2011, 21, 12917–12926. [Google Scholar] [CrossRef]
- Steunenberg, P.; Könst, P.M.; Scott, E.L.; Franssen, M.C.R.; Zuilhof, H.; Sanders, J.P.M. Polymerisation of β-alanine through catalytic ester–amide exchange. Eur. Polym. J. 2013, 49, 1773–1781. [Google Scholar] [CrossRef]
- Zarka, M.T.; Nuyken, O.; Weberskirch, R. Amphiphilic polymer supports for the asymmetric hydrogenation of amino acid precursors in water. Chem. Eur. J. 2003, 9, 3228–3234. [Google Scholar] [CrossRef] [PubMed]
- Kotre, T.; Zarka, M.T.; Krause, J.O.; Buchmeiser, M.R.; Weberskirch, R.; Nuyken, O. Design and application of amphiphilic polymeric supports for micellar catalysis. Macromol. Symp. 2004, 217, 203–214. [Google Scholar] [CrossRef]
- Rueda, J.C.; Zschoche, S.; Komber, H.; Krahl, F.; Arndt, K.F.; Voit, B. New thermo-sensitive graft copolymers based on a poly(n-isopropylacrylamide) backbone and functional polyoxazoline grafts with random and diblock structure. Macromol. Chem. Phys. 2010, 211, 706–716. [Google Scholar] [CrossRef]
- Zschoche, S.; Rueda, J.C.; Binner, M.; Komber, H.; Janke, A.; Arndt, K.F.; Lehmann, S.; Voit, B. Reversibly switchable pH- and thermoresponsive core-shell nanogels based on poly(NiPAAm)-graft-poly(2-carboxyethyl-2-oxazoline)s. Macromol. Chem. Phys. 2012, 213, 215–226. [Google Scholar] [CrossRef]
- Rueda, J.C.; Campos, E.; Komber, H.; Zschoche, S.; Haussler, L.; Voit, B. Synthesis and characterization of new pH- and thermo-responsive hydrogels based on n-isopropylacrylamide and 2-oxazolines. Des. Monomers Polym. 2014, 17, 208–216. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Fijten, M.W.M.; Thijs, H.M.L.; van Lankvelt, B.M.; Schubert, U.S. Microwave-assisted synthesis and properties of a series of poly(2-alkyl-2-oxazoline)s. Des. Monomers Polym. 2005, 8, 659–671. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouten, P.J.M.; Lava, K.; Van Hest, J.C.M.; Hoogenboom, R. Thermal Properties of Methyl Ester-Containing Poly(2-oxazoline)s. Polymers 2015, 7, 1998-2008. https://doi.org/10.3390/polym7101494
Bouten PJM, Lava K, Van Hest JCM, Hoogenboom R. Thermal Properties of Methyl Ester-Containing Poly(2-oxazoline)s. Polymers. 2015; 7(10):1998-2008. https://doi.org/10.3390/polym7101494
Chicago/Turabian StyleBouten, Petra J. M., Kathleen Lava, Jan C. M. Van Hest, and Richard Hoogenboom. 2015. "Thermal Properties of Methyl Ester-Containing Poly(2-oxazoline)s" Polymers 7, no. 10: 1998-2008. https://doi.org/10.3390/polym7101494
APA StyleBouten, P. J. M., Lava, K., Van Hest, J. C. M., & Hoogenboom, R. (2015). Thermal Properties of Methyl Ester-Containing Poly(2-oxazoline)s. Polymers, 7(10), 1998-2008. https://doi.org/10.3390/polym7101494