Highly Sulfonated Diamine Synthesized Polyimides and Protic Ionic Liquid Composite Membranes Improve PEM Conductivity
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials

2.2. Synthesis of 1,4-Bis(4-aminophenoxy-2-sulfonic acid) Benzenesulfonic Acid (pBABTS)

2.3. Synthesis of Sulfonated Polyimides

| Code | Diamines Mole in Feed (mmol) | Dianhydride ǂ (11.38 mmol) | IEC a(theory) (mmol/g) | IEC b(exp) (mmol/g) | |
|---|---|---|---|---|---|
| pBABTS | Diamine * | ||||
| SPI1pO | 5.69 | BAPP 5.69 | ODPA | 2.01 | 1.64 |
| SPI2pO | 6.83 | BAPP 4.55 | ODPA | 2.38 | 1.97 |
| SPI3pO | 7.97 | BAPP 3.41 | ODPA | 2.73 | 2.60 |
| SPI1nO | 5.69 | BAPN 5.69 | ODPA | 2.11 | 1.60 |
| SPI2nO | 6.83 | BAPN 4.55 | ODPA | 2.47 | 1.90 |
| SPI3nO | 7.97 | BAPN 3.41 | ODPA | 2.80 | 2.40 |
| SPI1fO | 5.69 | 9FDA 5.69 | ODPA | 2.10 | 1.65 |
| SPI2fO | 6.83 | 9FDA 4.55 | ODPA | 2.46 | 1.98 |
| SPI3fO | 7.97 | 9FDA 3.41 | ODPA | 2.80 | 2.30 |
| SPI1nN | 5.69 | BAPN 5.69 | NTDA | 2.24 | 1.72 |
| SPI2nN | 6.83 | BAPN 4.55 | NTDA | 2.62 | 2.06 |
| SPI3nN | 7.97 | BAPN 3.41 | NTDA | 2.97 | 2.38 |
| SPI1nS | 5.69 | BAPN 5.69 | DSDA | 1.98 | 1.44 |
| SPI2nS | 6.83 | BAPN 4.55 | DSDA | 2.31 | 1.68 |
| SPI3nS | 7.97 | BAPN 3.41 | DSDA | 2.64 | 1.95 |
| SPI1fS | 5.69 | 9FDA 5.69 | DSDA | 1.97 | 1.44 |
| SPI2fS | 6.83 | 9FDA 4.55 | DSDA | 2.31 | 1.67 |
| SPI3fS | 7.97 | 9FDA 3.41 | DSDA | 2.63 | 1.84 |
2.4. Preparation of SPI/PIL Composite Membranes
| Code | SPI | PIL * | Code | SPI | PIL * |
|---|---|---|---|---|---|
| SPI1pO/V40 | SPI1pO 60% | VIm 40% | SPI1nN/V40 | SPI1nN 60% | VIm 40% |
| SPI2pO/V40 | SPI2pO 60% | VIm 40% | SPI2nN/V40 | SPI2nN 60% | VIm 40% |
| SPI3pO/V40 | SPI3pO 60% | VIm 40% | SPI3nN/V40 | SPI3nN 60% | VIm 40% |
| SPI1nO/V40 | SPI1nO 60% | VIm 40% | SPI1nS/V40 | SPI1nS 60% | VIm 40% |
| SPI2nO/V40 | SPI2nO 60% | VIm 40% | SPI2nS/V40 | SPI2nS 60% | VIm 40% |
| SPI3nO/V40 | SPI3nO 60% | VIm 40% | SPI3nS/V40 | SPI3nS 60% | VIm 40% |
| SPI1fO/V40 | SPI1fO 60% | VIm 40% | SPI1fS/V40 | SPI1fS 60% | VIm 40% |
| SPI2fO/V40 | SPI2fO 60% | VIm 40% | SPI2fS/V40 | SPI2fS 60% | VIm 40% |
| SPI3fO/V40 | SPI3fO 60% | VIm 40% | SPI3fS/V40 | SPI3fS 60% | VIm 40% |
| SPI1fS/V30 | SPI1fS 70% | VIm 30% | |||
| SPI2fS/V30 | SPI2fS 70% | VIm 30% | |||
| SPI3fS/V30 | SPI3fS 70% | VIm 30% |
2.5. Characterization
3. Results and Discussion
3.1. Sulfonation of pBAB
3.2. Synthesis of Sulfonated Polyimides

3.3. Composite Membranes Structure Characterization

3.4. Thermal Properties

| Material | Td-10% (°C) | Tg (°C) | Material | Td-10% (°C) | Tg (°C) |
|---|---|---|---|---|---|
| SPI1pO | 319.4 | 281.4 | SPI1pO/V40 | 364.3 | 128.9 |
| SPI2pO | 295.0 | 235.2 | SPI2pO/V40 | 358.7 | 112.0 |
| SPI3pO | 270.0 | 203.6 | SPI3pO/V40 | 352.2 | 103.4 |
| SPI1nO | 329.5 | 319.0 | SPI1nO/V40 | 355.4 | 139.4 |
| SPI2nO | 314.5 | 296.9 | SPI2nO/V40 | 352.6 | 131.2 |
| SPI3nO | 295.2 | 281.7 | SPI3nO/V40 | 341.3 | 118.2 |
| SPI1fO | 263.9 | 254.8 | SPI1fO/V40 | 366.9 | 135.0 |
| SPI2fO | 254.0 | 245.2 | SPI2fO/V40 | 358.6 | 126.2 |
| SPI3fO | 243.4 | 230.5 | SPI3fO/V40 | 350.8 | 116.3 |
| SPI1nN | 334.3 | 301.7 | SPI1nN/V40 | 353.9 | 160.3 |
| SPI2nN | 296.4 | 286.1 | SPI2nN/V40 | 338.2 | 148.8 |
| SPI3nN | 271.5 | 260.2 | SPI3nN/V40 | 312.8 | 128.5 |
| SPI1nS | 304.7 | 289.5 | SPI1nS/V40 | 357.1 | 155.5 |
| SPI2nS | 275.8 | 260.4 | SPI2nS/V40 | 343.2 | 146.6 |
| SPI3nS | 253.6 | 224.3 | SPI3nS/V40 | 334.5 | 136.7 |
| SPI1fS | 282.1 | 247.5 | SPI1fS/V40 | 358.7 | 137.5 |
| SPI2fS | 276.9 | 234.5 | SPI2fS/V40 | 354.5 | 128.9 |
| SPI3fS | 233.7 | 224.7 | SPI3fS/V40 | 345.6 | 100.3 |
| SPI1fS/V30 | 357.1 | 141.7 | |||
| SPI2fS/V30 | 347.4 | 132.4 | |||
| [VIm][OTf] | 376.2 | 50.0 | SPI3fS/V30 | 340.9 | 123.5 |
3.5. Membrane Conductivity
3.5.1. Effect of Sulfonated Diamine on PEM Conductivity
3.5.2. Effect of Various Diamines in SPI on PEM Conductivity

3.5.3. Effect of Various Dianhydrides in SPI on PEM Conductivity

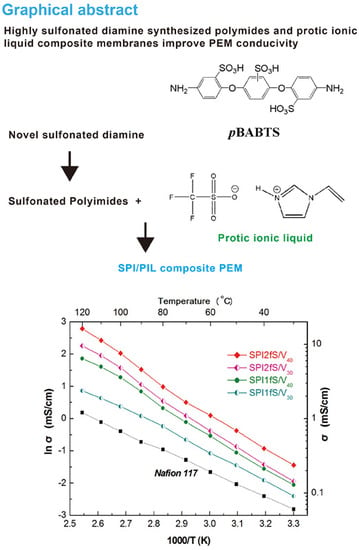
3.5.4. Conductivity Comparison with Nafion 117

3.6. Morphology Studies


4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chandan, A.; Hattenberger, M.; El-kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)—A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Water soluble polymers as proton exchange membranes for fuel cells. Polymers 2012, 4, 913–963. [Google Scholar] [CrossRef]
- Díaz, M.; Ortiz, A.; Ortiz, I. Progress in the use of ionic liquids as electrolyte membranes in fuel cells. J. Membrane Sci. 2014, 469, 379–396. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, C.H.; Guiver, M.D.; Lee, Y.M. Sulfonated hydrocarbon membranes for medium-temperature and low-humidity proton exchange membrane fuel cells (PEMFCs). Prog. Polym. Sci. 2011, 36, 1443–1498. [Google Scholar] [CrossRef]
- Marestin, C.; Gebel, G.; Diat, O.; Mercier, R. Sulfonated Polyimides. Adv. Polym. Sci. 2008, 216, 185–258. [Google Scholar]
- Yan, J.; Liu, C.; Wang, Z.; Xing, W.; Ding, M. Water resistant sulfonated polyimides based on 4,4′-binaphthyl-1,1′,8,8′-tetracarboxylic dianhydride (BNTDA) for proton exchange membranes. Polymer 2007, 48, 6210–6214. [Google Scholar] [CrossRef]
- Chen, B.K.; Tsay, S.Y.; Chiu, T.M. Synthesis and characterization of polyimide/silica hybrid nanocomposites. J. Appl. Polym. Sci. 2004, 94, 382–393. [Google Scholar] [CrossRef]
- Ye, Y.S.; Rick, J.; Hwang, B.J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Luo, J.; Conrad, O.; Vankelecom, I.F.J. Imidazolium methanesulfonate as a high temperature proton conductor. J. Mater. Chem. A 2013, 1, 2238–2247. [Google Scholar] [CrossRef]
- Doyle, M.; Choi, S.K.; Proulx, G. High-temperature proton conducting membranes based on perfluorinated ionomer membrane-ionic liquid composites. J. Electrochem. Soc. 2000, 147, 34–37. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Park, J.S.; Cho, E.K.; Yoon, Y.G.; Kim, C.S.; Lee, W.Y. Studies of high temperature proton conducting membranes containing hydrophilic/hydrophobic ionic liquids. Macromolecules 2009, 42, 2054–2062. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yasuda, T.; Watanabe, M. Fabrication of protic ionic liquid/sulfonated polyimide composite membranes for non-humidified fuel cells. J. Power Sources 2010, 195, 5909–5914. [Google Scholar] [CrossRef]
- Chen, B.K.; Wu, T.Y.; Kuo, C.W.; Peng, Y.C.; Shih, I.C.; Hao, L.; Sun, I.W. 4,4′-Oxydianiline (ODA) containing sulfonated polyimide/protic ionic liquid composite membranes for anhydrous proton conduction. Int. J. Hydrog. Energy 2013, 38, 11321–11330. [Google Scholar] [CrossRef]
- Chen, B.K.; Wong, J.M.; Wu, T.Y.; Chen, L.C.; Shih, I.C. Improving the conductivity of sulfonated polyimides as proton exchange membranes by doping of a protic ionic liquid. Polymers 2014, 6, 2720–2736. [Google Scholar] [CrossRef]
- Wu, T.Y.; Sun, I.W.; Gung, S.T.; Chen, B.K.; Wang, H.P.; Su, S.G. High conductivity and low viscosity Brønsted acidic ionic liquids with oligomeric anions. J. Taiwan Inst. Chem. Eng. 2011, 42, 874–881. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, B.K.; Hao, L.; Lin, K.F.; Sun, I.W. Thermophysical properties of a room temperature ionic liquid (1-methyl-3-pentyl-imidazolium hexafluorophosphate) with poly(ethylene glycol). J. Taiwan Inst. Chem. Eng. 2011, 42, 914–921. [Google Scholar] [CrossRef]
- Luo, J.; Hu, J.; Saak, W.; Beckhaus, R.; Wittstock, G.; Vankelecom, I.F.J.; Agerta, C.; Conrad, O. Protic ionic liquid and ionic melts prepared from methanesulfonic acid and 1H-1,2,4-triazole as high temperature PEMFC electrolytes. J. Mater. Chem. 2011, 21, 10426–10436. [Google Scholar] [CrossRef]
- Pibiri, I.; Pace, A.; Buscemi, S.; Causin, V.; Rastrelli, F.; Saielli, G. Oxadiazolyl-pyridines and perfluoroalkyl-carboxylic acids as building blocks for protic ionic liquids: crossing the thin line between ionic and hydrogen bonded materials. Phys. Chem. Chem. Phys. 2012, 14, 14306–14314. [Google Scholar] [CrossRef] [PubMed]
- Deligöz, H.; Yılmazoğlu, M. Development of a new highly conductive and thermomechanically stable complex membrane based on sulfonated polyimide/ionic liquid for high temperature anhydrous fuel cells. J. Power Sources 2011, 196, 3496–3502. [Google Scholar] [CrossRef]
- Schauer, J.; Sikora, A.; Pliškova, M.; Mališ, J.; Mazur, P.; Paidar, M.; Bouzek, K. Ion-conductive polymer membranes containing 1-butyl-3-methylimidazolium trifluoromethanesulfonate and 1-ethylimidazolium trifluoromethanesulfonate. J. Membrane Sci. 2011, 367, 332–339. [Google Scholar] [CrossRef]
- Van de Ven, E.; Chairuna, A.; Merle, G.; Benito, S.P.; Borneman, Z.; Nijmeijer, K. Ionic liquid doped polybenzimidazole membranes for high temperature proton exchange membrane fuel cell applications. J. Power Sources 2013, 222, 202–209. [Google Scholar] [CrossRef]
- Ye, Y.S.; Tseng, C.Y.; Shen, W.C.; Wang, J.S.; Chen, K.J.; Cheng, M.Y.; Rick, J.; Huang, Y.J.; Chang, F.C.; Hwang, B.J. A new graphene-modified protic ionic liquid-based composite membrane for solid polymer electrolytes. J. Mater. Chem. 2011, 21, 10448–10453. [Google Scholar] [CrossRef]
- Leu, T.S.; Wang, C.S. Synthesis and properties of copolyimides containing naphthalene group. Polymer 2002, 43, 7069–7074. [Google Scholar] [CrossRef]
- Gao, Y.; Robertson, G.P.; Guiver, M.D.; Ean, X.G.; Mikhailenko, S.D.; Wang, K.P.; Kaliaguine, S. Sulfonation of poly(phthalazinones) with fuming sulfuric acid mixtures for proton exchange membrane materials. J. Membr. Sci. 2003, 227, 39–50. [Google Scholar] [CrossRef]
- Luo, J.; Tan, T.V.; Conrad, O.; Vankelecom, I.F.J. 1H-1,2,4-Triazole as solvent for imidazolium methanesulfonate. Phys. Chem. Chem. Phys. 2012, 14, 11441–11447. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Ho, W.S.W. New poly(ethylene oxide) soft segment-containing sulfonated polyimide copolymers for high temperature proton-exchange membrane fuel cells. J. Membr. Sci. 2008, 313, 75–85. [Google Scholar] [CrossRef]
- Hirao, M.; Ito, K.; Ohno, H. Preparation and polymerization of new organic molten salts; N-alkylimidazolium salt derivatives. Electrochim. Acta 2000, 45, 1291–1294. [Google Scholar] [CrossRef]
- Ohno, H. Electrochemical Aspects of Ionic Liquids; Wiley Interscience: Hoboken, NJ, USA, 2005. [Google Scholar]
- Higashihara, T.; Matsumoto, K.; Ueda, M. Sulfonated aromatic hydrocarbon polymers as proton exchange membranes for fuel cells. Polymer 2009, 50, 5341–5357. [Google Scholar] [CrossRef]
- Ye, X.; Bai, H.; Ho, W.S. Synthesis and characterization of new sulfonated polyimides as proton-exchange membranes for fuel cells. J. membrane Sci. 2006, 279, 570–577. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.-K.; Wu, T.-Y.; Wong, J.-M.; Chang, Y.-M.; Lee, H.-F.; Huang, W.-Y.; Chen, A.F. Highly Sulfonated Diamine Synthesized Polyimides and Protic Ionic Liquid Composite Membranes Improve PEM Conductivity. Polymers 2015, 7, 1046-1065. https://doi.org/10.3390/polym7061046
Chen B-K, Wu T-Y, Wong J-M, Chang Y-M, Lee H-F, Huang W-Y, Chen AF. Highly Sulfonated Diamine Synthesized Polyimides and Protic Ionic Liquid Composite Membranes Improve PEM Conductivity. Polymers. 2015; 7(6):1046-1065. https://doi.org/10.3390/polym7061046
Chicago/Turabian StyleChen, Bor-Kuan, Tzi-Yi Wu, Jhong-Ming Wong, Yu-Ming Chang, Hsu-Feng Lee, Wen-Yao Huang, and Antonia F. Chen. 2015. "Highly Sulfonated Diamine Synthesized Polyimides and Protic Ionic Liquid Composite Membranes Improve PEM Conductivity" Polymers 7, no. 6: 1046-1065. https://doi.org/10.3390/polym7061046
APA StyleChen, B.-K., Wu, T.-Y., Wong, J.-M., Chang, Y.-M., Lee, H.-F., Huang, W.-Y., & Chen, A. F. (2015). Highly Sulfonated Diamine Synthesized Polyimides and Protic Ionic Liquid Composite Membranes Improve PEM Conductivity. Polymers, 7(6), 1046-1065. https://doi.org/10.3390/polym7061046