Abstract
Few changes have occurred in the use of various stabilizers over recent years. In the current literature, phosphate derivatives are used as anti-ageing additives in polymers, and the most popular of these are sterically hindering cyclic amines. However, most of these compounds are carcinogenic. Synthetic phenols have been increasingly used as antioxidants in food and in polymers. Ecological standards encourage the elimination of harmful additives in polymeric products that come in contact with food or with the human body. This article presents application of flavonoid (silymarin/flavonoligand) for polymer stabilization and use of natural phytocompounds such as color indicators of polymers ageing time. In this research, I propose two ways of application: traditional, during processing; and the new one, by using impregnation method. Based on the change of deformation energy (ageing coefficient K), FTIR, oxidative induction time (OIT) evaluated by differential scanning calorimetry (OIT), thermogravimetry analysis (TG), spectrophotometric color measurements in terms of CIE-Lab color space values, I confirmed the high antioxidant activity of flavonoids in EPM. They provide coloration of the polymeric materials that changes cyclically as a function of aging time. Additionally, the use of phytocompounds in polymers provides similar stabilizing effect to those of synthetic antioxidants.
1. Introduction
The polymer lifetime is determined by the effectiveness of the stabilizers. All of the polymers undergo degradation when exposed to an outside environment under sunlight. The preparation of new polymeric materials for technological applications requires a good understanding of the possible mechanisms that determine the oxidation processes in the materials.
The available literature suggests that there are several known major groups of stabilizers. The division is determined by the types of environmental factors against which the material should be protected [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. One of the most important groups of stabilizers are, undoubtedly, antioxidants. The polymer industry used synthetic antioxidants but it seems recently that more interest is directed towards natural substances with anti-aging properties. Natural antioxidants may be a new, environmentally friendly alternative to the aromatic amines commonly used in polymers.
Currently, natural antioxidants are of great interest to scientists because of their effect on biological processes in which they protect against free radicals, thereby preventing many undesired oxidative processes. Thus, the search for new sources of these compounds is ongoing. Studies to improve the structure of natural antioxidants to increase their effectiveness have also been performed. Because many of the synthetic antioxidants used to date have been gradually withdrawn due to their toxicity, the potential for natural antioxidants has increased, and the interest in them has increased accordingly. Natural antioxidants are typically present in many vegetables and fruits and are often responsible for their color. The largest group of natural antioxidants includes polyphenol compounds, such as phenolic acids, flavonoids, stilbenes, lignans and lycopene. Many environmental factors influence the beginning of the oxidation processes. Therefore, to prevent undesirable processes resulting in the destruction of living organisms and materials, appropriate antioxidants are used. From a chemical viewpoint, to be useful, an antioxidant should be thermally stable during every process because instability has negative effects. For example, the exposure of phosphoric compounds to the action of hydrolytic changes causes the formation of phosphoric acids, which initiate corrosion [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57].
An important parameter that is rarely reported in the literature is the stability of the antioxidant color. For example, amines cause color changes, which is a disadvantage of their use. An addition of even 1% antioxidant can change the product color; thus, it is necessary to determine the changes in the color of samples [58,59].
The physical properties of antioxidants, such as diffusion, solubility and lability can determine their antioxidizing activity. The technical effectiveness of a stabilizing system and the potential toxicity of the final product are important. Monomeric antioxidants containing vinyl, acrylic and methacrylic groups are added in low concentrations, and they are responsible for the formation of anti-oxidative bonds. Biological antioxidants, such as vitamin E [60,61,62] (alpha-tocopherol) [63], act through the mechanisms of chain breaking, enabling their high antioxidizing effectiveness at very low concentrations compared with synthetic stabilizers, most of which are toxic. The interaction between antioxidants providing enforced stabilization effects is known as a synergistic effect. I intend to investigate the phenomenon of homosynergism, which is based on the cooperation of two chemically similar antioxidants, as well as the phenomena of autosynergism of two different antioxidants in a single molecule and heterosynergism, the interaction of two antioxidants from two different functional classes, e.g., a primary antioxidant with a hydrogen peroxide deactivator [64,65,66,67,68]. In the protection against ageing, the best results are obtained by combining bound phenols, representing antioxidants that break chains, and phosphates as hydrogen peroxide deactivators. Phosphates can control the product color by reacting with colored phenols, resulting in the formation of colorless products. Good protection against photo-oxidation is provided by the mixture of a photo-antioxidant and stabilizers that act via a complementary antioxidizing mechanism. However, the simultaneous use of two stabilizers can yield undesirable antagonistic effects. This effect can be observed when using photo-antioxidants with metal dithionals in which an acceleration of dithional decomposition under the influence of phenol oxidation occurs [69,70,71]. Currently, various groups of antioxidants are commercially available, some of which undergo oxidation and reduction processes, while the available data in the literature address the activity of compounds to scavenge free radicals and the decomposition of oxidative forms. However, the literature data appear to require an update, focusing on the examination of the reaction mechanisms of natural antioxidants in oxidation processes including the intermediate forms created in various reaction stages, as well as an examination of the final oxidation products. It is important to determine the toxicity of the intermediate and final products, which requires the use of compatible and diversified test methods composed of a wide range of technology and chemical engineering. These determinations should be supported by quantum-chemical calculations to determine the suitability of the substrates tested to withstand oxidation and reduction processes. Additionally, theoretical calculations will provide the initial premises for practical experiments. The mechanism of the oxidation reaction is affected by several factors, such as reaction medium, solvents, pH, oxygen accessibility, and antioxidant structure, including the number of hydroxyl, carbonyl or carboxyl groups and other important functional groups.
It is well known from the literature that flavonoids show not only antioxidizing properties but also pro-oxidizing characteristics [72]; however, investigations into these properties have not been accurately considered and described. Mono- and dihydroxyflavonoids do not show oxidizing properties, while their derivatives containing many hydroxyl groups, especially in ring B, are characterized by a high pro-oxidizing activity in Fenton’s reactions. Most flavonoid molecules are combined with inactive carbohydrate groups whose presence can affect the activity of their interactions with microorganisms. It is well known that the primary plant functions include pigmentation, protection against UV radiation, control of auxin transport and free radical scavenging. The chemical nature of flavonoids depends on the degree of hydroxylation, the presence of other substituents and their conjugation, and the degree of polymerization. The structure of flavonoids is based on the carbon skeleton C6–C3–C6. Flavonoids are derived from 2-phenylchromane, which is composed of three phenol rings marked as A, B and C, each of which is characterized by different levels of hydroxylation and methoxylation, acetylation, glycosidation and isoprenylation. Flavonoids are mostly hydroxylated at positions 3, 5, 7, 3′, 4′, and 5′. Some of these hydroxyl groups are ethylated, acetylated or sulfonated. Benzene ring C is substituted at position 2 with ring B. Ring C can be a heterocyclic pirane (catechins and anthocyanins) or pyrone (flavones, flavonoles, flavanones) containing carbonyl groups at position C4 [73,74].
Therefore, the determination of the peroxidative capability of flavonoids depending on their structures is important. These investigations can considerably extend the current knowledge, indicating new application opportunities of flavonoids with specific properties.
Literature reports contain information about the capability of some flavonoids to inhibit the growth of microorganisms. However, the authors of these reports only mention evidence indicating the antibacterial and fungicidal actions of selected flavonoid derivatives.
The regulation of the European Union No. 10/2011 defines the acceptable use of additives in food packaging because during the processing or exploitation of the materials, additives can migrate from the packaging to the food [75].
2. Application of Flavonoids to Polymer Stabilization and the Use of Natural Phytocompounds as Color Indicators of Polymer Ageing Time
Anti-ageing substances are used to enhance the pro-ecological profile of the polymeric composites, enabling their use in products while complying with restrictive standards. The addition of anti-ageing substances derived from flavonoids considerably improves the resistance of polymeric composites to ageing, especially under UV irradiation and climatic conditions with variable air humidity and solar radiation. I present the preliminary results of the application of a flavonoid (silymarin) to an ethylene-propylene copolymer. The principal components of silymarin are silybin A, silybin B, isosilybin A, isosilybin B, silychristin A, silychristin B and silydianin [76].
Therefore the determination of the peroxidative capability of flavonoides depending on their structures is of paramount importance. Undoubtedly, these investigations can considerably extend the present state of knowledge, indicating new application opportunities of the flavonoide specific properties.
In some literature reports, one can find information about the capability of some flavonoides to inhibit the growth in microorganisms. However, their authors only mention that there are premises indicating the antibacterial and fungicidal actions of some selected flavonoide derivatives.
3. Experimental Section
3.1. Chemicals
The ethylene-propylene elastomer (EPM) having 41 wt% of propylene (Dutral CO-054). EPM used in this study was obtained from Mentedison (Ferrara, Italy). Dicumyl peroxide (DCP, from Fluka, Steinheim, Germany) was used as the cross-linking agent. 1,3,5-Triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione (Sigma Aldrich Chemie GmbH, Steinheim, Germany) was the cross-linking co-agent, and hexadecyltrimethylammonium bromide (CTAB, Sigma Aldrich Chemie GmbH, Steinheim, Germany) was the dispersing agent. Aerosil 380 silica (from Evonic Degussa GmbH, Frankfurt, Germany) was used as a filler. The silymarin–flavon ligand was purchased from Sigma GmbH (Steinheim, Germany).
3.2. Measurement Methods
3.2.1. EPM Impregnation with Silymarin in Chloroform
EPM rubber (an ethylene propylene rubber) was impregnated with silymarin (a mixture of anti-hepatotoxic flavonolignans from the fruit of Silybum marianum), obtained from Sigma Aldrich, which is available as a ready-made solution. Impregnation was performed with 5%, 10%, and 15% solutions of silymarin in chloroform (Sigma, anhydrous, ≥99%). Prior to applying chloroform to the EPM vulcanizate, drilling, cutting, and turning operations were completed, and the relative humidity was in equilibrium with the test environment. In the impregnation process, the dipping duration was at least 6 h, and the impregnation pool contained at least 1 L of impregnation material for every 0.4 m2 of polymer. The impregnated materials remained in a dryer for at least 48 h (T = 313 K).
3.2.2. Traditional Method of Processing EPM
Rubber blends were prepared using a laboratory mixing mill with rolls of 330 mm in length and 140 mm in diameter. The rotational speed of the front roll was Vp = 20 rpm, the friction ratio was 1.1, and the average temperature of the rolls was approximately 40 °C.
The vulcanization of the rubber blends was performed using steel vulcanization molds placed between the shelves of an electrically heated hydraulic press. Teflon films were used as spacers to prevent the adherence of the blends to the press plates. Samples were vulcanized at 160 °C under a pressure of 15 MPa for 30 min.
The tensile strength of the vulcanizates was tested (according to the ISO 37:2005) at room temperature with the crosshead speed 500 mm/min, using a Zwick 435 universal testing machine (RoellGroup, Ulm, Germany) equipped with a 1 kN load cell. Five dumbbell specimens were used for each blend.
3.2.3. Accelerated Ageing
Thermal ageing characteristics were determined according to the PN-82/C-04216 standard. Samples were exposed to air at an elevated temperature (383 K) for 10 days in a dryer with thermo-circulation.
Photo-oxidation/UV ageing was performed using a UV 2000 apparatus from Atlas (Chicago, IL, USA). The measurement was performed for 420 h and consisted of two alternately repeating segments with the following parameters: daily segment (UVA radiation intensity E = 0.7 W/m2, λ = 340 nm, temperature 60 °C, duration 8 h), night segment (no UV radiation, temperature 50 °C, duration 4 h). Photo-oxidation (UV ageing) characteristics were determined according to the ISO 4892-3 standard and using the following equations:
Climatic ageing was performed using a Weather–Ometer (Atlas, Ci 4000,Chicago, IL, USA). The test was based on two variable segments simulating daytime and nighttime conditions, and the samples were subjected to two different cycles, as follows: day cycle (radiation intensity E = 40 W/m2 = 0.144 MJ/m2 over a λ range of 300–400 nm), temperature 60 °C, duration 240 min, humidity 80%, rain water on), and night cycle (no radiation, temperature 50 °C, humidity 60%, duration 120 min). The UVA part of the spectrum constitutes 6% of the total irradiance in natural sunlight. The UV radiant exposure per year in Florida is 286 MJ/m2, and 3.7 months of ageing in a Weather Ometer Chamber provides an equivalent amount of radiation energy. Material weathering tests by Xenon were performed according to the ISO 4892-2 standard (accelerated weathering simulates the damaging effects to materials and coatings of long-term outdoor exposure).
The ageing coefficient (K) was calculated according to the relationship.
3.2.4. The Dispersion Degree of Additives
The dispersion degree of the fillers in the elastomeric matrix was evaluated from images obtained using a LEO 1530 scanning electron microscope SEM HITACHI S800 (Tokyo, Japan). The samples tested consisted of vulcanizate fracture surfaces in liquid nitrogen dusted with carbon.
3.2.5. Attenuated Total Reflectance Fourier Transforms Infrared (ATR-FTIR) Spectroscopy
FTIR spectra were tested in the range of 3000–700 cm−1 using an FTIR Nicolet 6700 FTIR (Thermo Scientific, Waltham, MA, USA) with an attenuated total reflexion diamond crystal accessory (ATR). The measurement parameters were as follows: 280 scans; resolution was set to 8 cm−1; DTGS/KBr detector was employed.
3.2.6. Color Measurement
The color of the obtained vulcanizates was measured using a CM-3600d spectrophotometer (Konica Minolta, Osaka, Japan). The radiation source consisted of four xenon tube impulses. The spectral range of the apparatus was 360–740 nm, and the change in color dE*ab was calculated as follows:
The CIE-Lab scale is an approximately uniform color scale in which the differences between points plotted in the color space correspond to visual differences between the colors plotted. The CIE-Lab color space is organized in a cube form. The L* axis runs from top to bottom. The maximum value for L* is 100, which represents a perfect reflecting diffuser. The minimum value for L* is zero, which represents the color black. The a* and b* axes have no specific numerical limits. A positive value for a* is red; a negative a* is green; a positive b* is yellow; and a negative b* is blue.
3.2.7. The Oxygen Induction time (OIT)
OIT test was performed on a Mettler Toledo differential scanning calorimeter (DSC) instrument (Greifensee, Switzerand). Samples with a mass of 4 mg were heated from room temperature to the test temperature, 220 °C, at a rate of 20 °C/min under a nitrogen atmosphere. After 5 min at 220 °C, the gas was changed from nitrogen to air at a flow rate of 60 mL/min. When all of the antioxidants were consumed, the sample began to oxidize, producing a deviation in the baseline. The OIT was measured as the time between the change to airflow and the intersection with a tangent from the maximum derivative after oxidation began. Two analyses of each sample were performed to ensure the accuracy of the results. The OIT index was determined according to the PN EN 728:1999 standard [77], “Plastic piping system and ducting system. Polyolefin pipes and fitting determination of induction time” and the ISO 11357-6:2002 standard “Plastics-DSC-Part 6: Determination of oxygen induction time” [78,79].
3.2.8. Thermogravimetric Analysis (TGA)
TGA was performed using a Universal V1.12E TA instrument (Greifensee, Switzerand) to obtain the initial temperature of degradation and the maximum thermal degradation temperatures. The heating rate was maintained at 10 °C/min from 30–700 °C under an inert N2 atmosphere.
4. Results and Discussion (Application of Flavonoids for Polymer Stabilization and Use Natural Phytocompounds such as Color Indicator of Polymers Ageing Time)
The anti-ageing substances used enhance the pro-ecological profile of the composites, making it possible to use them in products to be in conformity with restrictive standards. The addition of anti-ageing substances derived from flavonoids considerable improves the resistance of polymeric composites to ageing, especially under UV irradiation and climatic conditions with variable air humidity and solar radiation. Below we present preliminary results of application flavonoid (silymarin) in ethylene-propylene copolymer. The principal components of silymarin are silybin, isosilybin, silychristin and silydianin (Figure 1) [80,81,82,83,84,85,86,87].
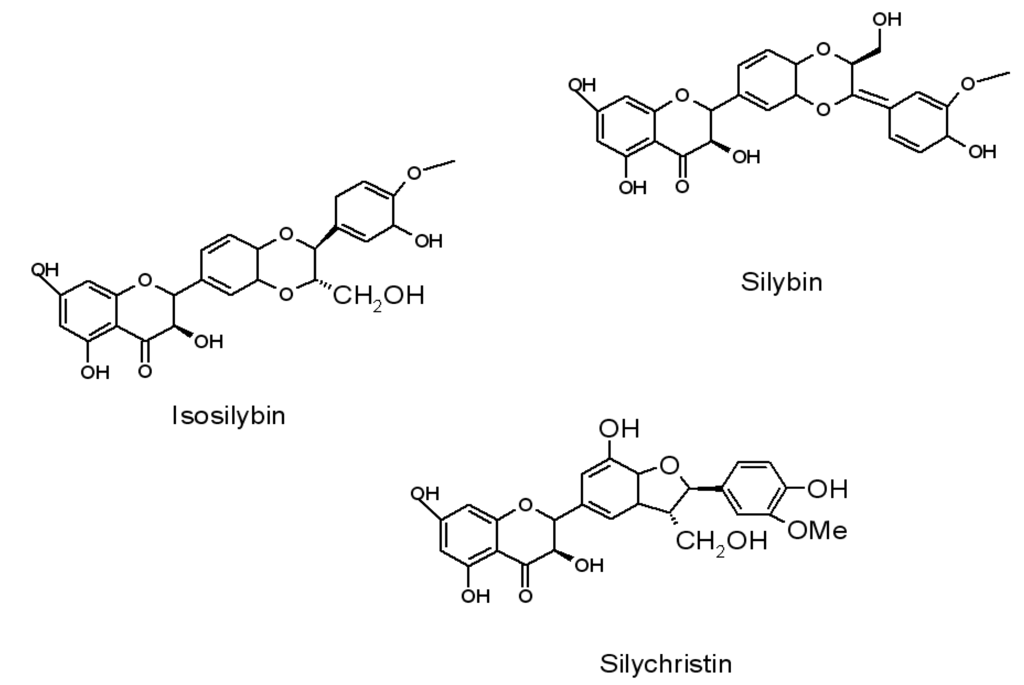
Figure 1.
Structure of silymarin compounds.
Important aspect of investigation was to confirm the presence silymarin in the polymer as well as the determination of its amount. Fourier transform infrared spectroscopy was done for the EPM samples after and before impregnation. The spectra obtained for the impregnated EPM copolymer Figure 2 shows a larger peak related to silymarin band at 3650 and 1640 cm−1. This response is coherent with the high silymarin solubility and the higher silymarin impregnation capacity in chloroform of silymarin of EPM vulcanizate. Concentration of antioxidant can be monitored by 3650 and 1640 cm−1 (Figure 2). Based on intensity change in the FTIR spectrum of selected bands, we can get information about the approximate contents of antioxidant impregnated of ethylene-propylene rubber. The intensity of OH group at 3650 cm−1 for EPM containing silymarin (4.5%) added during processing is similar to the intensity of this group in EPM impregnated by 15% solution of silymarin in chloroform. Peak at 3650 cm−1 of EPM impregnated by 10% solution of silymarin is two times less intense than the sample formed by the traditional method. Based on phenolic groups concentration at the 3650 cm−1 by FTIR, it follows that impregnation with 10% and 15% solution of silymarin leads to the concentration of this antioxidant in the polymer matrix, respectively, ~2% and ~4%.
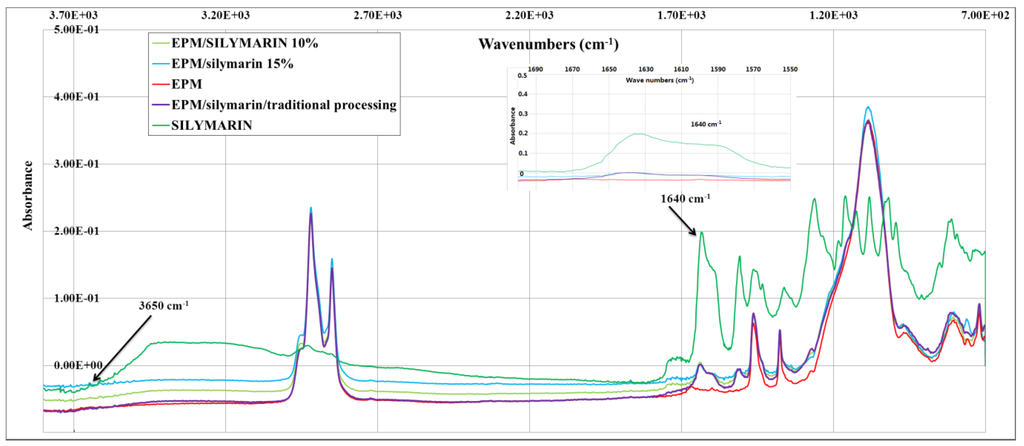
Figure 2.
Figure 2. FTIR spectra for EPM stabilized with silymarin.
The second step was to investigate the thermal stability of silymarin and to evaluate its effect on the thermal decomposition of the EPM rubber vulcanizate. The thermooxidative stability in a polymeric material is an important material selection criterion for commercial applications. The result of the thermogravimetric analysis of silymarin powder and EPM stabilized with silymarin is shown in Table 1. Silymarin has a wide range of thermal stability, which is characteristic of a natural antioxidant. The TGA isothermal profile for silymarin showed that this compound presents only three decomposition steps. The first mass loss in the TGA curve is associated with dehydration processes from 40 to 120 °C. The mass loss of silymarin associated with the thermal degradation of the sample is observed only at approximately 240 °C (weight loss = 38.43%). The peak with an onset temperature at 60 °C suggested that the molecule rearranged, corresponding to the transformation of the hydrated molecule to the anhydrous form. The second and third stages are associated with the process of thermal decomposition.
The thermal stability of polymers depends on various factors. It can be observed that the sample containing silymarin shows higher stability than the non-stabilized EPM. The TGA result shows that EPM undergoes thermal degradation beginning at 267 °C with a total mass loss of 62.24%; however, for EPM stabilized with silymarin, the temperature of decomposition is observed at 280 °C. The second peak in the TGA curve of EPM corresponds to the decomposition an approximate 13% mass loss in the DTG curves at 471 °C for the reference sample and 474 °C for the vulcanizate with the flavonoid (Table 1).

Table 1.
Thermogravimetric characteristics of silymarin and EPM copolymer stabilized with silymarin.
| Material | Atmosphere | Decomposition stage | ||
|---|---|---|---|---|
| First | Second | Third | ||
| Silymarin | Weight loss (%) | 0.63 | 0.89 | 38.43 |
| Temperature range (°C) | 58–99 | 108–150 | From 240 | |
| EPM | Weight loss (%) | 62.24 | 13.81 | – |
| Temperature range (°C) | 267–408 | From 471 | – | |
| EPM/silymarin | Weight loss (%) | 66.33 | 13.05 | – |
| Temperature range (°C) | 283–406 | From 474 | – | |
We used two methods of application of the flavonoids in polymer matrix. The first method was via traditional addition during processing on a Brabender mixer. The second method, which is economically advantageous, was via the addition of silymarin by impregnation in chloroform. Based on the deformation energy changes (ageing factor, K), we evaluated the effect of silymarin on EPM rubber vulcanizate regarding protection against photo-ageing (UV), weathering and thermooxidation. The effects of silymarin on EPM rubber stabilization are shown below (Figure 3).
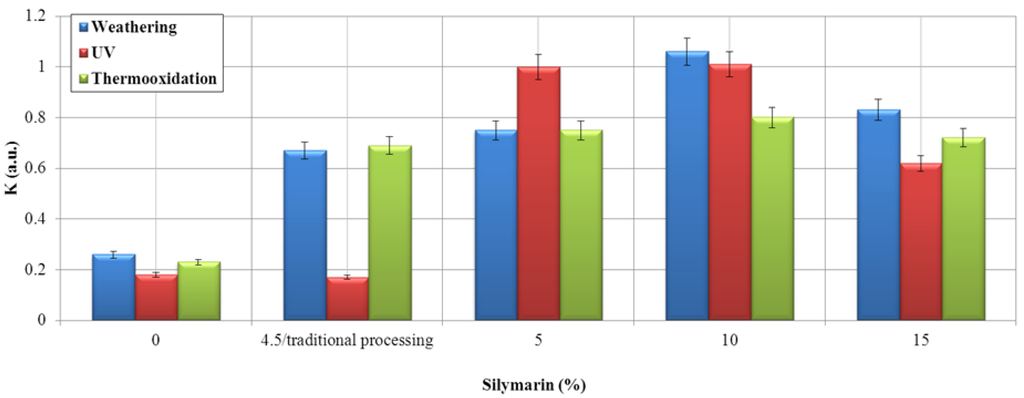
Figure 3.
Ageing coefficient of EPM composite modified with the addition of silymarin via the impregnation method.
Impregnation is defined as the process of infusing or saturating a material with substances. In technical applications, impregnation is used to modify the properties of bulk substances by chemically binding functionalizing substances (impregnating) to a surface. Based on the graph, we conclude that the addition of silymarin significantly improved the ability of the ethylene-propylene copolymer to resist ageing. The effect of UV radiation and weathering on the vulcanizate of EPM containing silymarin resulted in minor degradation of the vulcanizate compared with the reference sample. The optimum content of the antioxidant in the polymer is over the range of 5% to 10%. Above 10%, we observe the effect of the pro-oxidation material. The silymarin that was added during the polymer processing did not protect against UV radiation.
Silymarin impregnated into the EPM vulcanizates significantly improved the ageing resistance of the fabric to UV exposure and temperature. UV ageing of a material begins on the surface. If silymarin were added during polymer processing, its migration from the bulk into the surface layer of the material would be hindered. Impregnation of the antioxidant eliminates this problem.
The OIT test, as performed using a DSC, is used to predict the thermo-oxidative performance of polymeric materials.
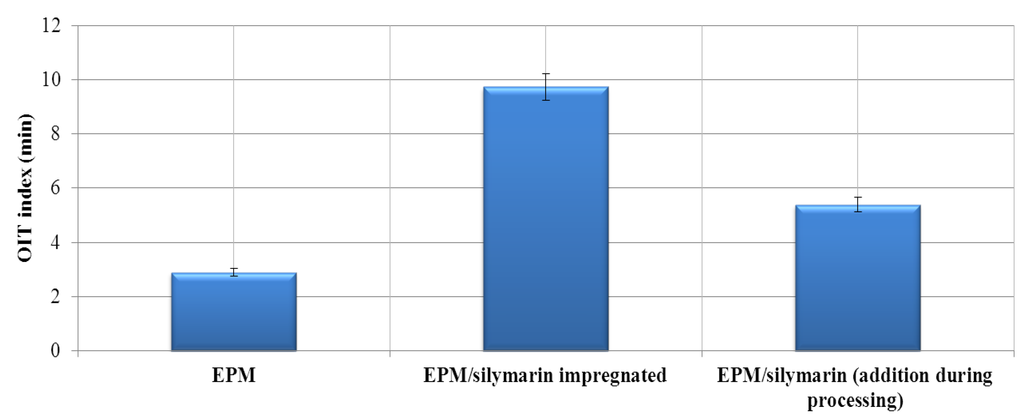
Figure 4.
Oxygen induction time of EPM composite with silymarin.
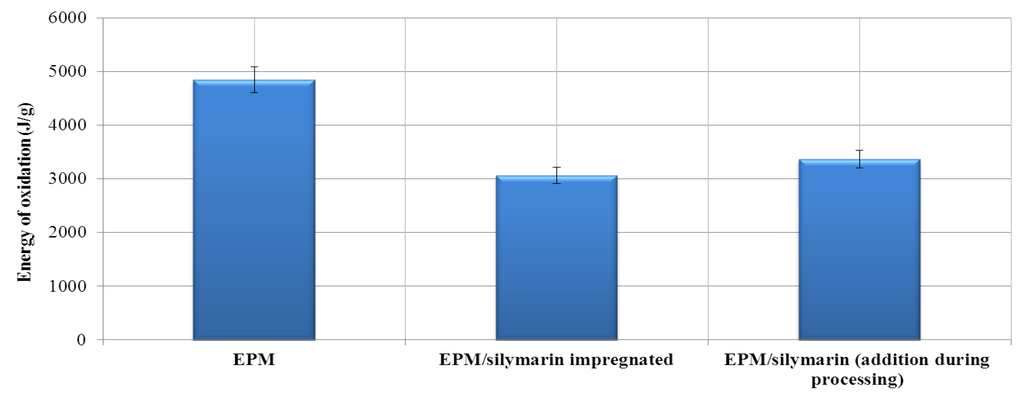
Figure 5.
Energy of induction oxidation of EPM composite with silymarin.
OIT measurements provide a valuable characterization parameter associated with the long-term stabilities of polyolefin materials. The oxygen induction time determined by DSC also allowed confirmation that silymarin influenced the stabilization of the elastomer under investigation (Figure 4 and Figure 5). Based on the results, we conclude that the addition of silymarin significantly prolonged the oxidation induction time of the EPM vulcanizate. The OIT index of the reference vulcanizate containing silymarin added during processing was longer by approximately 0.53 min compared with the reference sample. The addition of silymarin by impregnation increased the OIT of the EPM vulcanizates by as much as 4.35 min. A proposed mechanism of oxidation of the ethylene-propylene rubber stabilized with silymarin is shown below (Scheme 1, Scheme 2 and Scheme 3).
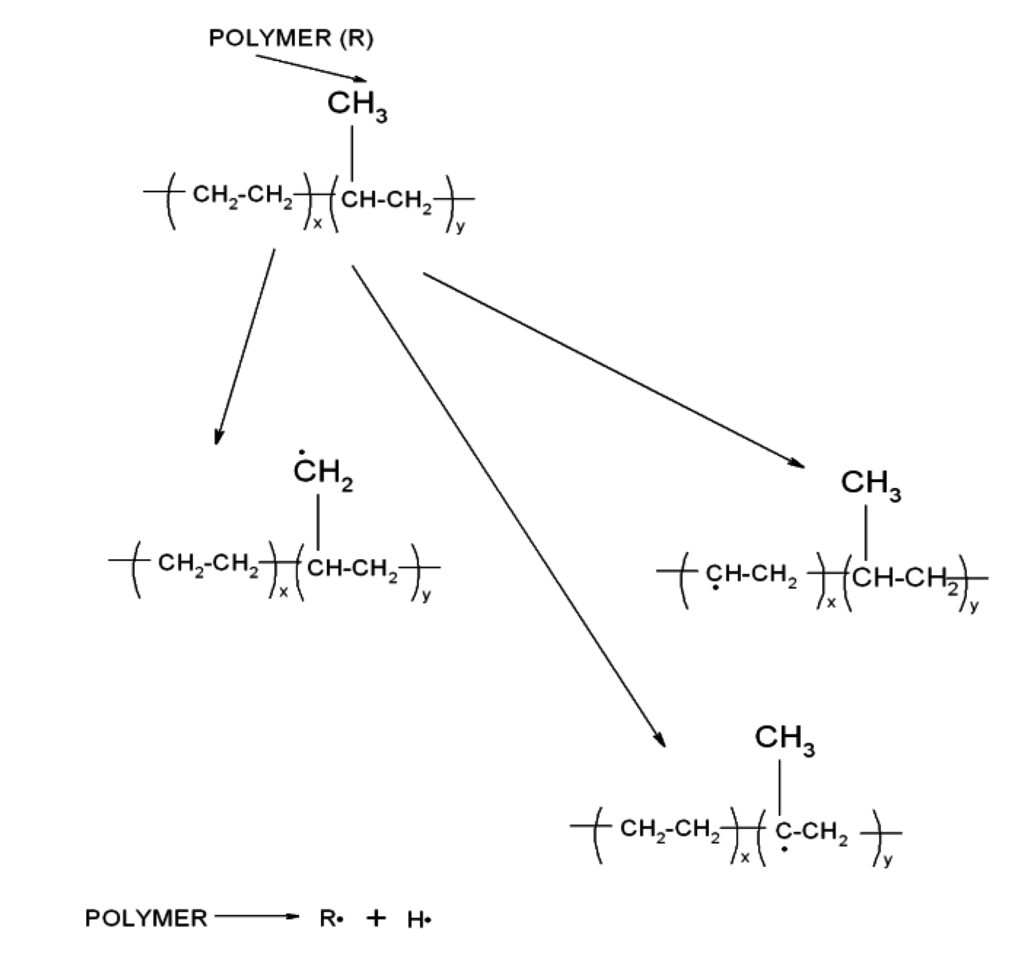
Scheme 1.
The first step of polymer oxidation is initiation (initiation can be caused by oxygen, heat or UV radiation).
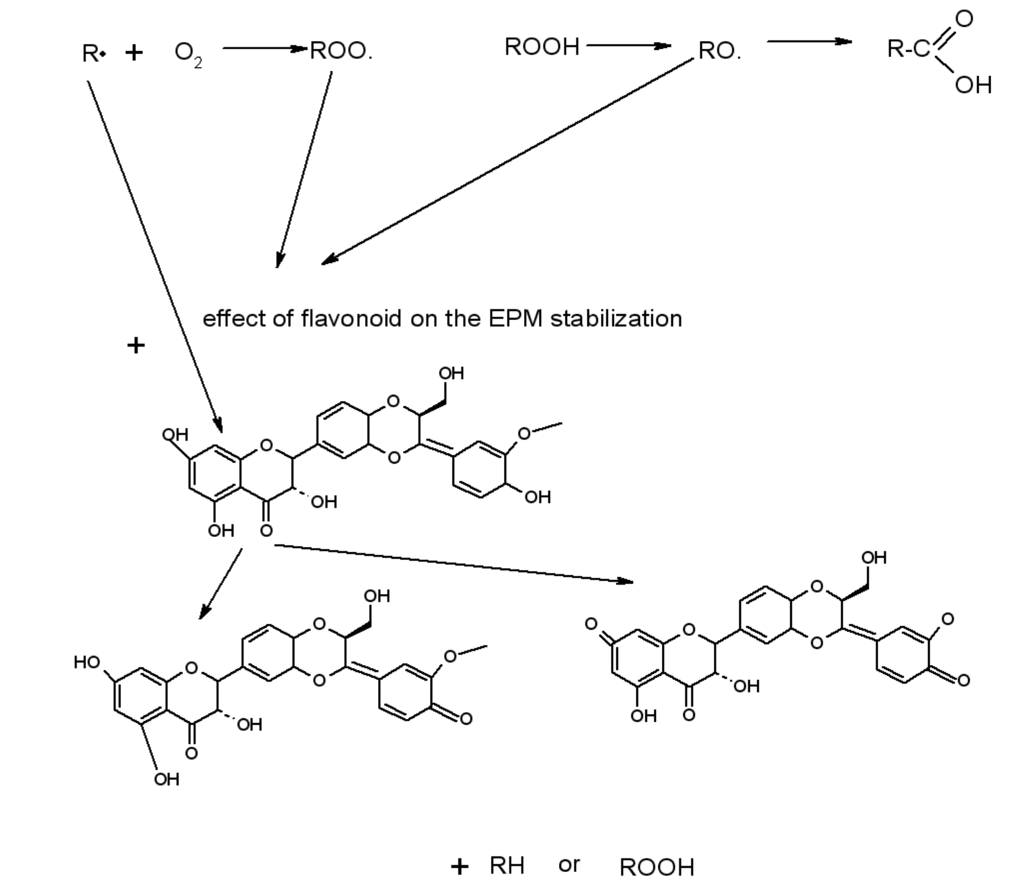
Scheme 2.
The effect of silymarin (trapping the free radicals R· (alkyl) or ROO· (peroxy) in the ethylene-propylene copolymer (EPM) oxidation process.
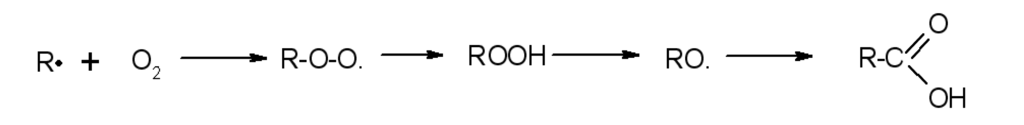
Scheme 3.
The third step produces an oxidizing peroxide, which is highly unstable (when the polymer is destabilized with an antioxidant).
The rate of polymer oxidation depends on various factors and is given by the following equation [79]:
where Ia is the absorbed light intensity at wavelength λ, Ea is the activation energy, T is the absolute temperature, and λ1 and λ2 are the range of the radiation wavelength.
Flavonoid Influence on the Color Stability of Polymeric Materials
Pigments responsible for the appearance of colors in plants are classified into several groups, as follows: chlorophylls, carotenoids (carotenes, xanthophylls), flavonoids (chalcones, anthocyanins, flavones, flavonols) and betalains (betaxanthin, betacyanin). Flavonoids are pigments (or co-pigments) in plants, protecting them from UV-radiation and free radical scavengers and attracting pollinators. Flavonoids appear in nearly all tissues of different plants, and their color is influenced by many factors, such as the number of hydroxyl and methoxyl groups present. If many OH groups are present, then the color is blue, whereas the presence of many –OCH3 groups direct the color towards red.
These antioxidants undergo characteristic changes under the action of various environmental factors [82,83,84]. Therefore, we tested the influence of silymarin on the color of EPM rubber vulcanizates and examined the color change of these materials as a function of ageing time. The results revealed that silymarin could be used as a natural color indicator of polymer ageing time.
Discoloration is one of the most critical problems that arises during ageing. Artificial weathering induced significant color changes in EPM vulcanizates. The color changes are discussed primarily in terms of the color coordinates measured in the CIE-Lab space. Figure 6, Figure 7, Figure 8 and Figure 9 show the changes of the a, b and dE*ab parameters after the ageing process. The UV degradation of the materials starts at the surface layer, resulting in a marked color change of the EPM sample.
The artificial weathering produced a substantially stronger discoloration compared with thermooxidation and UVA radiation. Parameter a is changed to a positive value after ageing, i.e., it was changed to the red color in the CIE-Lab. The change in parameter a is more noticeable for samples containing impregnated silymarin.
Parameter b of the sample is changed to a yellow color on the b axis (Figure 7 and Figure 8). In contrast, the sample with antioxidant added during processing experiences a color change toward the blue shades after weathering and thermooxidation.
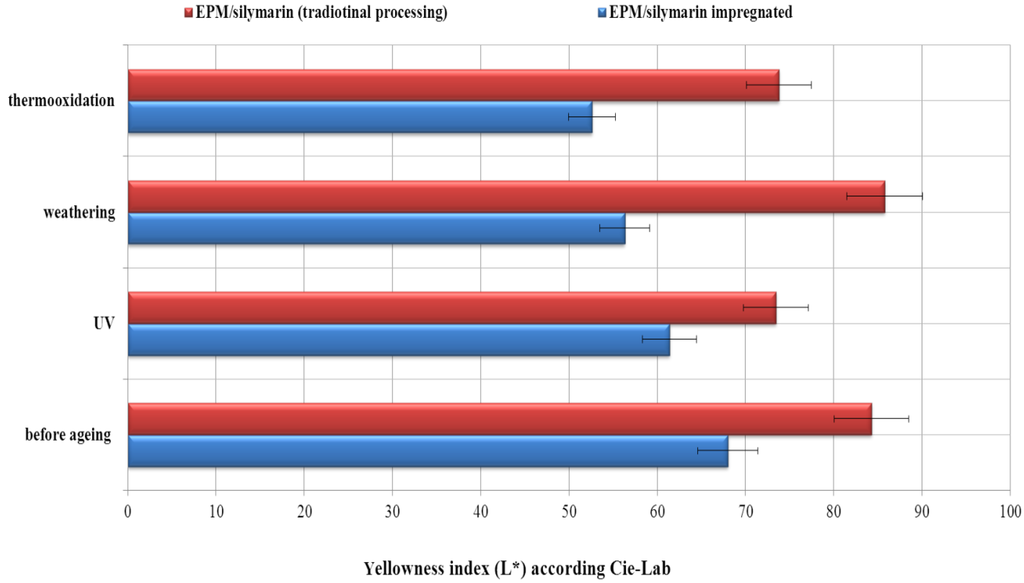
Figure 6.
Effect of silymarin (10%) on the color of the EPM material. L index (L*—level of light or dark over the range 0–100) tested in the CIE-Lab coordinate space.
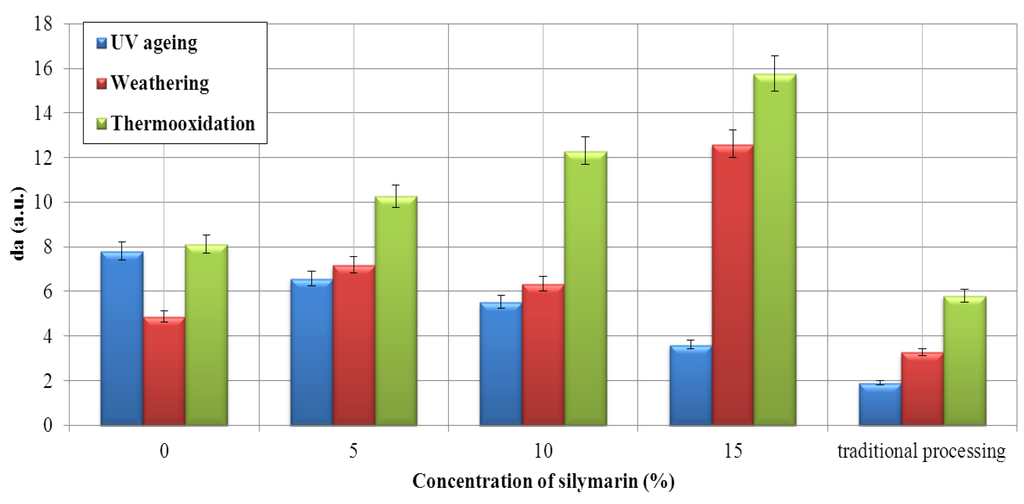
Figure 7.
Effect of silymarin on the color of the EPM material. The a index (the red/green coordinate, with +a* indicating red and −a* indicating green) tested in the CIE-Lab coordinate space.
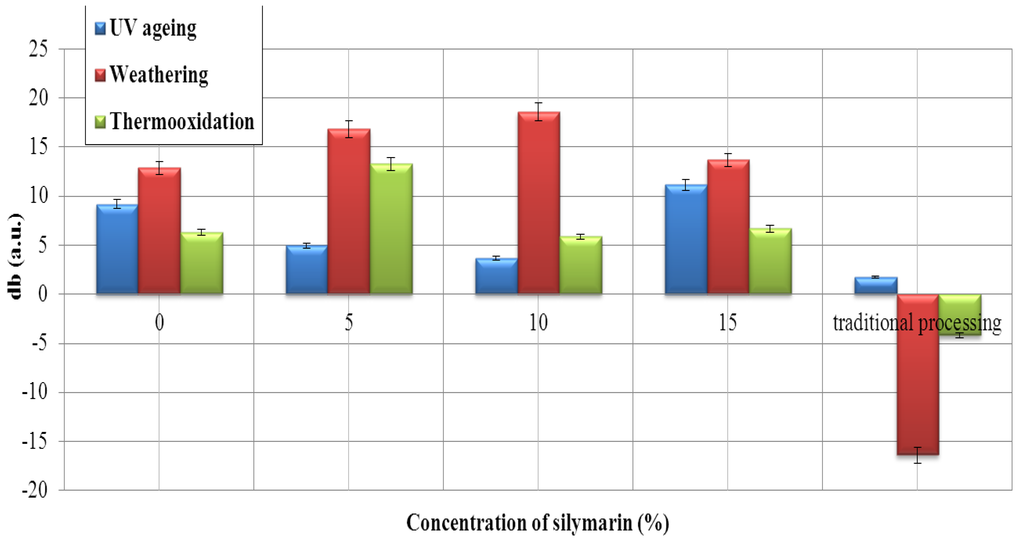
Figure 8.
Effect of silymarin on the color of EPM material. The b index (the yellow/blue coordinate, with +b* indicating yellow and −b* indicating blue) tested in the CIE-Lab coordinate space.
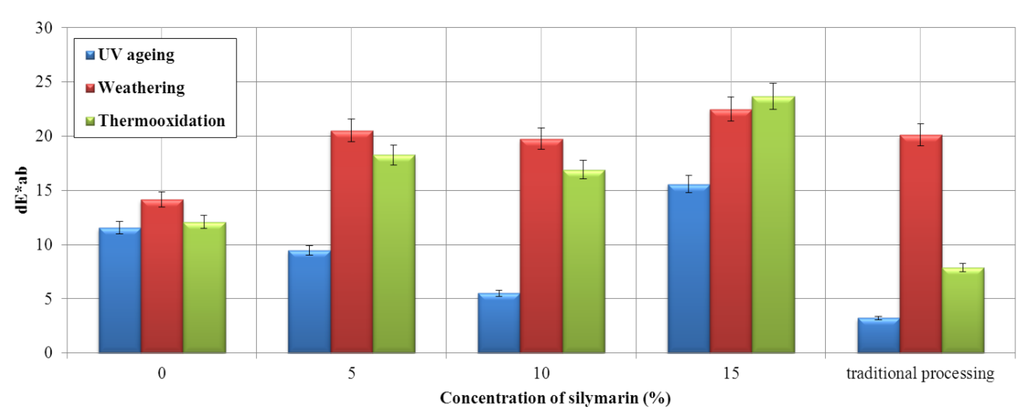
Figure 9.
Effect of silymarin on the color of the EPM material. The dE*ab index (ΔE* = (ΔL*2 + Δa*2 + Δb*2)1/2) tested in the CIE-Lab coordinate space. ΔL* is the degree of lightness difference, Δa* is the red/green difference, and Δb* is the yellow/blue difference.
The dE*ab parameter (Figure 9), indicating the degree of color change of the samples, shows that the greatest changes after weathering and thermooxidation occur for the sample impregnated with silymarin.
Materials containing flavonoid undergo a color change due to the applied ageing factors, confirming that these natural substances can be used as color indicators of ageing time. Thus, these polymers can be used as packaging materials. Below is a graph of color change, dE*ab, as a function of the ageing time of the EPM rubber vulcanizate containing silymarin. The greatest recorded color change occurred under the influence of UVA radiation, with a maximum change after 25 days (Figure 10).
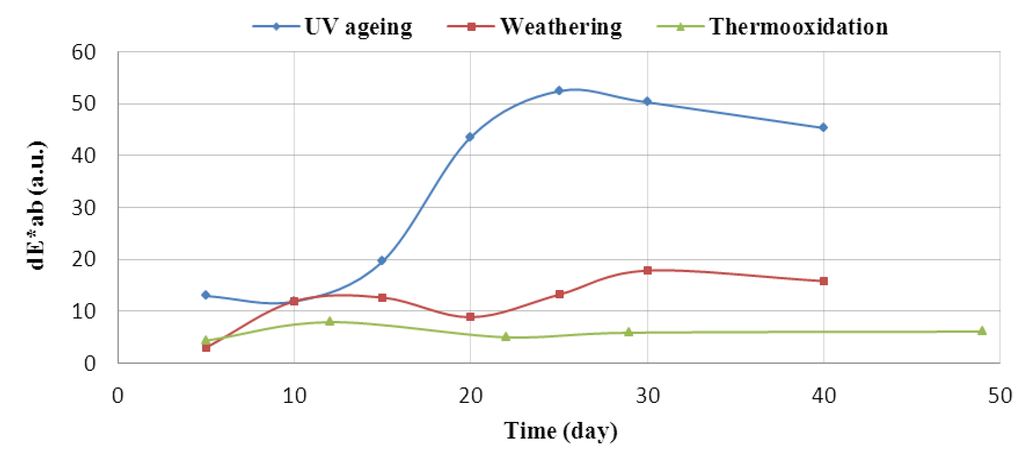
Figure 10.
Effect of silymarin (10%) on the color of the EPM material in ageing time tests. The dE*ab index (ΔE* = (ΔL*2 + Δa*2 + Δb*2)1/2) was tested in the CIE-Lab coordinate space, with ΔL* as the degree of lightness difference, Δa* being the red/green difference, Δb* being the yellow/blue difference.
The color of the EPM sample containing silymarin added during processing is more stable than the sample impregnated with silymarin. The color change may be due to oxidation of the antioxidant during processing and vulcanization. When anti-ageing substances are impregnated into the polymer, the influence of processing on color is minimized. The change in the color of the vulcanized rubber EPM can be observed by the human eye. The color change is significant; therefore, it appears that silymarin can be used as a natural indicator of ageing time. The most visible color change occurs in the sample ontaining 10% EPM antioxidant (Figure 11).
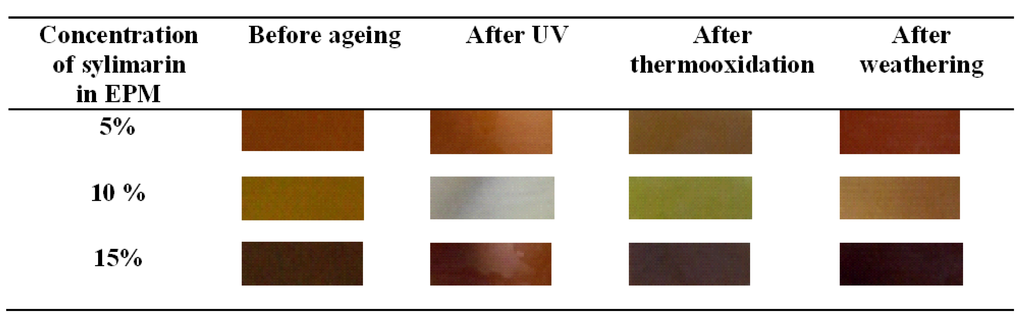
Figure 11.
Image of the EPM composite containing silymarin before and after ageing.
5. Conclusions
Based on the results, we conclude that silymarin can be used as a natural anti-ageing additive for polymers. The addition of silymarin considerably improves the stabilization of the polymer. Natural antioxidants are an ecologically attractive alternative to aromatic amines with steric hindrance or synthetic polyphenols currently used in industry. Silymarin was demonstrated as a natural indicator of polymer ageing time. Materials susceptible to color change under the influence of specified weathering factors can be used in certain applications, e.g., food packaging.
Acknowledgments
This study was supported by Ministry of Science of Higher Education IP 2012 037072.
Conflicts of Interest
The author declares no conflict of interest.
References
- Kriston, I.; Orbán-Mester, Á.; Nagy, G.; Staniek, P.; Földes, E.; Pukánszky, B. Melt stabilisation of Phillips type polyethylene, Part II: Correlation between additive consumption and polymer properties. Polym. Degrad. Stab. 2009, 94, 1448–1456. [Google Scholar] [CrossRef]
- Zweifel, H. Stabilization of Polymeric Materials; Springer: Berlin, Germany, 1998; p. 44. [Google Scholar]
- Holmström, A.; Sörvik, E.M. Thermal degradation of polyethylene in a nitrogen atmosphere of low oxygen content. II. Structural changes occurring in low-density polyethylene at an oxygen content less than 0.0005%. J. Appl. Polym. Sci. 1974, 18, 761–778. [Google Scholar] [CrossRef]
- Holström, A.; Sörvik, E.M. Thermal degradation of polyethylene in a nitrogen atmosphere of low oxygen content. III. Structural changes occurring in low-density polyethylene at oxygen contents below 1.2%. J. Appl. Polym. Sci. 1974, 18, 779–804. [Google Scholar] [CrossRef]
- Massey, S.; Adnot, A.; Rjeb, A.; Roy, D. Action of water in the degradation of low-density polyethylene studied by X-ray photoelectron spectroscopy. Express. Polym. Lett. 2007, 8, 506–515. [Google Scholar] [CrossRef]
- Földes, E.; Lohmeijer, J. Relationship between chemical l structure and performance of primary antioxidants in PBD. Polym. Degrad. Stab. 1999, 66, 31–39. [Google Scholar] [CrossRef]
- Földes, E.; Lohmeijer, J. Study of the effects of additive interaction in polymer stabilization. Polym. Prepr. 2001, 42, 365–366. [Google Scholar]
- Anandhan, S.; Viknesh, C.J.; Othman, N.; Sasidharan, S. A new processing additive for natural rubber from agricultural waste. Kaut. Schuk Gummi Kunst. 2011, 64, 44–51. [Google Scholar]
- Fenollar, O.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Lopez, J.; Balart, R. Effect of the epoxidized linseed oil concentration as natural plasticizer in vinyl plastisols. J. Mater. Sci. 2010, 45, 4406–4413. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Comparison of sorbitol and glycerol as plasticizers for thermoplastic starch in TPS/PLA blends. J. Appl. Polym. Sci. 2011, 119, 2439–2448. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosanfilms. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Bueno-Ferrer, C.; Garrigós, M.C.; Jiménez, A. Characterization and thermal stability of poly(vinyl chloride) plasticized with epoxidized soybean oil for food packaging. Polym. Degrad. Stab. 2010, 95, 2207–2221. [Google Scholar] [CrossRef]
- Yurttas, H.C.; Shafer, H.W.; Warthesen, J.J. Antioxidant activity of nontocopherol hazelnut (Corylus spp.) phenolics. J. Food Sci. 2000, 65, 276–280. [Google Scholar] [CrossRef]
- Zalacain, A.; Carmona, M.; Lorenzo, C.; Blazquez, I.; Alonso, G.L. Antiradical efficiency of different vegetable tannin extracts. J. Am. Leather Chem. Assoc. 2002, 97, 137–142. [Google Scholar]
- Schwarzenbach, K.; Gilg, B.; Muller, D.; Knobloch, G.; Pauquet, J.R.; Rota-Graziosi, P. Antioxidants. In Plastic Additives Handbook; Zweifel, H., Ed.; Hanser: Munich, Germany, 2001; pp. 29–31. [Google Scholar]
- Torikai, A.; Takeuchi, A.; Nagaya, S.; Fueki, K. Photodegradation of polyethylene: Effect of crosslinking on the oxygenated products and mechanical properties. Polym. Photochem. 1986, 7, 199–211. [Google Scholar] [CrossRef]
- Carlsson, D.J.; Wile, D.M. The photodegradation of polypropylene films. II. Photolysis of ketonic oxidation products. Macromolecules 1969, 2, 587–597. [Google Scholar] [CrossRef]
- Philippart, J.L.; Sinturel, C.; Arnaud, R.; Gardette, J.L. In fluence of the exposure parameters on the mechanism of photooxidation of polypropylene. Polym. Degrad. Stab. 1999, 64, 213–225. [Google Scholar] [CrossRef]
- Mita, I.; Jellinek, H.H. Aspects of Degradation and Stabilization of Polymers; Elsevier: Amsterdam, The Netherlands, 1978; p. 24. [Google Scholar]
- Kellen, T. Polymer Degradation; Van Nostrand Reinhold: New York, NY, USA, 1983; p. 7. [Google Scholar]
- Ivan, B.; Kellen, T.; Tudos, F.; Jellinek, H.H.; Kachi, H. Degradation and Stabilisation of Polymers; Elsevier: Amsterdam, The Netherlands, 1989; volume 2, p. 570. [Google Scholar]
- Cooray, B.; Scott, G.; Scott, G. Developments in Polymer Stabilisation–2; Applied Science Publishers: London, UK, 1980; p. 419. [Google Scholar]
- Al-Malaika, S.; Ashley, H.; Issenhuth, S. The antioxidant role of α-tocopherol in polymers. I. The nature of transformation products of α-tocopherol formed during melt processing of LDPE. J. Polym. Sci. A 1994, 32, 3099–3113. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Goodwin, C.; Issenhuth, S.; Burdick, D. The antioxidant role of alpha-tocopherol in polymers II. Melt stabilising effect inpolypropylene. Polym. Degrad. Stab. 1999, 64, 145–156. [Google Scholar] [CrossRef]
- Al-Malaika, S.; Issenhuth, S. The antioxidant role of alpha-tocopherol in polymers III. Nature of transformation products during polyolefins extrusion. Polym. Degrad. Stab. 1999, 65, 143–151. [Google Scholar] [CrossRef]
- Masek, A.; Zaborski, M.; Kosmalska, A. Derivatives of flavonoides as anti-aging substances in elastomers. Compt. Rendus Chim. 2011, 14, 483–488. [Google Scholar] [CrossRef]
- Peng, Z.; Liang, X.; Zhang, Y.; Zhang, Y. Reinforcement of EPDM by in situ prepared zinc dimethacrylate. J. Appl. Polym. Sci. 2002, 84, 1339–1345. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Basfar, A.A. Evaluation of some antioxidants in radiation vulcanized ethylene-propylene diene (EPDM) rubber. Nucl. Instrum. Meth. B 2001, 185, 346–350. [Google Scholar] [CrossRef]
- Noronha, F.; Angelini, J.; Góis, N.; Mei, L. Performance development requirementsfor elastomers of electric power network insulators. J. Mater. Process. Technol. 2005, 162, 102–108. [Google Scholar] [CrossRef]
- Li, Z.; Chen, S.; Zhang, J.; Shi, D. Influence of different antioxidants on cure kinetics and aging behaviours of ethylene propylene diene rubber/low density polyethylene blends. Plast. Rubber Compos. 2009, 38, 187–194. [Google Scholar] [CrossRef]
- Tsepalov, V.F.; Kharitonova, A.A.; Gladyshev, G.P.; Emanuel, N.M. Determination of the rate constants and inhibition coefficients of phenol antioxidants with the aid of model chain reactions. Kinet. Catal. 1977, 18, 1034–1041. [Google Scholar]
- Gugumus, F. New trends in the stabilization of polyolefin fibers. Polym. Degrad. Stab. 1994, 44, 273–297. [Google Scholar] [CrossRef]
- Thompson, C.R.; Moore, J.C.; Saal, S.F.; Swan, H.S. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Khabbaz, F.; Albertsson, A.C. Rapid test methods for analysing degradable polyolefins with a pro-oxidant system. J. Appl. Polym. Sci. 2001, 79, 2309–2316. [Google Scholar] [CrossRef]
- Kelen, T. Polymer Degradation; Van Nostrand Reinhold: New York, NY, USA, 1983; p. 421. [Google Scholar]
- Dixon, R.K. Thermal ageing predictions from an Arrhenius plot with only one data point. IEEE T. Dielect. El. In. 1980, E1–15, 331–340. [Google Scholar]
- Gugumus, F. The Use of Accelerated Tests in the Evaluation of Antioxidantsand Light Stabilizers. In Developments in Polymer Stabilization; Scott, G., Ed.; Applied Science Publishers: Barking, UK, 1987; pp. 239–289. [Google Scholar]
- Scoponi, M.; Pradella, F.; Carassiti, V. Photodegradable polyolefins. Photo-oxidation mechanisms of innovative polyolefin copolymers containing double bonds. Coordin. Chem. Rev. 1993, 125, 219–230. [Google Scholar] [CrossRef]
- Davis, A.; Sims, D. Weathering of Polymers; Applied Science Publishers: London, UK, 1983; pp. 55–57. [Google Scholar]
- Gijsman, P.; Hennekens, J.; Janssen, K. Polymer Durability, Degradation, Stabilization and Lifetime Prediction; Clough, R.L., Billingham, N.C., Gillen, K.T., Eds.; Advances in Chemistry Series 249; American Chemical Society: Washington, DC, USA, 1996; pp. 622–648. [Google Scholar]
- Thorat, H.B.; Prabhu, C.S.; Sureshkumar, K.; Pandya, M.V. Stabilization of ethylene-propylene copolymer against g-ray induced degradation. Radiat. Phys. Chem. 1998, 51, 215. [Google Scholar]
- Zaharescu, T.; Giurginca, M.; Jipa, S.; Podina, C. Radiochemical oxidation of ethylene–propylene elastomers in the presence of some phenolic antioxidants. Polym. Degrad. Stab. 1999, 63, 245–251. [Google Scholar] [CrossRef]
- Mitra, S.; Ghanbari-Siahkali, A.; Kingshott, P.; Hvilsted, S.; Almdal, K. An investigation on changes in chemical properties of pure ethylene-propylene-diene rubber in aqueous acidic environments. Mater. Chem. Phys. 2006, 98, 248–255. [Google Scholar] [CrossRef]
- Rivaton, A.; Cambon, S.; Gardette, J.L. Radiochemical ageing of ethylene–propylene–diene monomer elastomers. 1. Mechanism of degradation under inert atmosphere. J. Polym. Sci. Polym. Chem. 2004, 42, 1239–1248. [Google Scholar] [CrossRef]
- Gillen, K.T.; Clough, R.L.; Wise, J. Prediction of Elastomer Lifetimes from Accelerated Thermal-Ageing Experiments, ASC 249. In Polymer Durability; American Chemical Society Publications: Washington, DC, USA, 1993; pp. 557–575. [Google Scholar]
- Arakawa, K.; Seguchi, T.; Hayakawa, N.; Machi, S. Radiation-induced oxidation of polymers. Effect of antioxidant and antirad agent on oxygen consumption and gas evolution. J. Polym. Sci. 1983, 21, 1173–1181. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Zaborski, M. Characteristics of curcumin using cyclic voltammetry, UV–VIS, fluorescence and thermogravimetric analysis. Electrochem. Acta 2013, 107, 441–447. [Google Scholar] [CrossRef]
- Heller, H.J. Photochemistry of dyed and pigmented polymers. Eur. Polym. J. Suppl. 1969, 99–105. [Google Scholar]
- Rabek, J.F. Polymer photodegradation-mechanisms and experimental methods. Chapman Hall: Cambridge, UK, 1995; pp. 300–600. [Google Scholar]
- Rabek, J.F. Polymer Photodegradation of Polymers: Physical Characteristic and Applications; Springer-Verlag: Berlin, Germany, 1996. [Google Scholar]
- Ranby, B.G.; Rabek, J.F. Photodegradation, Photooxidation and Photostabilization of Polymers; John Wiley & Sons: New York, NY, USA, 1975; pp. 101–102. [Google Scholar]
- Masek, A.; Chrzescijanska, E.; Zaborski, M.; Piotrowska, M. Dodecyl gallate as a pro-ecological antioxidant for food packing materials. Compt. Rendus Chim. 2014, 17, 1116–1127. [Google Scholar] [CrossRef]
- Wypych, J. Handbook of Polymers; Chem. Tec. Publishing: Toronto, Ontario, Canada, 2012; pp. 200–450. [Google Scholar]
- Cirillo, G.; Iemma, F. Antioxidant Polymers: Synthesis, Properties, and Applications; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Ryun Oh, D.; Hyun-Kyu, K.; Lee, N.; Ho Chae, K.; Kaang, S.; Sung Lee, M.; Hyeon Kim, T. Synthesis of new polymeric antioxidants. Bull. Korean Chem. Soc. 2001, 22, 629–632. [Google Scholar]
- Masek, A.; Zaborski, M.; Chrześciajańska, E. Morin hydrate as pro-ecological antioxidant and pigment for polyolefin polymers. Compt. Rendus Chim. 2013, 16, 990–996. [Google Scholar] [CrossRef]
- Masek, A.; Zaborski, M. Polymer biocomposites ENR/PCL from renewable resources. Compt. Rendus Chim. 2014, 17, 944–951. [Google Scholar] [CrossRef]
- Masek, A.; Zaborski, M.; Piotrowska, M. Controlled degradation of biocomposites ENR/PCL containing natural antioxidants. Compt. Rendus Chim. 2014, 17, 1128–1135. [Google Scholar] [CrossRef]
- Masek, A.; Chrześcijańska, E.; Zaborski, M. Antioxidative Properties of silymarin, 7-aminoflavone, neohesperidin dihydrochalcone and trihydroxyethylenorutin studied by the electrochemical methods. Int. J. Electrochem. Sci. 2014, 9, 7875–7889. [Google Scholar]
- Masek, A.; Chrześcijańska, E.; Zaborski, M. Electrochemical properties of catechin in non-aqueous media. Int. J. Electrochem. Sci. 2015, 10, 2504–2514. [Google Scholar]
- Masek, A.; Chrześcijańska, E.; Zaborski, M. Voltammetric and FTIR spectroscopic studies of the oxidation of retinyl propionate at Pt electrode in non-aqueous media. Int. J. Electrochem. Sci. 2014, 6, 6809–6820. [Google Scholar]
- Garcia, J.P.D.; Hsieh, M.F.; Doma, B.T., Jr.; Peruelo, D.C.; Chen, I.-H.; Lee, H.-M. Synthesis of Gelatin-γ-polyglutamic acid-based hydrogel for the in vitro controlled release of epigallocatechin gallate (EGCG) from Camellia sinensis. Polymers 2014, 6, 39–58. [Google Scholar] [CrossRef]
- Rijo, P.; Matias, D.; Fernandes, A.S.; Simões, M.F.; Nicolai, M.; Reis, C.P. Antimicrobial plant extracts encapsulated into polymeric beads for potential application on the skin. Polymers 2014, 6, 479–490. [Google Scholar] [CrossRef]
- Ibrahim, S.; Daik, R.; Abdullah, I. Functionalization of liquid natural rubber via oxidative degradation of natural rubber. Polymers 2014, 6, 2928–2941. [Google Scholar] [CrossRef]
- Webb, H.K.; Arnott, J.; Crawford, R.J.; Ivanova, E.P. Plastic degradation and its environmental implications with special reference to poly(ethylene terephthalate). Polymers 2013, 5, 1–18. [Google Scholar] [CrossRef]
- Pretsch, T. Review on the functional determinants and durability of shape memory polymers. Polymers 2010, 2, 120–158. [Google Scholar] [CrossRef]
- Das, S.K.; Vasudevan, D.M. Protective effects of silymarin, a milk thistle (silybium marianum) derivative on ethanol-induced oxidative stress in liver. Indian J. Biochem. Biophys. 2006, 43, 306–311. [Google Scholar] [PubMed]
- Galhardi, F.; Mesquita, K.; Monserrat, J.M.; Barros, D.M. Effect of silymarin on biochemical parameters of oxidative stress in aged and young rat brain. Food Chemi. Toxicol. 2009, 47, 2655–2660. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Meleth, S.; Sharma, S.D. Silymarin, a flavonoid from milk thistle (Silybum marianum L.), inhibits UV-induced oxidative stress through targeting infiltrating CD11b+ cells in mouse skin. Photochem. Photobiol. 2008, 84, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Korman, N.J.; Mukhtar, H. Agarwal, R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J. Natl. Cancer Inst. 1997, 89, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Zaborski, M.; Chrześcijańska, E. Electrochemical oxidation of flavonoid derivatives. Food Chem. 2011, 127, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Chobot, V.; Kubicova, L.; Bachmann, G.; Hadacek, F. Versatile redox chemistry complicates antioxidant capacity assessment: Flavonoids as milieu-Dependent anti- and pro-oxidants. Int. J. Mol. Sci. 2013, 14, 11830–11841. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Mattivi, F.; de Rosso, M.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, stilbenes and flavonols. Int. J. Mol. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef] [PubMed]
- Stobiecki, M.; Kachlicki, P.; Grotewold, E. The Science of Flavonoids; Springer: New York, NY, USA, 2006; pp. 47–70. [Google Scholar]
- The regulation of the European Union No. 10/2011. Available online: https://www.fsai.ie/uploadedFiles/Reg10_2011.pdf (accessed on 14 January 2011).
- Kvasnika, F.; Biba, B.; Sevcík, R.; Voldrich, M.; Krátká, J. Analysis of the active components of silymarin. J Chromatogr. A 2003, 990, 239–245. [Google Scholar] [CrossRef]
- PN-EN 728:1999. “Plastic Piping Systems and Ducting Systems. Polyolefin Pipes and Fittings. Determination of Oxidation Induction Time”. Available online: http://www.pkn.pl (accessed on 28 January 1999).
- ISO 11357–6:2002. “Plastics-DSC-Part 6: Determination of Oxygen Induction Time”. 2002. Available online: http://www.iso.org/iso/catalogue_detail.htm?csnumber=42718 (accessed on 21 June 2002).
- Dabkowski, Z. Lifetime prediction for polymer materials using OIT measurements by the DSC method. Polimery 2005, 50, 231–215. [Google Scholar]
- Griesbach, R. Biochemistry and genetics of flower colour. Plant Breed. Rev. 2005, 25, 89–114. [Google Scholar]
- Kevan, P.; Giurfa, M.; Chittka, L. Why are there so many and so few white flowers? Trends in Plant Sci. 1996, 1, 280–284. [Google Scholar] [CrossRef]
- Niyogi, K. Safety values for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–560. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Deroles, S.; Bennett, R.; Davies, K. New insight into the structures and formation of anthocyanic vacuolar inclusions in flower petals. BMC Plant Biol. 2006, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Shiono, M. Structure of the blue cornflower pigment. Nature 2005, 436, 791. [Google Scholar] [CrossRef] [PubMed]
- Młodzińska, E. Survey of plant pigments: molecular and environmental determinants of plant colors. Acta Biol. Crac. Ser. Bot. 2009, 51, 7–16. [Google Scholar]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Rao, V.; Balachandran, B.; Shen, H.; Logan, A.; Rao, L. In vitro and in vivo antioxidant properties of the plant-based supplement greens. Int. J. Mol. Sci. 2011, 12, 4896–4908. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).