Synthesis and Characterization of Alternating Polymers Incorporating Boron-Chelated Heterochrysene Units
Abstract
:1. Introduction

2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of Monomer 5
2.3. Synthesis of Boc-poly
2.4. Synthesis of poly-1
2.5. Synthesis of BF2-poly
2.6. Synthesis of BPh2-poly
2.7. Characterizations
3. Results and Discussion
3.1. Synthetic Approach and Characterizations

| No. | Mn | Mw | Mw/Mn |
|---|---|---|---|
| Boc-poly | 1.41 × 104 g/mol | 4.47 × 104 g/mol | 3.17 |
| poly-1 | 1.03 × 104 g/mol | 1.99 × 104 g/mol | 1.93 |
| BF2-poly | 1.13 × 104 g/mol | 2.10 × 104 g/mol | 1.86 |
| BPh2-poly | 1.24 × 104 g/mol | 2.63 × 104 g/mol | 2.12 |




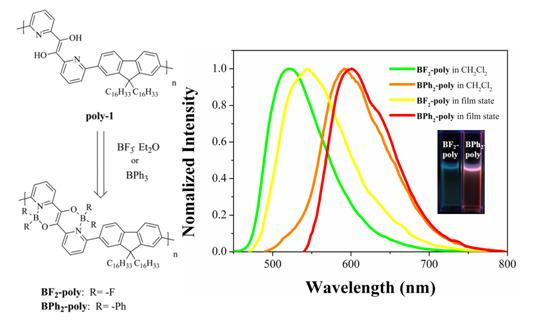
3.2. UV–vis Absorption and Photoluminescence Studies

| Samples | λmax | λem | Stokes Shift | Φf |
|---|---|---|---|---|
| 1 | 410 nm | 486 nm | 3810 cm−1 | 0.63 |
| 2 | 468 nm | 573 nm | 3920 cm−1 | 0.32 |
| BF2-poly | 433 nm | 520 nm | 3860 cm−1 | 0.23 |
| BPh2-poly | 481 nm | 592 nm | 3900 cm−1 | 0.11 |

3.3. Electrochemical Analyses
| Polymers | HOMO (eV) | LUMO (eV) | band Gap (eV) |
|---|---|---|---|
| BF2-poly | −5.89 | −3.55 | 2.34 |
| BPh2-poly | −5.31 | −3.20 | 2.11 |

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dong, H.; Zhu, H.; Meng, Q.; Gong, X.; Hu, W. Organic photoresponse materials and devices. Chem. Soc. Rev. 2012, 41, 1754–1808. [Google Scholar] [CrossRef] [PubMed]
- Bendikov, M.; Wudl, F.; Perepichka, D.F. Tetrathiafulvalenes, oligoacenenes, and their buckminsterfullerene derivatives: The brick and mortar of organic electronics. Chem. Rev. 2004, 104, 4891–4946. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, J.; Li, Y.; Li, Y. Aggregate nanostructures of organic molecular materials. Acc. Chem. Res. 2010, 43, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, Y.; Liu, H.; Yin, X.; Li, Y. Construction of heterostructure materials toward functionality. Chem. Soc. Rev. 2011, 40, 4506–4524. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Chao, D.; Qi, X.; Xiong, Q.; Zhang, Y.; Tu, J.; Zhang, H.; Fan, H.J. Controllable growth of conducting polymers shell for constructing high-quality organic/inorganic core/shell nanostructures and their optical-electrochemical properties. Nano Lett. 2013, 13, 4562–4568. [Google Scholar] [CrossRef] [PubMed]
- Suraru, S.L.; Zschieschang, U.; Klauk, H.; Wurthner, F. A core-extended naphthalene diimide as a p-channel semiconductor. Chem. Commun. 2011, 47, 11504–11506. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles: Preparation, properties, functionalization and biological applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Ang, T.; Ooi, Z.; Sonar, P.; Lin, T.; Neo, W.; Song, J.; Hobley, J. Optical characterization of the hole polaron in a series of diketopyrrolopyrrole polymers used for organic photovoltaics. Polymers 2015, 7, 69–90. [Google Scholar] [CrossRef]
- Clar, E. Polycyclic hydrocarbons; Academic Press: London, UK, 1964. [Google Scholar]
- Li, G.; Wu, Y.; Gao, J.; Wang, C.; Li, J.; Zhang, H.; Zhao, Y.; Zhao, Y.; Zhang, Q. Synthesis and physical properties of four hexazapentacene derivatives. J. Am. Chem. Soc. 2012, 134, 20298–20301. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.Y.; Zhou, F.; Gao, J.; Li, G.; Wang, C.; Xu, Q.F.; Zhang, Q.; Lu, J.M. Synthesis, characterization, and nonvolatile ternary memory behavior of a larger heteroacene with nine linearly fused rings and two different heteroatoms. J. Am. Chem. Soc. 2013, 135, 14086–14089. [Google Scholar] [CrossRef] [PubMed]
- Kivelson, S.; Chapman, O.L. Polyacene and a new class of quasi-one-dimensional conductors. Phys. Rev. B 1983, 28, 7236–7243. [Google Scholar] [CrossRef]
- Bendikov, M.; Duong, H.M.; Starkey, K.; Houk, K.N.; Carter, E.A.; Wudl, F. Oligoacenes: Theoretical prediction of open-shell singlet diradical ground states. J. Am. Chem. Soc. 2004, 126, 7416–7417. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Duong, H.M.; Liu, Y.; Shi, W.; Ji, L.; Li, G.; Li, S.; Liu, X.W.; Ma, J.; Wudl, F.; et al. Synthesis and structure characterization of a stable nonatwistacene. Angew. Chem. Int. Ed. 2012, 51, 6094–6098. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, L.; Liu, T.; Yu, Y.; Liu, H.; Li, Y.; Zhu, D. Anthraceno–perylene bisimides: The precursor of a new acene. Org. Lett. 2011, 13, 5692–5695. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Jazdzyk, M.; Stein, N.N.; Prusevich, P.; Miller, G.P. Design, synthesis, and characterization of a persistent nonacene derivative. J. Am. Chem. Soc. 2010, 132, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Tonshoff, C.; Bettinger, H.F. Photogeneration of octacene and nonacene. Angew. Chem. Int. Ed. 2010, 49, 4125–4128. [Google Scholar] [CrossRef] [PubMed]
- Nepomnyashchii, A.B.; Bard, A.J. Electrochemistry and electrogenerated chemiluminescence of bodipy dyes. Acc. Chem. Res. 2012, 45, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Liu, T.W.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Hsieh, M.C.; Lin, J.C.; Chang, T.C. Selective photodynamic therapy based on aggregation-induced emission enhancement of fluorescent organic nanoparticles. Biomaterials 2012, 33, 897–906. [Google Scholar] [CrossRef]
- Tamgho, I.S.; Hasheminasab, A.; Engle, J.T.; Nemykin, V.N.; Ziegler, C.J. A new highly fluorescent and symmetric pyrrole-BF2 chromophore: BOPHY. J. Am. Chem. Soc. 2014, 136, 5623–5626. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef] [PubMed]
- Frath, D.; Massue, J.; Ulrich, G.; Ziessel, R. Luminescent materials: Locking π-conjugated and heterocyclic ligands with boron(III). Angew. Chem. Int. Ed. Engl. 2014, 53, 2290–2310. [Google Scholar] [CrossRef] [PubMed]
- Nagai, A.; Kokado, K.; Nagata, Y.; Chujo, Y. 1,3-diketone-based organoboron polymers: Emission by extending π-conjugation along a polymeric ligand. Macromolecules 2008, 41, 8295–8298. [Google Scholar] [CrossRef]
- Qin, Y.; Kiburu, I.; Shah, S.; Jakle, F. Synthesis and characterization of organoboron quinolate polymers with tunable luminescence properties. Macromolecules 2006, 39, 9041–9048. [Google Scholar] [CrossRef]
- Zhang, H.; Hong, X.; Ba, X.; Yu, B.; Wen, X.; Wang, S.; Wang, X.; Liu, L.; Xiao, J. Synthesis, physical properties, and photocurrent behavior of strongly emissive boron-chelate heterochrysene derivatives. Asian J. Org. Chem. 2014, 3, 1168–1172. [Google Scholar] [CrossRef]
- Chen, H.; He, M.; Pei, J.; Liu, B. End-group analysis of blue light-emitting polymers using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 2002, 74, 6252–6258. [Google Scholar] [CrossRef] [PubMed]
- Tolman, C.A.; Seidel, W.C.; Gerlach, D.H. Triarylphosphine and ethylene complexes of zerovalent nickel, palladium, and platinum. J. Am. Chem. Soc. 1972, 94, 2669–2676. [Google Scholar] [CrossRef]
- Heek, T.; Fasting, C.; Rest, C.; Zhang, X.; Wurthner, F.; Haag, R. Highly fluorescent water-soluble polyglycerol-dendronized perylene bisimide dyes. Chem. Commun. 2010, 46, 1884–1886. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Ma, S.; Li, D.; Wu, Y.; Ba, X. Synthesis and Characterization of Alternating Polymers Incorporating Boron-Chelated Heterochrysene Units. Polymers 2015, 7, 1192-1204. https://doi.org/10.3390/polym7071192
Zhang H, Ma S, Li D, Wu Y, Ba X. Synthesis and Characterization of Alternating Polymers Incorporating Boron-Chelated Heterochrysene Units. Polymers. 2015; 7(7):1192-1204. https://doi.org/10.3390/polym7071192
Chicago/Turabian StyleZhang, Hailei, Shuli Ma, Dongqin Li, Yonggang Wu, and Xinwu Ba. 2015. "Synthesis and Characterization of Alternating Polymers Incorporating Boron-Chelated Heterochrysene Units" Polymers 7, no. 7: 1192-1204. https://doi.org/10.3390/polym7071192
APA StyleZhang, H., Ma, S., Li, D., Wu, Y., & Ba, X. (2015). Synthesis and Characterization of Alternating Polymers Incorporating Boron-Chelated Heterochrysene Units. Polymers, 7(7), 1192-1204. https://doi.org/10.3390/polym7071192