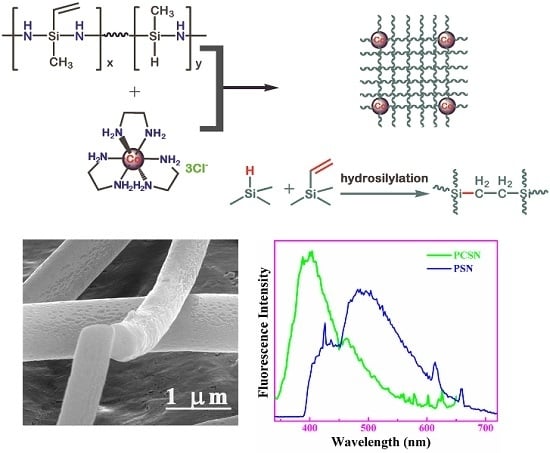
Synthesis of Novel Cobalt-Containing Polysilazane Nanofibers with Fluorescence by Electrospinning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Co/Polysilazane
3. Results and Discussion
3.1. Synthesis of the Polymer Precursors
3.2. Polymer Nanofibers
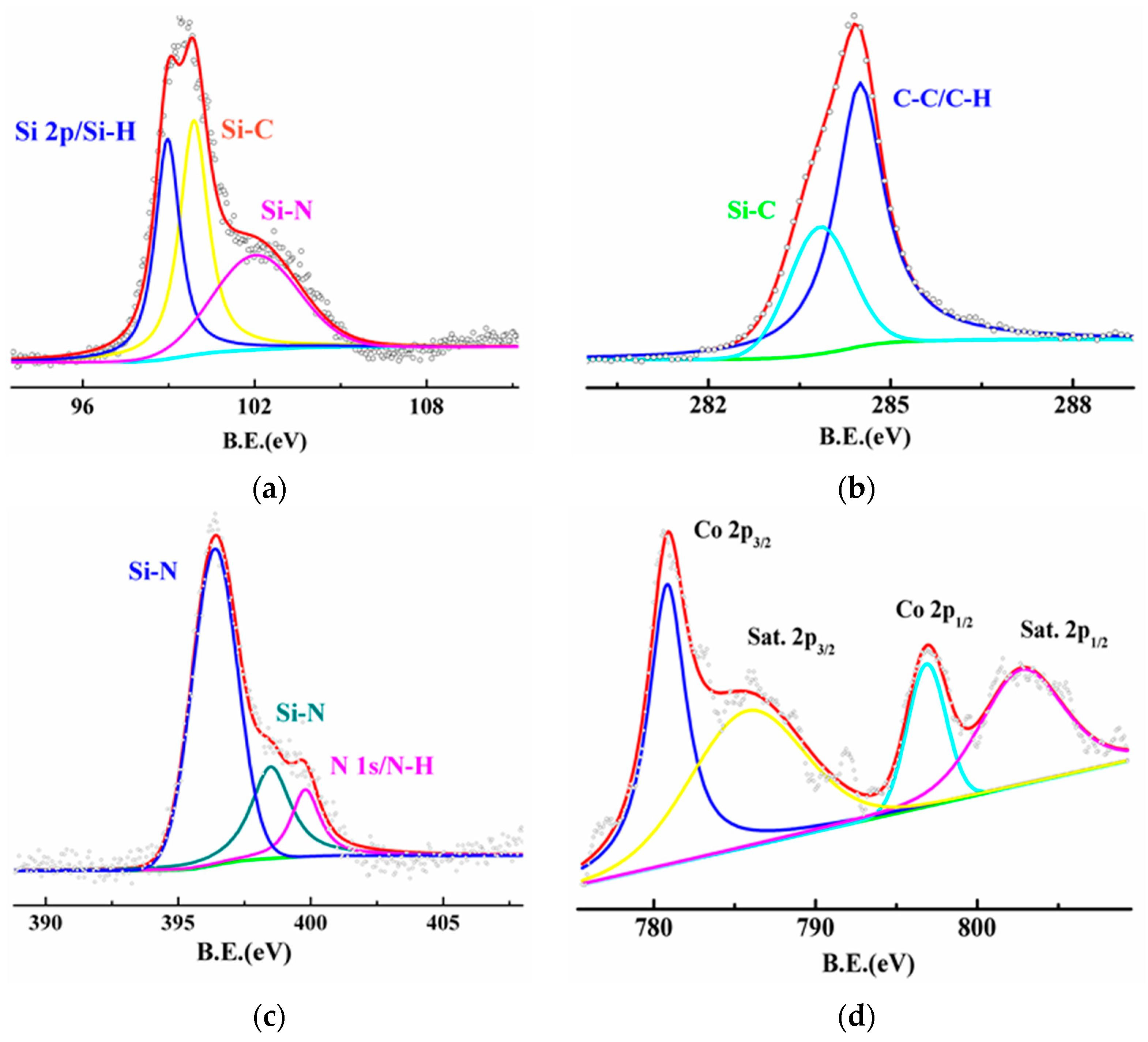
3.3. Structure Analysis
3.4. Thermal Stability
3.5. Fluorescence
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hide, F.; DÍaz-GarcÍa, M.A.; Schwartz, B.J.; Heeger, A.J. New developments in the photonic applications of conjugated polymers. Acc. Chem. Res. 1997, 30, 430–436. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.Z. Optical properties and applications of hybrid semiconductor nanomaterials. Coord. Chem. Rev. 2009, 253, 3015–3041. [Google Scholar] [CrossRef]
- Dou, L.; Liu, Y.; Hong, Z.; Li, G.; Yang, Y. Low-bandgap Near-IR conjugated polymers/molecules for organic electronics. Chem. Rev. 2015, 115, 12633–12665. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; You, J.; Hong, Z.; Xu, Z.; Li, G.; Street, R.A.; Yang, Y. 25th Anniversary Article: A decade of organic/polymeric photovoltaic research. Adv. Mater. 2013, 25, 6642–6671. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.A.J.; Nelson, J. Factors limiting device efficiency in organic photovoltaics. Adv. Mater. 2013, 25, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, J. New insights in the study of pyrene excimer fluorescence to characterize macromolecules and their supramolecular assemblies in solution. Langmuir 2012, 28, 6527–6538. [Google Scholar] [CrossRef] [PubMed]
- Winnik, M.A. End-to-end cyclization of polymer chains. Acc. Chem. Res. 1985, 18, 73–79. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Celebioglu, A.; Bayir, S.; Gorur, M.; Doganci, E.; Yilmaz, F.; Uyar, T. Highly fluorescent pyrene-functional polystyrene copolymer nanofibers for enhanced sensing performance of TNT. ACS Appl. Mater. Interfaces 2015, 7, 21038–21046. [Google Scholar] [CrossRef] [PubMed]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Menapace, I.; Mera, G.; Riedel, R.; Erdem, E.; Eichel, R.-A.; Pauletti, A.; Appleby, G.A. Luminescence of heat-treated silicon-based polymers: Promising materials for LED applications. J. Mater. Sci. 2008, 43, 5790–5796. [Google Scholar] [CrossRef]
- Wang, W.Z.; Fan, Q.L.; Cheng, F.; Zhao, P.; Huang, W. Sonochemical synthesis of novel blue-emissive, water-soluble, cationic polysilanes as fluorescent sensors. J. Polym. Sci. Polym. Chem. 2006, 44, 3513–3525. [Google Scholar] [CrossRef]
- Magdalena, G.Z.; Reinold, M.L.; Kaspar, J.; Sasikumar, V.P.; Soraru, G.D.; Riedel, R. New insights into understanding irreversible and reversible Lithium storage within SiOC and SiCN ceramics. Nanomaterials 2015, 5, 233–245. [Google Scholar]
- Zaheer, M.; Schmalz, T.; Motz, G.; Kempe, R. Polymer derived non-oxide ceramics modified with late transition metals. Chem. Soc. Rev. 2012, 41, 5102–5116. [Google Scholar] [CrossRef] [PubMed]
- Seyferth, D.; Lang, H.; Sobon, C.A.; Borm, J.; Tracy, H.J.; Bryson, N. Chemical modification of preceramic polymers: Their reactions with transition metal complexes and transition metal powders. J. Inorg. Organomet. Polym. 1992, 2, 59–77. [Google Scholar] [CrossRef]
- Kong, J.; Kong, M.; Zhang, X.; Chen, L.; An, L. Magnetoceramics from the bulk pyrolysis of polysilazane cross-linked by polyferrocenylcarbosilanes with hyperbranched topology. ACS Appl. Mater. Interfaces 2013, 5, 10367–10375. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.; Mera, G.; Riedel, R.; Soraru, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Colombo, P.; Carturan, S.M.; Pippel, E.; Woltersdorf, J. Growth of one-dimensional nanostructures in porous polymer-derived ceramics by catalyst-assisted pyrolysis. Part II: Cobalt catalyst. J. Am. Ceram. Soc. 2010, 93, 3709–3719. [Google Scholar] [CrossRef]
- Vakifahmetoglu, C.; Pippel, E.; Woltersdorf, J.; Colombo, P. Growth of one-dimensional nanostructures in porous polymer-derived ceramics by catalyst-assisted pyrolysis. Part I: Iron catalyst. J. Am. Ceram. Soc. 2010, 93, 959–968. [Google Scholar] [CrossRef]
- Lu, S.; Jin, T.; Bao, M.; Yamamoto, Y. Cobalt-catalyzed hydroalkylation of 60 fullerene with active alkyl bromides: Selective synthesis of monoalkylated fullerenes. J. Am. Chem. Soc. 2011, 133, 12842–12848. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, D.W.; Nam, K.B.; Gim, Y.H.; Ko, H.S.; Seo, D.K.; Lee, G.H.; Kim, Y.H.; Kim, S.W.; Oh, T.S.; et al. Continuous bundles of aligned electrospun PAN nano-fiber using electrostatic spiral collector and converging coil. Polymer 2016, 84, 52–58. [Google Scholar] [CrossRef]
- Dzenis, Y.A. Spinning continuous fibers for nanotechnology. Science 2004, 304, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Wilfert, J.; Hagen, V.R.; Fiz, R.; Jansen, M.; Mathur, S. Electrospinning of preceramic polymers for the preparation of SiBNC felts and their modification with semiconductor nanowires. J. Mater. Chem. 2012, 22, 2099–2104. [Google Scholar] [CrossRef]
- Harrison, R.H.; Steele, J.A.M.; Chapman, R.; Gormley, A.J.; Chow, L.W.; Mahat, M.M.; Podhorska, L.; Palgrave, R.G.; Payne, D.J.; Hettiaratchy, S.P.; et al. Modular and versatile spatial functionalization of tissue engineering scaffolds through fiber-initiated controlled radical polymerization. Adv. Funct. Mater. 2015, 25, 5748–5757. [Google Scholar] [CrossRef] [PubMed]
- Bounioux, C.; Avrahami, R.; Vasilyev, G.; Patil, N.; Zussman, E.; Yerushalmi-Rozen, R. Single-step electrospinning of multi walled carbon nanotubes-Poly(3-octylthiophene) hybrid nano-fibers. Polymer 2016, 86, 15–21. [Google Scholar] [CrossRef]
- Savoji, H.; Rana, D.; Matsuura, T.; Tabe, S.; Feng, C. Development of plasma and/or chemically induced graft co-polymerized electrospun poly(vinylidene fluoride) membranes for solute separation. Sep. Purif. Technol. 2013, 108, 196–204. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Hua, D.; Xiong, R.; Zhao, J.; Rao, W.; Huang, S.; Zhan, X.; Chen, F.; Huang, C. Electrospun fibers for oil-water separation. RSC Adv. 2016, 6, 12868–12884. [Google Scholar] [CrossRef]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Roger, J.A. High performance piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef] [PubMed]
- Prince, J.A.; Rana, D.; Matsuura, T.; Ayyanar, N.; Shanmugasundaram, T.S.; Singh, G. Nanofiber based triple layer hydro-philic/-phobic membrane—A solution for pore wetting in membrane distillation. Sci. Rep. 2014, 4, 6949. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Camposeo, A.; Pisignano, D. Active polymer nanofibers for photonics, electronics, energy generation and micromechanics. Prog. Polym. Sci. 2015, 43, 48–95. [Google Scholar] [CrossRef]
- Clendenning, S.B.; Fournier-Bidoz, S.; Pietrangelo, A.; Yang, G.; Han, S.; Brodersen, P.M.; Yip, C.M.; Lu, Z.; Ozin, G.A.; Manners, I. Ordered 2D arrays of ferromagnetic Fe/Co nanoparticle rings from a highly metallized metallopolymer precursor. J. Mater. Chem. 2004, 14, 1686–1690. [Google Scholar] [CrossRef]
- Luzio, A.; Canesi, V.E.; Bertarelli, C.; Caironi, M. Electrospun polymer fibers for electronic applications. Materials 2014, 7, 906–947. [Google Scholar]
- Malatesta, F.; Carrara, G.; Colombini, M.P.; Giacomelli, A. Activity coefficients of electrolytes from the, E.M.F. of liquid membrane cells. II—Multicharged electrolyte solutions. J. Solut. Chem. 1993, 22, 733–749. [Google Scholar] [CrossRef]
- Kim, D.; Dhand, V.; Rhee, K.; Park, S.J. Study on the effect of silanization and improvement in the tensile behavior of graphene-chitosan-composite. Polymers 2015, 7, 527–551. [Google Scholar] [CrossRef]
- Bazarjani, M.S.; Mueller, M.M.; Kleebe, H.J.; Juettke, Y.; Voigt, I.; Yazdi, M.B.; Alff, L.; Riedel, R.; Gurlo, A. High-temperature stability and saturation magnetization of superparamagnetic nickel nanoparticles in microporous polysilazane-derived ceramics and their gas permeation properties. ACS Appl. Mater. Interfaces 2014, 6, 12270–12278. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Hapis, S.; Breitzke, H.; Xu, Y.; Fasel, C.; Kleebe, H.J.; Buntkowsky, G.; Riedel, R.; Inoescu, E. Single-source-precursor synthesis of hafnium-containing ultrahigh-temperature ceramic nanocomposites (UHTC-NCs). Inorg. Chem. 2014, 53, 10443–10455. [Google Scholar] [CrossRef] [PubMed]
- Kraabel, B.; Moses, D.; Heeger, A.J. Direct observation of the intersystem crossing in poly(3-octylthiophene). J. Chem. Phys. 1995, 103, 5102–5108. [Google Scholar] [CrossRef]
- Gao, S.; Fan, H.; Chen, Y.; Li, L.; Bando, Y.; Golberg, D. One stone, two birds: Gastrodia elata-derived heteroatom-doped carbon materials for efficient oxygen reduction electrocatalyst and as fluorescent decorative materials. Nano Energy 2013, 2, 1261–1270. [Google Scholar] [CrossRef]
- Ionescu, E.; Kleebe, H.J.; Riedel, R. Silicon-containing polymer-derived ceramic nanocomposites (PDC-NCs): Preparative approaches and properties. Chem. Soc. Rev. 2012, 41, 5032–5052. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Bernard, S.; Gervais, C.; Miele, P.; Singh, G. Facile synthesis and high rate capability of silicon carbonitride/boron nitride composite with a sheet-like morphology. J. Phys. Chem. C. 2015, 119, 2783–2791. [Google Scholar] [CrossRef]
- Bhandavat, R.; Gurpreet, S. Synthesis, characterization, and high temperature stability of Si(B)CN-coated carbon nanotubes using a boron-modified poly(ureamethylvinyl)silazane chemistry. J. Am. Ceram. Soc. 2012, 95, 1536–1543. [Google Scholar] [CrossRef]
- Gunes, I.S.; Cao, F.; Jana, S.C. Evaluation of nanoparticulate fillers for development of shape memory polyurethane nanocomposites. Polymer 2008, 49, 2223–2234. [Google Scholar] [CrossRef]
- Girardon, J.S.; Lermontov, A.S.; Gengembre, L.; Chernavskii, P.A.; Griboval-Constant, A.; Khodakov, A.Y. Effect of cobalt precursor and pretreatment conditions on the structure and catalytic performance of cobalt silica-supported Fischer–Tropsch catalysts. J. Catal. 2005, 230, 339–352. [Google Scholar] [CrossRef]
- Uraoka, Y.; Tadanaga, K.; Tatsumisago, M. Preparation and characterization of methylsilsesquioxane thin film containing tris(ethylenediamine)cobalt(III) chloride as a photobase generator. Chem. Mater. 2010, 22, 6125–6129. [Google Scholar] [CrossRef]
- Li, Y.; Kroke, E.; Riedel, R.; Fasel, C.; Gervais, C.; Babonneau, F. Thermal cross-linking and pyrolytic conversionof poly(ureamethylvinyl)silazanes tosilicon-based ceramics. Appl. Organomet. Chem. 2001, 820–832. [Google Scholar] [CrossRef]
- Ionescu, E.; Papendorf, B.; Kleebe, H.-J.; Breitzke, H.; Nonnenmacher, K.; Buntkowsky, G.; Riedel, R. Phase separation of a hafnium alkoxide-modified polysilazane upon polymer-to-ceramic transformation—A case study. J. Eur. Ceram. Soc. 2012, 32, 1873–1881. [Google Scholar] [CrossRef]
- Song, E.J.; Kang, J.; You, G.R.; Park, G.J.; Kim, Y.; Kim, S.-J.; Kim, C.; Harrison, R.G. A single molecule that acts as a fluorescence sensor for zinc and cadmium and a colorimetric sensor for cobalt. Dalton Trans. 2013, 42, 15514–15520. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Aoki, Y.; Takahara, S.; Wakasa, M. Fluorescent spherical particle formation from solid polysilanes by the aid of laser ablation and photochemical reaction. J. Photopolym. Sci. Technol. 2010, 23, 371–378. [Google Scholar] [CrossRef]
- Cho, K.S.; Park, N.M.; Kim, T.Y.; Kim, K.H.; Sung, G.Y.; Shin, J.H. High efficiency visible electroluminescence from silicon nanocrystals embedded in silicon nitride using a transparent doping layer. Appl. Phys. Lett. 2005, 86, 071909. [Google Scholar] [CrossRef]
- Pham, T.A.; Kim, D.-P.; Lim, T.-W.; Park, S.-H.; Yang, D.-Y.; Lee, K.-S. Three-dimensional SiCN ceramic microstructures via nano-stereolithography of inorganic polymer photoresists. Adv. Funct. Mater. 2006, 16, 1235–1241. [Google Scholar] [CrossRef]
- Shankar, R.; Sahoo, U.; Shahi, V. Synthesis and characterization of fluorescent polymer-metal nanocomposites comprising poly(silylene-co-silyne)s and silver nanoparticles. Macromolecules 2011, 44, 3240–3249. [Google Scholar] [CrossRef]
- Yang, B.; Tian, L.; Zhang, H.; Zhang, W.; Xu, H.; Xie, Z.; Lu, P.; Zhang, M.; Yu, J.; Lu, D.; et al. Nature of zinc(II)-induced ionochromic effect of bipyridine-containing conjugated polymers: An electrostatic interaction mechanism. J. Phys. Chem. B 2006, 110, 16846–16851. [Google Scholar] [CrossRef] [PubMed]







| Sample | Si (wt %) | N (wt %) | C (wt %) | O (wt %) | Co (wt %) | Sum formula |
|---|---|---|---|---|---|---|
| PSN | 43.61 | 25.74 | 27.55 | 3.10 | 0 | Si1.56N1.84C2.29O0.19 |
| Polycobaltsilazane | 33.35 | 27.75 | 28.67 | 2.87 | 7.36 | Si1.19N1.98C2.39Co0.125O0.17 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Jia, D.; Yang, Z.; Duan, X.; Chen, Q.; Zhou, Y. Synthesis of Novel Cobalt-Containing Polysilazane Nanofibers with Fluorescence by Electrospinning. Polymers 2016, 8, 350. https://doi.org/10.3390/polym8100350
Zhang Q, Jia D, Yang Z, Duan X, Chen Q, Zhou Y. Synthesis of Novel Cobalt-Containing Polysilazane Nanofibers with Fluorescence by Electrospinning. Polymers. 2016; 8(10):350. https://doi.org/10.3390/polym8100350
Chicago/Turabian StyleZhang, Qian, Dechang Jia, Zhihua Yang, Xiaoming Duan, Qingqing Chen, and Yu Zhou. 2016. "Synthesis of Novel Cobalt-Containing Polysilazane Nanofibers with Fluorescence by Electrospinning" Polymers 8, no. 10: 350. https://doi.org/10.3390/polym8100350