Atomistic Modelling of Confined Polypropylene Chains between Ferric Oxide Substrates at Melt Temperature
Abstract
:1. Introduction
2. Models and Methods
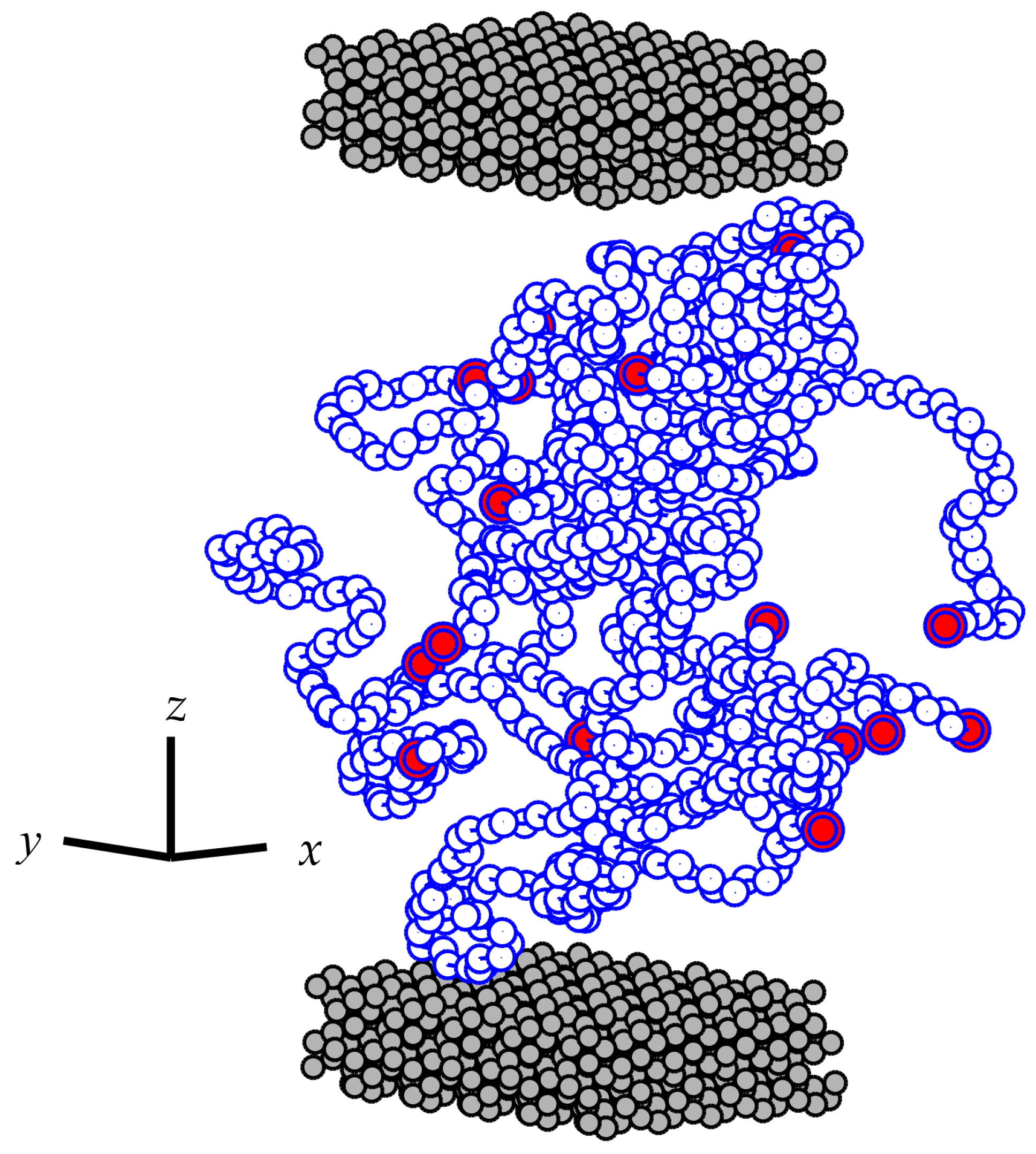
2.1. Molecular Model
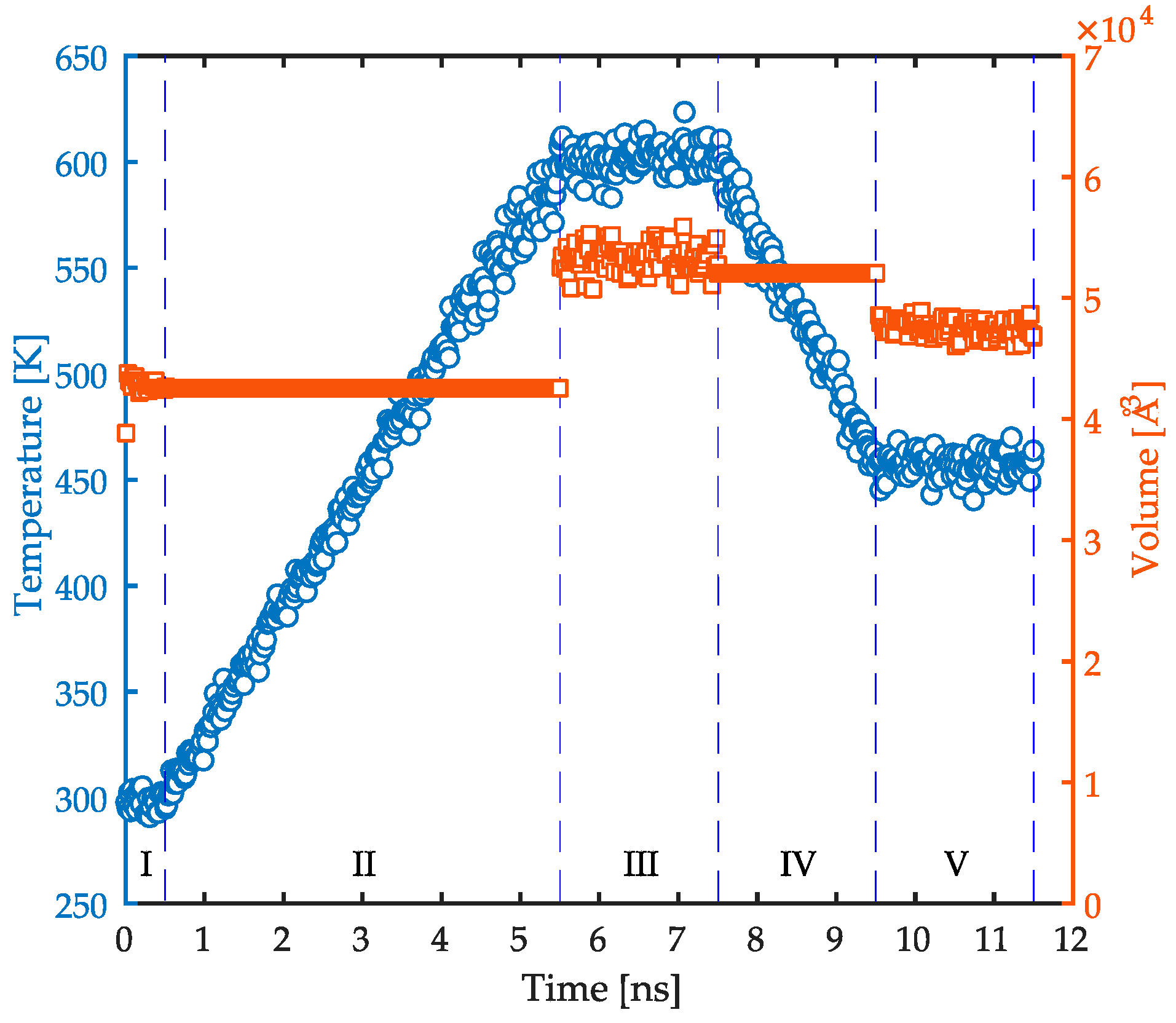
2.2. Simulations Methodology
3. Results and Discussion
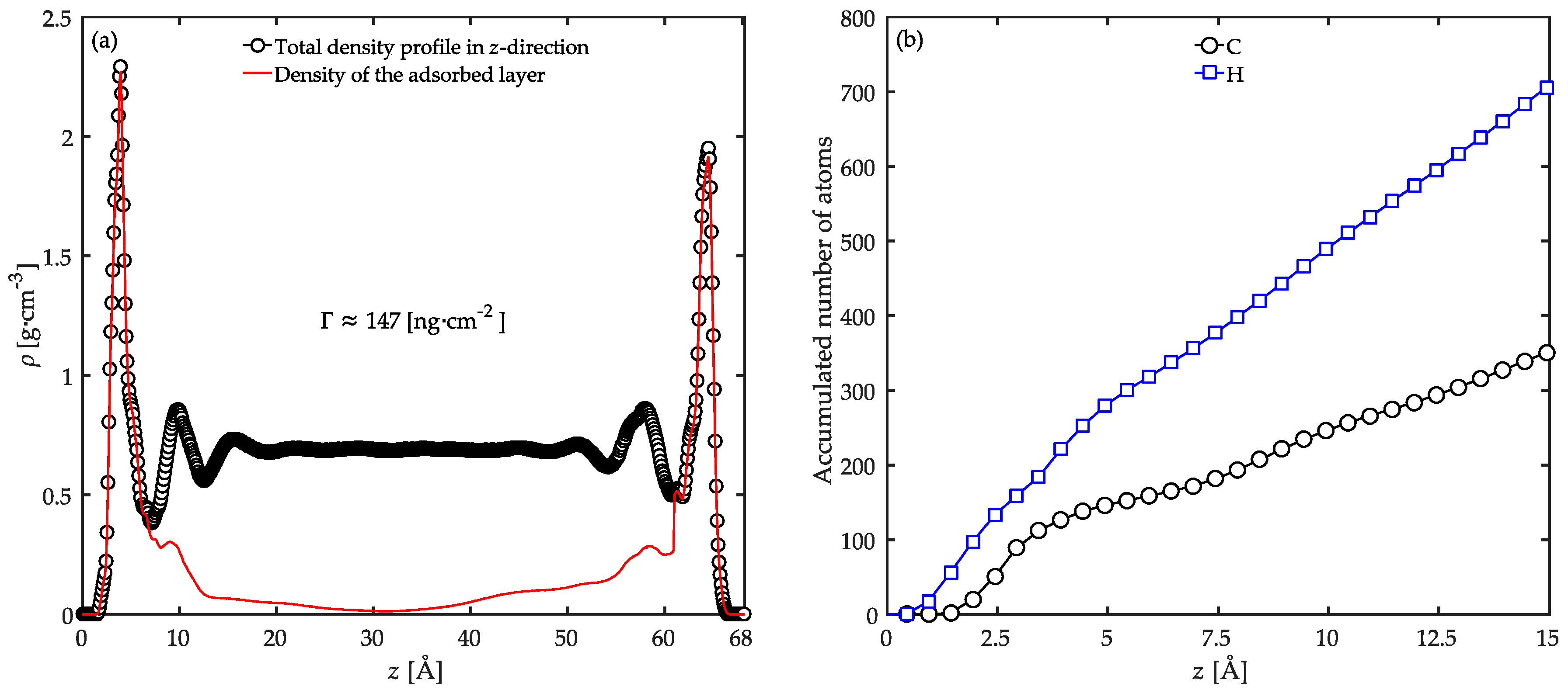
3.1. Local Density Profile
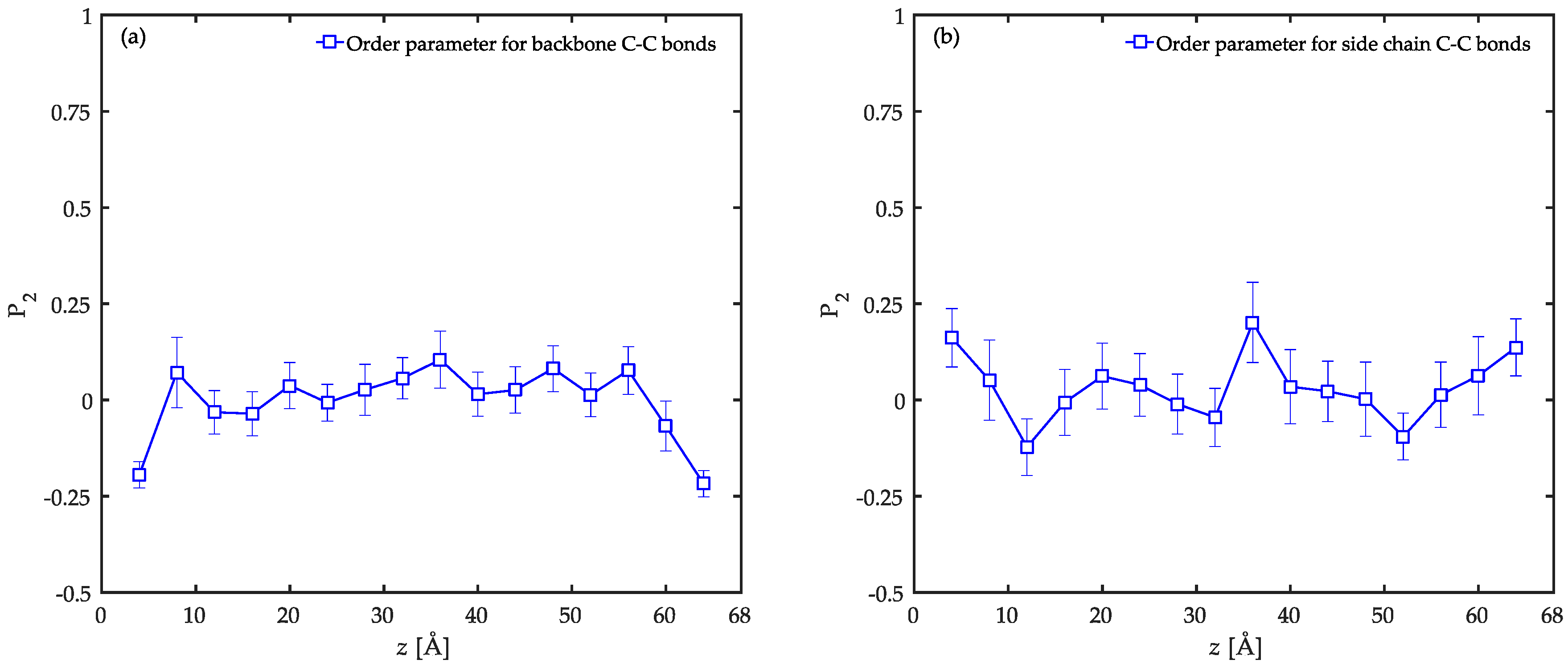
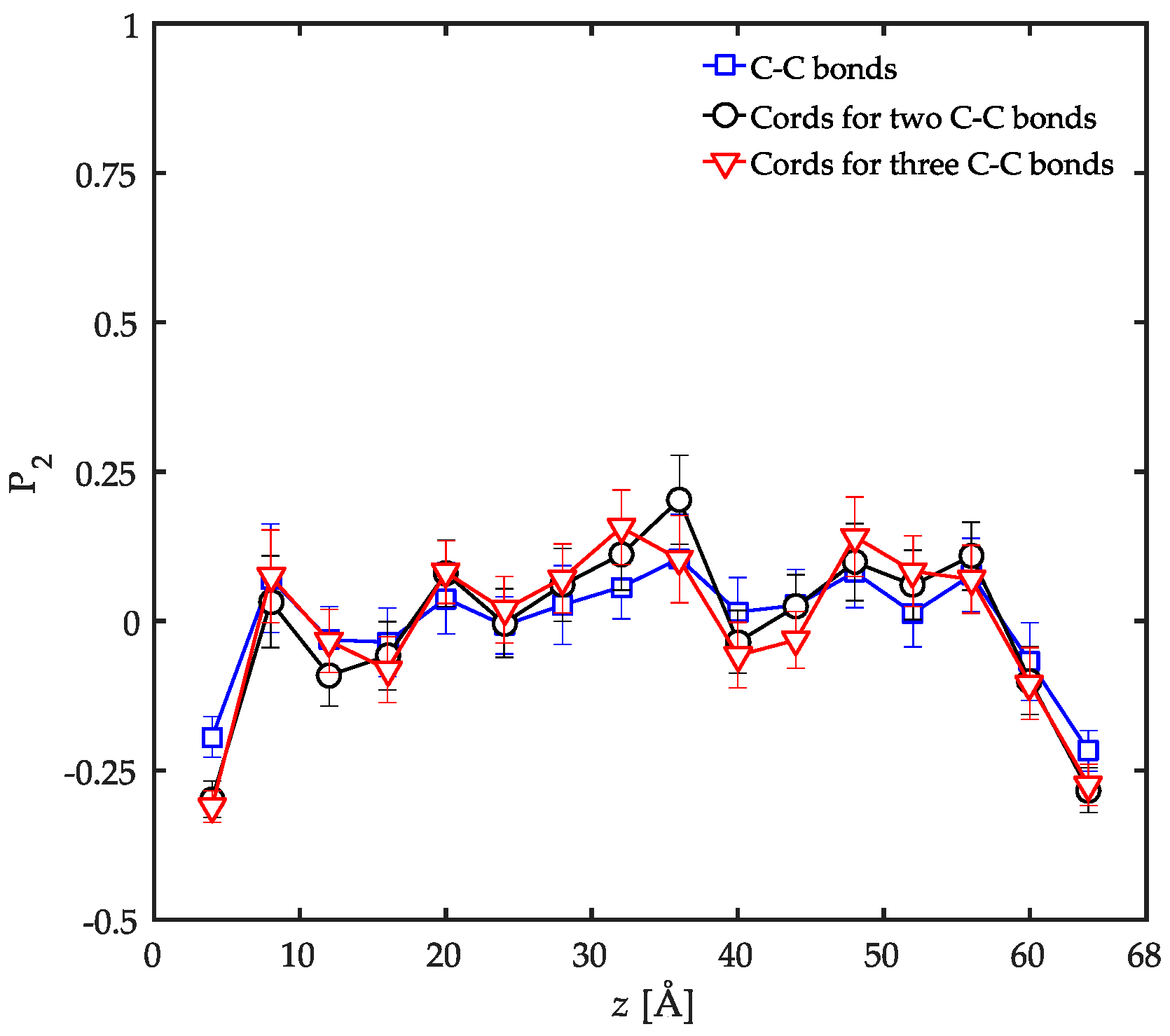
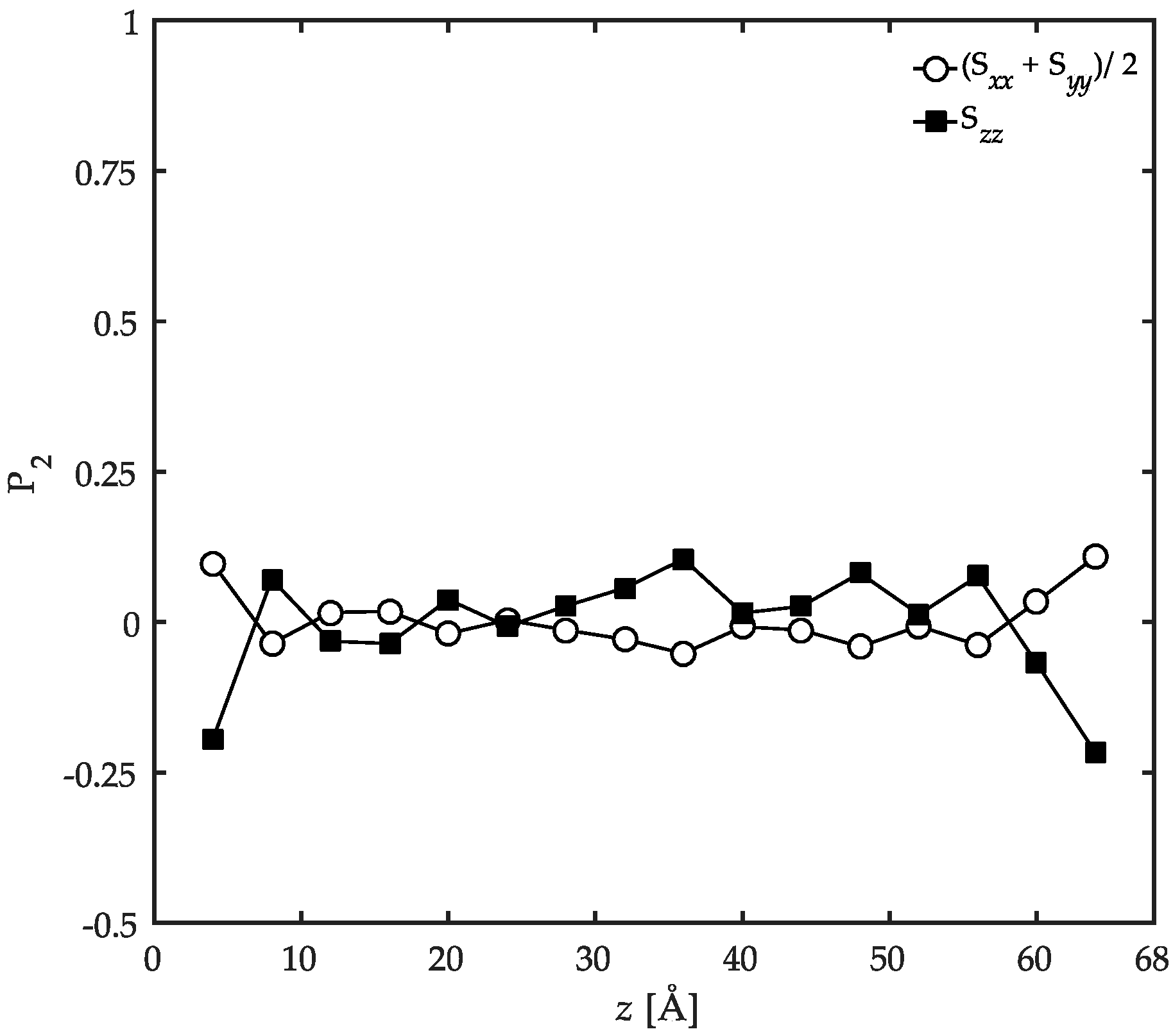
3.2. Conformational Characteristics of the Confined Film
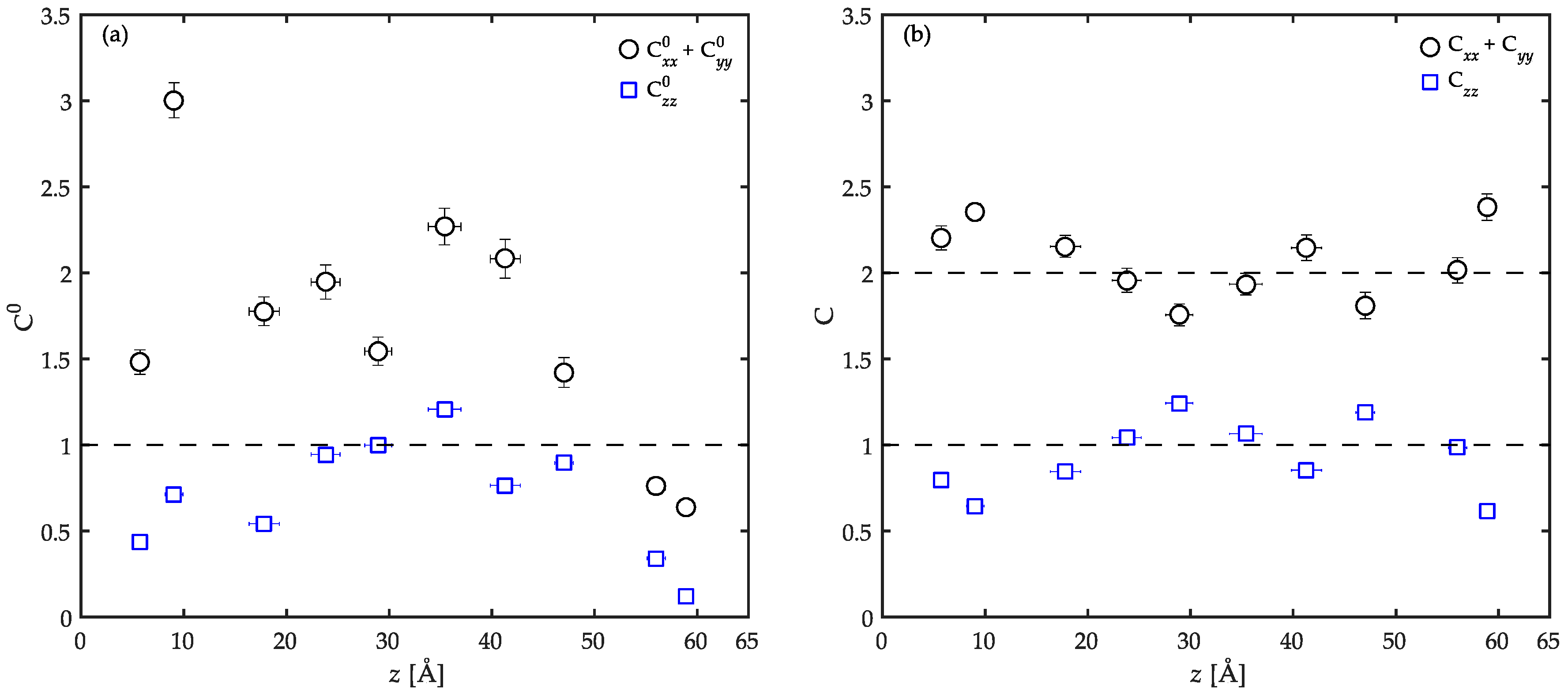
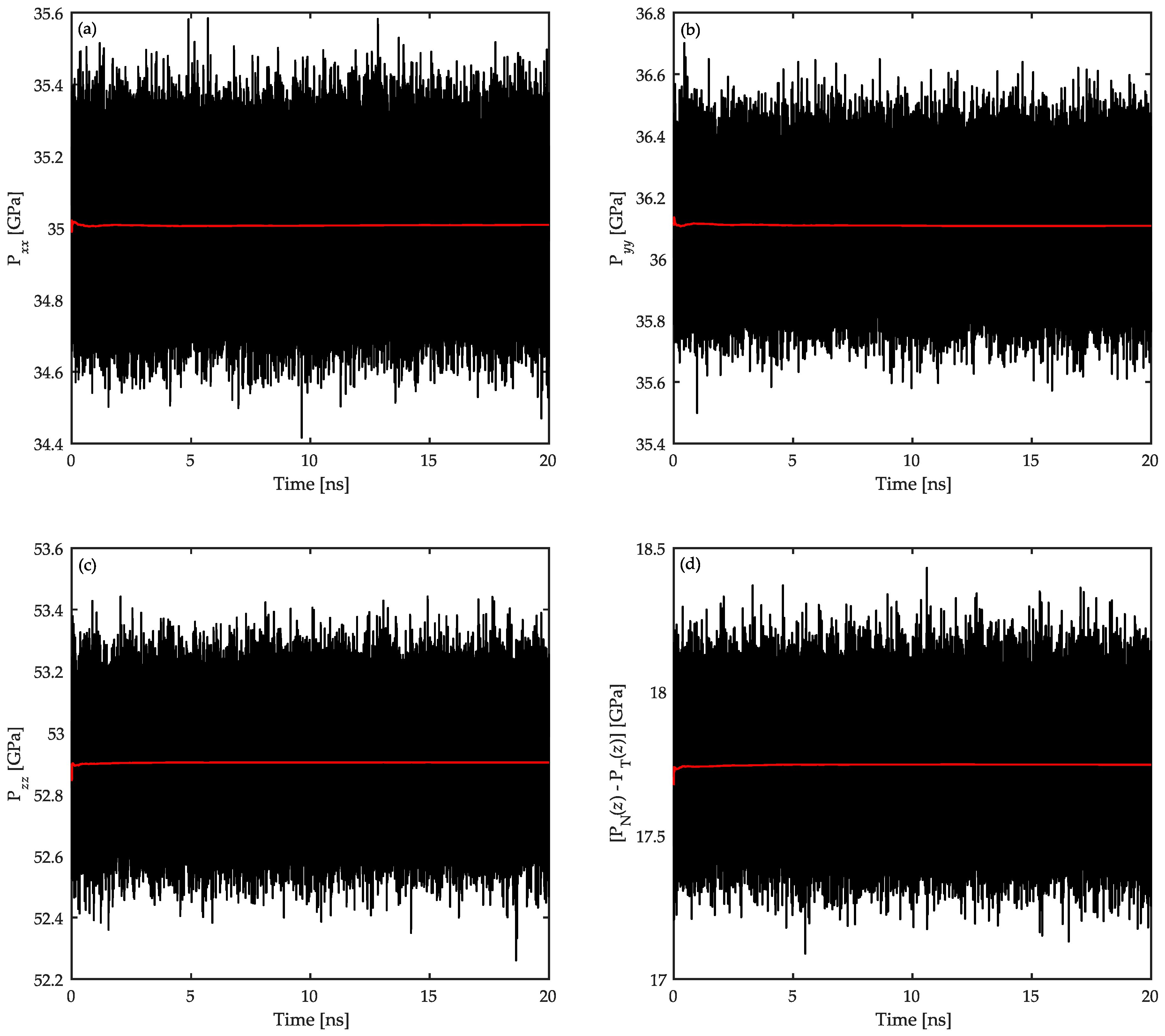
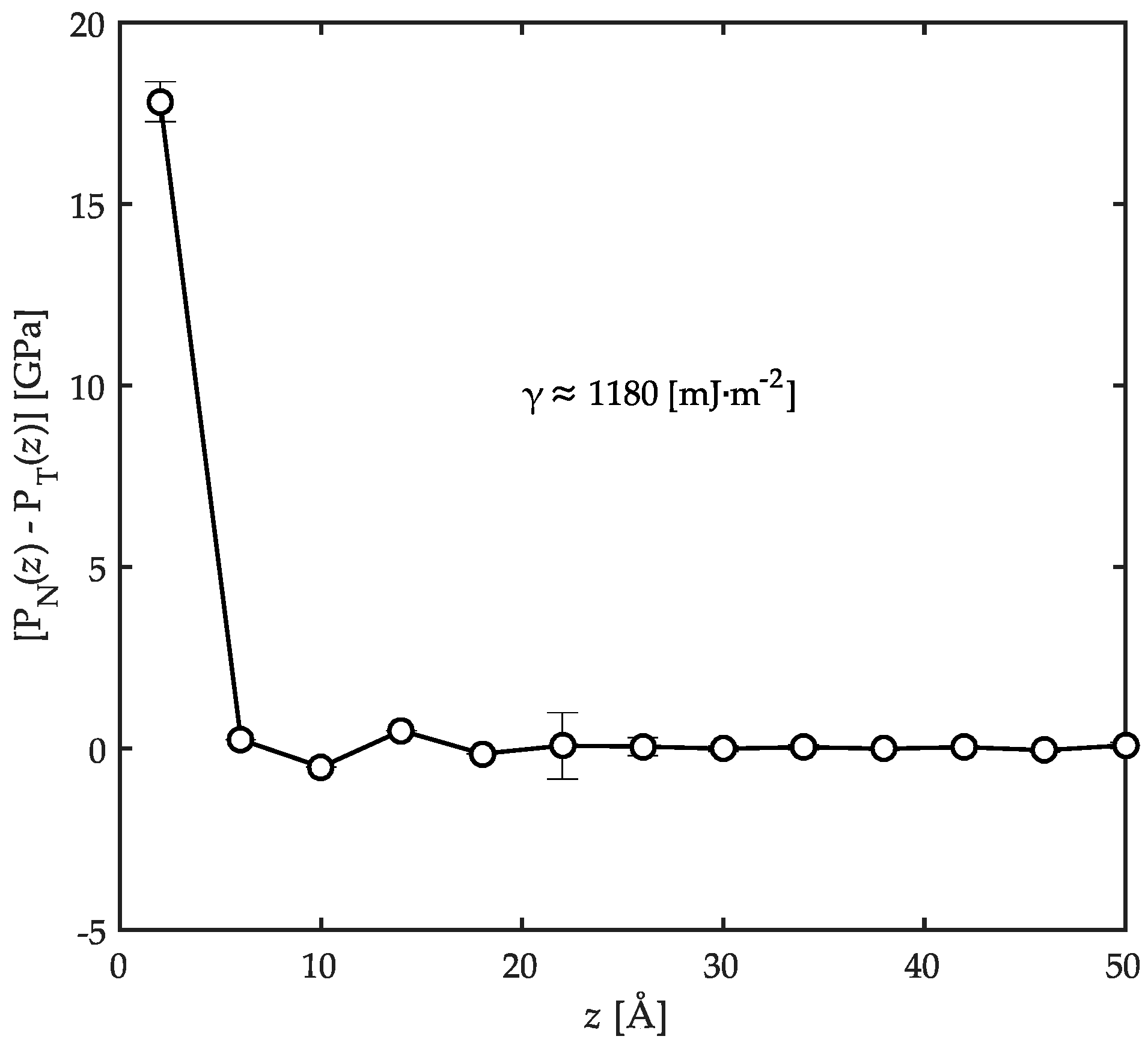
3.3. Surface Tension Evaluation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zamarion, V.M.; Khan, M.K.; Schlesinger, M.; Bsoul, A.; Walus, K.; Hamad, W.Y.; MacLachlan, M.J. Photonic metal-polymer resin nanocomposites with chiral nematic order. Chem. Commun. 2016, 52, 7810–7813. [Google Scholar] [CrossRef] [PubMed]
- Konkol, M.; Kondracka, M.; Kowalik, P.; Próchniak, W.; Michalska, K.; Schwedt, A.; Merkens, C.; Englert, U. Decomposition of the mixed-metal coordination polymer—A preparation route of the active Ag/Yb2O3 catalyst for the deN2O process. Appl. Catal. B 2016, 190, 85–92. [Google Scholar] [CrossRef]
- Schwartzkopf, M.; Buffet, A.; Korstgens, V.; Metwalli, E.; Schlage, K.; Benecke, G.; Perlich, J.; Rawolle, M.; Rothkirch, A.; Heidmann, B.; et al. From atoms to layers: In situ gold cluster growth kinetics during sputter deposition. Nanoscale 2013, 5, 5053–5062. [Google Scholar] [CrossRef] [PubMed]
- Minnai, C.; Milani, P. Metal-polymer nanocomposite with stable plasmonic tuning under cyclic strain conditions. Appl. Phys. Lett. 2015, 107, 073106. [Google Scholar] [CrossRef]
- Taki, K.; Nakamura, S.; Takayama, T.; Nemoto, A.; Ito, H. Direct joining of a laser-ablated metal surface and polymers by precise injection molding. Microsyst. Technol. 2016, 22, 31–38. [Google Scholar] [CrossRef]
- Gonzalez-Gutierrez, J.; Duretek, I.; Kukla, C.; Poljšak, A.; Bek, M.; Emri, I.; Holzer, C. Models to predict the viscosity of metal injection molding feedstock materials as function of their formulation. Metals 2016, 6, 129. [Google Scholar] [CrossRef]
- Gwon, T.M.; Kim, J.H.; Choi, G.J.; Kim, S.J. Mechanical interlocking to improve metal–polymer adhesion in polymer-based neural electrodes and its impact on device reliability. J. Mater. Sci. 2016, 51, 6897–6912. [Google Scholar] [CrossRef]
- Agarwala, M.K.; Jamalabad, V.R.; Langrana, N.A.; Safari, A.; Whalen, P.J.; Danforth, S.C. Structural quality of parts processed by fused deposition. Rapid Prototyp. J. 1996, 2, 4–19. [Google Scholar] [CrossRef]
- Johnston, K.; Harmandaris, V. Hierarchical simulations of hybrid polymer-solid materials. Soft Matter 2013, 9, 6696–6710. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Gutierrez, J.; Beulke, G.; Emri, I. Powder injection molding of metal and ceramic parts. In Some Critical Issues for Injection; Wang, J., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Rissanou, A.; Power, A.; Harmandaris, V. Structural and dynamical properties of polyethylene/graphene nanocomposites through molecular dynamics simulations. Polymers 2015, 7, 390–417. [Google Scholar] [CrossRef]
- Daoulas, K.; Harmandaris, V.A.; Mavrantzas, V.G. Detailed atomistic simulation of a polymer melt/solid interface: Structure, density, and conformation of a thin film of polyethylene melt adsorbed on graphite. Macromolecules 2005, 38, 5780–5795. [Google Scholar] [CrossRef]
- Gooneie, A.; Schuschnigg, S.; Holzer, C. Orientation of anisometric layered silicate particles in uncompatibilized and compatibilized polymer melts under shear flow. Macromol. Theory Simul. 2016, 25, 85–98. [Google Scholar] [CrossRef]
- Gooneie, A.; Schuschnigg, S.; Holzer, C. Coupled orientation and stretching of chains in mesoscale models of polydisperse linear polymers in startup of steady shear flow simulations. Macromol. Theory Simul. 2016, 25, 170–186. [Google Scholar] [CrossRef]
- Alejandre, J.; Tildesley, D.J.; Chapela, G.A. Molecular dynamics simulation of the orthobaric densities and surface tension of water. J. Chem. Phys. 1995, 102, 4574–4583. [Google Scholar] [CrossRef]
- Shi, B.; Sinha, S.; Dhir, V.K. Molecular dynamics simulation of the density and surface tension of water by particle-particle particle-mesh method. J. Chem. Phys. 2006, 124. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.W.; Campos, T.; Cividanes, L.S.; Simonetti, E.; Thim, G.P. Adsorbed water on iron surface by molecular dynamics. Appl. Surf. Sci. 2016, 362, 70–78. [Google Scholar] [CrossRef]
- Negreiros, F.R.; Pedroza, L.S.; Dalpian, G.M. Effect of charges on the interaction of a water molecule with the Fe2O3(0001) surface. J. Phys. Chem. C 2016, 120, 11918–11925. [Google Scholar] [CrossRef]
- Mendonça, A.; Malfreyt, P.; Pádua, A. Interactions and ordering of ionic liquids at a metal surface. J. Chem. Theory Comput. 2012, 8, 3348–3355. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, C.; Canongia Lopes, J.N.; Da Piedade, M. All-atom force field for molecular dynamics simulations on organotransition metal solids and liquids. Application to M(CO)n (M = Cr, Fe, Ni, Mo, Ru, or W) compounds. J. Phys. Chem. A 2013, 117, 11107–11113. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.F.; Theodorou, D.N. Molecular dynamics simulation of a glassy polymer surface. Macromolecules 1991, 24, 6283–6294. [Google Scholar] [CrossRef]
- Matsuda, T.; Smith, G.D.; Winkler, R.G.; Yoon, D.Y. Stochastic dynamics simulations of n-alkane melts confined between solid surfaces: Influence of surface properties and comparison with scheutjens-fleer theory. Macromolecules 1995, 28, 165–173. [Google Scholar] [CrossRef]
- Harmandaris, V.A.; Daoulas, K.; Mavrantzas, V.G. Molecular dynamics simulation of a polymer melt/solid interface: Local dynamics and chain mobility in a thin film of polyethylene melt adsorbed on graphite. Macromolecules 2005, 38, 5796–5809. [Google Scholar] [CrossRef]
- Larsen, G.; Cheng, Z.Q.; Barriere, T.; Liu, B.S.; Gelin, J.-C. Particle-polymer interactions analysis for metal powder injection molding feedstock: A molecular-dynamics simulation with dissipative particle dynamics using ESPResSo. AIP Conf. Proc. 2011, 1353, 109–114. [Google Scholar]
- Kacar, G.; Peters, E.; de With, G. Structure of a thermoset polymer near an alumina substrate as studied by dissipative particle dynamics. J. Phys. Chem. C 2013, 117, 19038–19047. [Google Scholar] [CrossRef]
- Anastassiou, A.; Mavrantzas, V.G. Molecular structure and work of adhesion of poly(n-butyl acrylate) and poly(n-butyl acrylate-co-acrylic acid) on α-quartz, α-ferric oxide, and α-ferrite from detailed molecular dynamics simulations. Macromolecules 2015, 48, 8262–8284. [Google Scholar] [CrossRef]
- Liu, H.; Espe, M.; Modarelli, D.A.; Arias, E.; Moggio, I.; Ziolo, R.F.; Heinz, H. Interaction of substituted poly(phenyleneethynylene)s with ligand-stabilized CdS nanoparticles. J. Mater. Chem. A 2014, 2, 8705–8711. [Google Scholar] [CrossRef]
- Ta, T.D.; Tieu, A.K.; Zhu, H.; Kosasih, B. Adsorption of normal-alkanes on Fe(110), FeO(110), and Fe2O3(0001): Influence of iron oxide surfaces. J. Phys. Chem. C 2015, 119, 12999–13010. [Google Scholar] [CrossRef]
- Abraham, J.W.; Strunskus, T.; Faupel, F.; Bonitz, M. Molecular dynamics simulation of gold cluster growth during sputter deposition. J. Appl. Phys. 2016, 119, 185301. [Google Scholar] [CrossRef]
- Heinz, H. Adsorption of biomolecules and polymers on silicates, glasses, and oxides: Mechanisms, predictions, and opportunities by molecular simulation. Curr. Opin. Chem. Eng. 2016, 11, 34–41. [Google Scholar] [CrossRef]
- Kerber, S.J.; Tverberg, J. Stainless steel surface analysis. Adv. Mater. Process. 2000, 33–36. [Google Scholar]
- Heinz, H.; Lin, T.-J.; Kishore Mishra, R.; Emami, F.S. Thermodynamically consistent force fields for the assembly of inorganic, organic, and biological nanostructures: The INTERFACE force field. Langmuir 2013, 29, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Heinz, H.; Vaia, R.A.; Farmer, B.L.; Naik, R.R. Accurate simulation of surfaces and interfaces of face-centered cubic metals using 12-6 and 9-6 lennard-jones potentials. J. Phys. Chem. C 2008, 112, 17281–17290. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrence and Uses, 2nd ed.; Wiley-VCH Verlag: Berlin, Germany, 2003. [Google Scholar]
- Martin, L.; Bilek, M.M.; Weiss, A.S.; Kuyucak, S. Force fields for simulating the interaction of surfaces with biological molecules. Interface Focus 2016, 6, 20150045. [Google Scholar] [CrossRef] [PubMed]
- Maple, J.R.; Hwang, M.-J.; Stockfisch, T.P.; Dinur, U.; Waldman, M.; Ewig, C.S.; Hagler, A.T. Derivation of class II force fields. I. Methodology and quantum force field for the alkyl functional group and alkane molecules. J. Comput. Chem. 1994, 15, 162–182. [Google Scholar] [CrossRef]
- MacKerell, A.D.; Bashford, D.; Bellott, M.; Dunbrack, R.L.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef] [PubMed]
- Sun, H. Compass: An ab initio force-field optimized for condensed-phase applications—Overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Heinz, H.; Koerner, H.; Anderson, K.L.; Vaia, R.A.; Farmer, B.L. Force field for mica-type silicates and dynamics of octadecylammonium chains grafted to montmorillonite. Chem. Mater. 2005, 17, 5658–5669. [Google Scholar] [CrossRef]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity—A rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Batista, E.R.; Friesner, R.A. A self-consistent charge-embedding methodology for ab initio quantum chemical cluster modeling of ionic solids and surfaces: Application to the (001) surface of hematite (α-Fe2O3). J. Phys. Chem. B 2002, 106, 8136–8141. [Google Scholar] [CrossRef]
- LAMMPS. Available online: http://lammps.sandia.gov/ (accessed on 5 August 2016).
- Theodorou, D.N.; Suter, U.W. Detailed molecular structure of a vinyl polymer glass. Macromolecules 1985, 18, 1467–1478. [Google Scholar] [CrossRef]
- Theodorou, D.N.; Suter, U.W. Atomistic modeling of mechanical properties of polymeric glasses. Macromolecules 1986, 19, 139–154. [Google Scholar] [CrossRef]
- Lange, J.; De Souza, F.G., Jr.; Nele, M.; Tavares, F.W.; Segtovich, I.; Da Silva, G.; Pinto, J.C. Molecular dynamic simulation of oxaliplatin diffusion in poly(lactic acid-co-glycolic acid). Part A: Parameterization and validation of the force-field CVFF. Macromol. Theory Simul. 2016, 25, 45–62. [Google Scholar] [CrossRef]
- Goicochea, A.G. Adsorption and disjoining pressure isotherms of confined polymers using dissipative particle dynamics. Langmuir 2007, 23, 11656–11663. [Google Scholar] [CrossRef] [PubMed]
- Gooneie, A.; Schuschnigg, S.; Holzer, C. Dissipative particle dynamics models of orientation of weakly-interacting anisometric silicate particles in polymer melts under shear flow. Macromol. Theory Simul. 2016, 25, 287–302. [Google Scholar] [CrossRef]
- Mansfield, K.F.; Theodorou, D.N. Atomistic simulation of a glassy polymer surface. Macromolecules 1990, 23, 4430–4445. [Google Scholar] [CrossRef]
- Semino, R.; Ramsahye, N.A.; Ghoufi, A.; Maurin, G. Microscopic model of the metal-organic framework/polymer interface: A first step toward understanding the compatibility in mixed matrix membranes. ACS Appl. Mater. Interfaces 2016, 8, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Nosé, S. Constant temperature molecular dynamics methods. Prog. Theor. Phys. Suppl. 1991, 103, 1–46. [Google Scholar] [CrossRef]
- Hoover, W.G. Constant-pressure equations of motion. Phys. Rev. A 1986, 34, 2499–2500. [Google Scholar] [CrossRef]
- Verlet, L. Computer “Experiments” on classical fluids. I. Thermodynamical properties of lennard-jones molecules. Phys. Rev. 1967, 159, 98–103. [Google Scholar] [CrossRef]
- Verlet, L. Computer “experiments” on classical fluids. II. Equilibrium correlation functions. Phys. Rev. 1968, 165, 201–214. [Google Scholar] [CrossRef]
- Mansfield, K.F.; Theodorou, D.N. Atomistic simulation of a glassy polymer/graphite interface. Macromolecules 1991, 24, 4295–4309. [Google Scholar] [CrossRef]
- Hansen, F.Y.; Herwig, K.W.; Matthies, B.; Taub, H. Intramolecular and lattice melting in n-alkane monolayers. Phys. Rev. Lett. 1999, 83, 2362–2365. [Google Scholar] [CrossRef]
- Scheutjens, J.M.H.M.; Fleer, G.J. Statistical theory of the adsorption of interacting chain molecules. 1. Partition function, segment density distribution, and adsorption isotherms. J. Phys. Chem. 1979, 83, 1619–1635. [Google Scholar] [CrossRef]
- Scheutjens, J.M.H.M.; Fleer, G.J. Statistical theory of the adsorption of interacting chain molecules. 2. Train, loop, and tail size distribution. J. Phys. Chem. 1980, 84, 178–190. [Google Scholar] [CrossRef]
- Gooneie, A.; Nazockdast, H.; Shahsavan, F. Effect of selective localization of carbon nanotubes in PA6 dispersed phase of PP/PA6 blends on the morphology evolution with time, part 1: Droplet deformation under simple shear flows. Polym. Eng. Sci. 2015, 55, 1504–1519. [Google Scholar] [CrossRef]
- Gooneie, A.; Nazockdast, H.; Shahsavan, F. Effect of selective localization of carbon nanotubes in PA6 dispersed phase of PP/PA6 blends on the morphology evolution with time, part 2: Relaxation of deformed droplets after cessation of flow. Polym. Eng. Sci. 2016, 56, 51–60. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gooneie, A.; Gonzalez-Gutierrez, J.; Holzer, C. Atomistic Modelling of Confined Polypropylene Chains between Ferric Oxide Substrates at Melt Temperature. Polymers 2016, 8, 361. https://doi.org/10.3390/polym8100361
Gooneie A, Gonzalez-Gutierrez J, Holzer C. Atomistic Modelling of Confined Polypropylene Chains between Ferric Oxide Substrates at Melt Temperature. Polymers. 2016; 8(10):361. https://doi.org/10.3390/polym8100361
Chicago/Turabian StyleGooneie, Ali, Joamin Gonzalez-Gutierrez, and Clemens Holzer. 2016. "Atomistic Modelling of Confined Polypropylene Chains between Ferric Oxide Substrates at Melt Temperature" Polymers 8, no. 10: 361. https://doi.org/10.3390/polym8100361






