Effect of Microwave Treatment of Graphite on the Electrical Conductivity and Electrochemical Properties of Polyaniline/Graphene Oxide Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microwave Treatment of Graphite
2.3. Synthesis of M-GO
2.4. Synthesis of PANI/M-GO Composites
2.5. Electrical Conductivity Measurements
2.6. Electrochemical Measurements
2.7. Characterizations and Analysis
3. Results and Discussion
3.1. Effect of the Microwave Treatment of Graphite and Feeding Ratio on the Electrical Conductivity of PANI/GO Composites
3.2. Effect of the Microwave Treatment of Graphite on the CV Curves of the PANI/GO Composites
3.3. Effect of the Microwave Treatment of Graphite on the Cycle Stability of the PANI/GO Composites
3.4. Effect of the Microwave Treatment of Graphite on the Microstructure of PANI/GO Composites
3.4.1. TEM Observations
3.4.2. SEM Observations
3.4.3. FT-IR Spectra
3.4.4. XRD Analysis
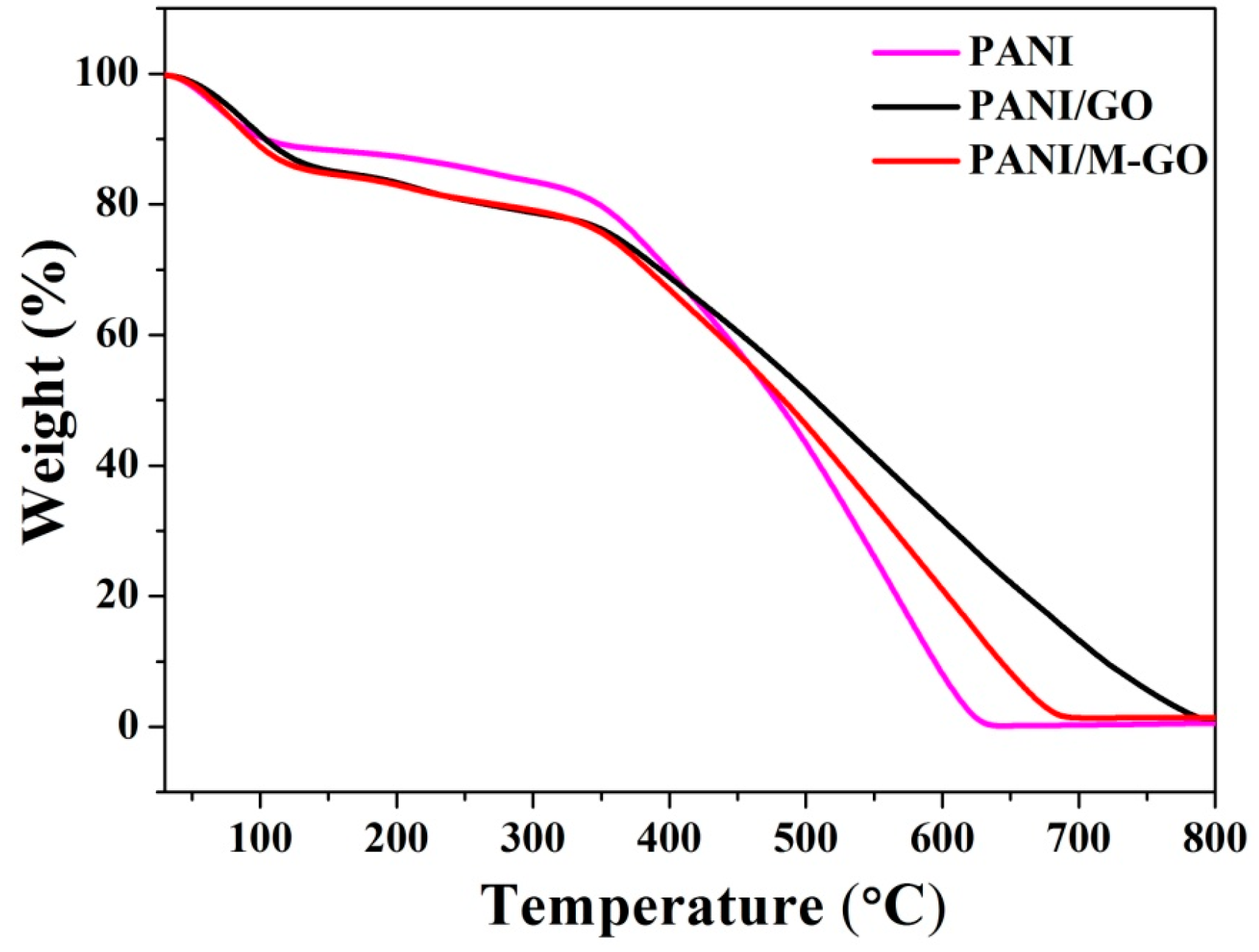
3.4.5. TG Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, Y.; Mosseler, J.A.; He, Z.; Ni, Y. Imparting cellulosic paper of high conductivity by surface coating of dispersed graphite. Ind. Eng. Chem. Res. 2014, 53, 10119–10124. [Google Scholar] [CrossRef]
- Kodama, M.; Yamashita, J.; Soneda, Y.; Hatori, H.; Nishimura, S.; Kamegawa, K. Structural characterization and electric double layer capacitance of template carbons. Mater. Sci. Eng. B 2004, 108, 156–161. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Wang, G.; Tong, Y.; Li, Y. Flexible solid-state supercapacitors: Design, fabrication and applications. Energy Environ. Sci. 2014, 7, 2160–2181. [Google Scholar] [CrossRef]
- Huang, B.; Kang, G.; Ni, Y. Electrically conductive fibre composites prepared from polypyrrole-engineered pulp fibres. Chem. Eng. 2005, 83, 896–903. [Google Scholar] [CrossRef]
- Mao, H.; Wu, X.; Qian, X.; An, X. Conductivity and flame retardancy of polyaniline-deposited functional cellulosic paper doped with organic sulfonic acids. Cellulose 2013, 21, 697–704. [Google Scholar] [CrossRef]
- Lei, Y.; Qian, X.; Shen, J.; An, X. Integrated reductive/adsorptive detoxification of Cr(VI)-contaminated water by polypyrrole/cellulose fibercomposite. Ind. Eng. Chem. Res. 2012, 51, 10408–10415. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Z.; Mao, L.; Hua, L.; Jiao, S. A high-performance supercapacitor based on a polythiophene/multiwalled carbon nanotube composite by electropolymerization in an ionic liquid microemulsion. Mater. Chem. A 2014, 2, 17024–17030. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-S.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C.; Modi, G.; Naidoo, D.; Kumar, P. A polyvinyl alcohol-polyaniline based electro-conductive hydrogel for controlled stimuli-actuable release of indomethacin. Polymers 2011, 3, 150–172. [Google Scholar] [CrossRef]
- Shen, X.; Tang, Y.; Zhou, D.; Zhang, J.; Guo, D.; Friederichs, G. Improving the electtroconductivity and mechanical properties of cellulosic paper with multi-wallled carbon nanotube/polyaniline nanocomposites. J. Bioresour. Bioprod. 2016, 1, 48–54. [Google Scholar]
- Du, Y.; Shen, S.Z.; Yang, W.; Donelson, R.; Cai, K.; Casey, P.S. Simultaneous increase in conductivity and seebeck coefficient in a polyaniline/graphene nanosheets thermoelectric nanocomposite. Synth. Met. 2012, 161, 2688–2692. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Tang, Y.; He, Z.; Mosseler, J.A.; Ni, Y. Production of highly elect-conductive celulosic paper via surface coting of carbon nanotube/graphene oxide nanocomposites using nanocrystalline cellulosic as a binder. Cellulose 2014, 21, 4569–4581. [Google Scholar] [CrossRef]
- Chieng, B.; Ibrahim, N.; Yunus, W.; Hussein, M. Poly(lactic acid)/Poly(ethylene glycol) polymer nanocomposites: Effects of graphene nanoplatelets. Polymers 2013, 6, 93–104. [Google Scholar] [CrossRef]
- Song, K.; Zhang, Y.; Meng, J.; Green, E.C.; Tajaddod, N.; Li, H.; Minus, M.L. Structural polymer-based carbon nanotube composite fibers: Understanding the processing–structure–performance relationship. Materials 2013, 6, 2543–2577. [Google Scholar] [CrossRef]
- Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang, X. Graphene oxide doped polyaniline for supercapacitors. Electrochem. Commun. 2009, 11, 1158–1161. [Google Scholar] [CrossRef]
- Bissessur, R.; Liu, P.K.Y.; White, W.; Scully, S.F. Encapsulation of polyanilines into graphite oxide. Langmuir 2006, 22, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang, X. Effect of graphene oxide on the properties of its composite with polyaniline. ACS Appl. Mater. Interfaces 2010, 2, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Venkanna, M.; Sreeramulu, V.; Sundaresan, S.; Caroline, C.; Vinod, R.D.; Chakraborty, A.K. Optical, electrical, and electrochemical properties of graphene based water soluble polyaniline composites. J. Appl. Polym. Sci. 2015, 42766, 1–9. [Google Scholar]
- Gedela, V.R.; Srikanth, V.V.S.S. Electrochemically active polyaniline nanofibers (PANi NFs) coated graphene nanosheets/PANi NFs composite coated on different flexible substrates. Synth. Met. 2014, 193, 71–76. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.S.; Bozoklu, G.; Cai, W.; Rodney, S.R. Graphene oxide papers modified by divalent ions—Enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Song, M. Recent advance in functionalized graphene/polymer nanocomposites. J. Mater. Chem. 2014, 20, 7906–7915. [Google Scholar] [CrossRef]
- Ke, R.; Zhang, X.; Wang, L.; Zhang, C.; Zhang, S.; Mao, C.; Niu, H.; Song, J.; Jin, B.; Tian, Y. Electrochemiluminescence sensor based on graphene oxide/polypyrrole/CdSe nanocomposites. J. Alloy. Compd. 2015, 622, 1027–1032. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zu, S.Z.; Han, B.H.; Wei, Z. Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets withsynergistic effect for energy storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.A.; Choi, H.J.; Shin, Y.R.; Chang, D.W.; Dai, L.M.; Baek, J.B. Polyaniline-grafted reduced graphene oxide for efficient electrochemical supercapacitors. ACS Nano 2012, 6, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, X.; Wang, X.; Meng, L.; Wang, H.; Peng, G.; Wang, X.; Mu, X. Effective saccharification of lignocellulosic biomass over hydrolysis residue derived solid acid under microwave irradiation. Green Chem. 2012, 14, 2162–2167. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Q.; Yan, X.; Li, H.; Zhang, P.; Wang, L.; Zhou, T.; Li, Y.; Ding, L. Rapid preparation of expanded graphite by microwave irradiation for the extractionof triazine herbicides in milk samples. Food Chem. 2016, 263, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Fan, Z.; Luo, G.; Zheng, C.; Xie, D. A rapid and efficient method to prepare exfoliated graphite by microwave irradiation. Carbon 2008, 47, 337–339. [Google Scholar] [CrossRef]
- Kwon, O.; Choi, S.; Park, K.; Kwon, Y. The preparation of exfoliated graphite by using microwave. J. Ind. Eng. Chem. 2003, 9, 743–747. [Google Scholar]
- Wu, X.; Xu, H.; Xu, P.; Shen, Y.; Lu, L.; Shi, J.; Fu, J.; Zhao, H. Microwave-treated graphite felt as the positive electrode for all vanadium redox flow battery. J. Power Sources 2014, 263, 104–109. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Huang, J.; Kaner, R.B. A general chemica route to polyaniline nanofibers. J. Am. Chem. Soc. 2004, 126, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, G.; Yu, Z.; Qi, J. The effect of graphite oxide on the thermoelectric properties of polyaniline. Carbon 2012, 50, 3064–3073. [Google Scholar] [CrossRef]
- Doi, M.; Edwards, S.D. Dynamics of concentrated polymer systems. Part 1. Brownian motion in the equilibrium state. J. Chem. Soc. Faraday Trans. 1978, 74, 1789–1801. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Velamakanni, A.; Piner, R.D.; Ruoff, R.S. Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 2010, 48, 2118–2122. [Google Scholar] [CrossRef]
- Aboutalebi, S.H.; Chidembo, A.T.; Salari, M.; Konstantinov, K.; Wexler, D.; Liu, H.K.; Dou, S.X. Comparison of GO, GO/MWCNTs composite and MWCNTs as potential electrode materials for supercapacitors. Energy Environ. Sci. 2011, 4, 1855–1865. [Google Scholar] [CrossRef]
- Cong, H.-P.; Ren, X.-C.; Wang, P.; Yu, S.-H. Flexible graphene–polyaniline composite paper for high-performance supercapacitor. Energy Environ. Sci. 2013, 6, 1185–1191. [Google Scholar] [CrossRef]
- Xu, G.; Wang, N.; Wei, J.; Lv, L.; Zhang, J.; Chen, Z.; Xu, Q. Preparation of graphene oxide/polyaniline nanocomposite with assistance of supercritical carbon dioxide for supercapacitor electrodes. Ind. Eng. Chem. Res. 2012, 51, 14390–14398. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, M.; Qu, H.; Zhang, X.; Wei, H.; Luo, Z.; Colorado, H.A.; Wei, S.; Guo, Z. Interfacial polymerized polyaniline/graphite oxide nanocomposites toward electrochemical energy. Polymer 2012, 53, 5953–5964. [Google Scholar] [CrossRef]
- Wang, M.; Tang, Q.; Xu, P.; He, B.; Lin, L.; Chen, H. Counter electrodes from polyaniline–graphene complex/graphene oxide multilayers for dye–sensitized solar cells. Electrochim. Acta 2014, 137, 175–182. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, S.; Zhang, M.; Jia, M. Preparation of sulfonated graphene–polyaniline nanofiber composites by oil/water interfacial polymerization and their application for supercapacitors. Synth. Met. 2013, 168, 58–64. [Google Scholar] [CrossRef]
- Jin, Y.; Jia, M. Preparation and electrochemical capacitive performance of polyaniline nanofiber-graphene oxide hybrids by oil–water interfacial polymerization. Synth. Met. 2014, 189, 47–52. [Google Scholar] [CrossRef]
- Wei, H.; Zhu, J.; Wu, S.; Wei, S.; Guo, Z. Electrochromic polyaniline/graphite oxide nanocomposites with endured electrochemical energy storage. Polymer 2013, 54, 1820–1831. [Google Scholar] [CrossRef]
- He, W.; Zhang, W.; Li, Y.; Jing, X. A high concentration graphene dispersion stabilized by polyaniline nanofibers. Synth. Met. 2012, 162, 1107–1113. [Google Scholar] [CrossRef]
- Li, J.; Xie, H.; Li, Y.; Liu, J.; Li, Z. Electrochemical properties of graphene nanosheets/polyaniline nanofibers composites as electrode for supercapacitors. J. Power Sources 2011, 196, 10775–10781. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Li, Z.; Yang, S.; Wang, J. Fabrication of free-standing graphene/polyaniline nanofibers composite paper via electrostatic adsorption for electrochemical supercapacitors. New J. Chem. 2011, 35, 369–374. [Google Scholar] [CrossRef]









© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Hu, X.; Liu, D.; Guo, D.; Zhang, J. Effect of Microwave Treatment of Graphite on the Electrical Conductivity and Electrochemical Properties of Polyaniline/Graphene Oxide Composites. Polymers 2016, 8, 399. https://doi.org/10.3390/polym8110399
Tang Y, Hu X, Liu D, Guo D, Zhang J. Effect of Microwave Treatment of Graphite on the Electrical Conductivity and Electrochemical Properties of Polyaniline/Graphene Oxide Composites. Polymers. 2016; 8(11):399. https://doi.org/10.3390/polym8110399
Chicago/Turabian StyleTang, Yanjun, Xiulan Hu, Dongdong Liu, Daliang Guo, and Junhua Zhang. 2016. "Effect of Microwave Treatment of Graphite on the Electrical Conductivity and Electrochemical Properties of Polyaniline/Graphene Oxide Composites" Polymers 8, no. 11: 399. https://doi.org/10.3390/polym8110399




