Miscibility, Morphology and Crystallization Behavior of Poly(Butylene Succinate-co-Butylene Adipate)/Poly(Vinyl Phenol)/Poly(l-Lactic Acid) Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Fourier Transform Infrared Spectroscopy (FTIR) Characterization
2.4. Differential Scanning Calorimetry (DSC)
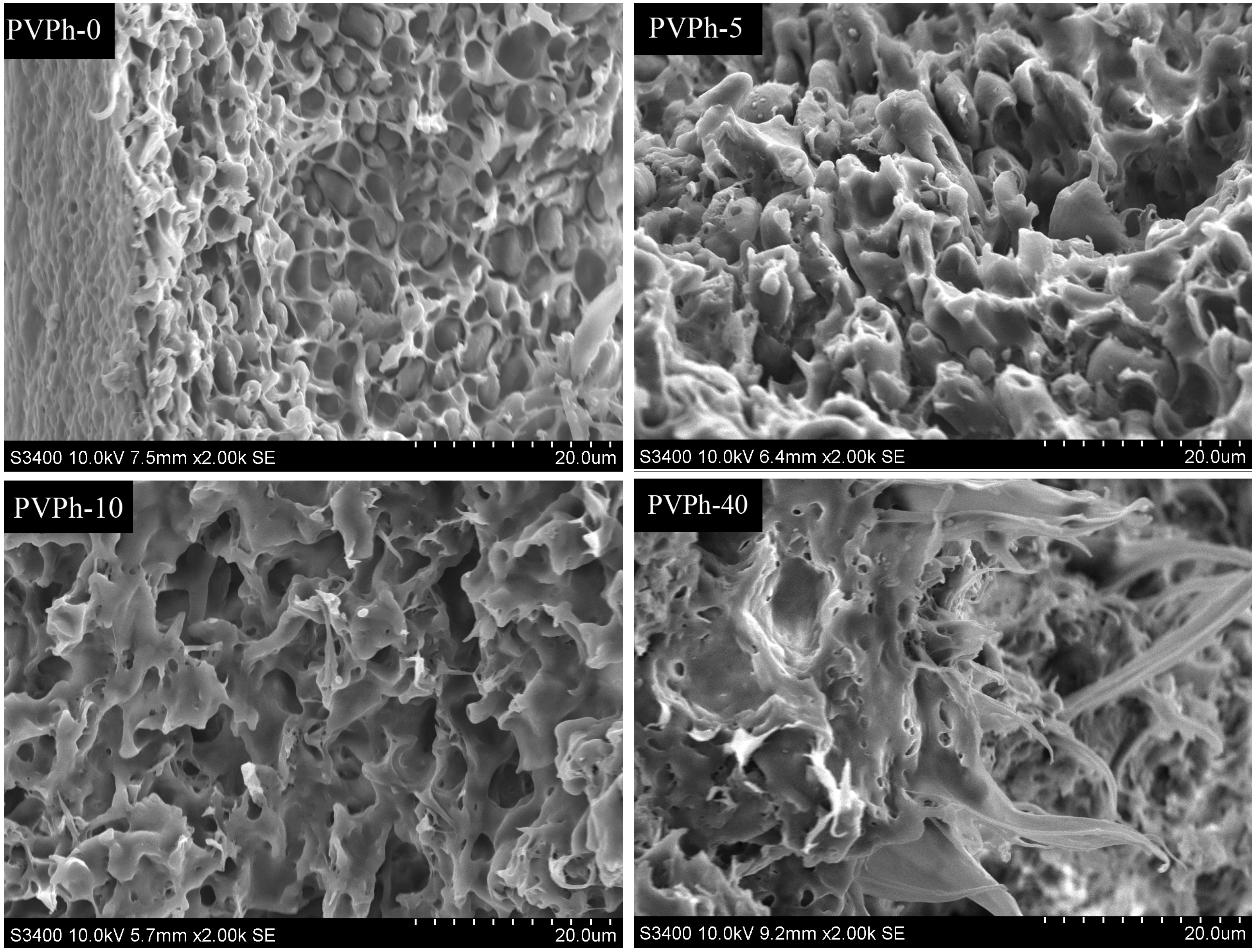
2.5. Scanning Electron Microscope (SEM)
2.6. Wide Angle X-ray Diffraction (WAXD)
3. Results
3.1. FTIR Analysis
3.2. Miscibility of PBSA/PVPh/PLLA Blends
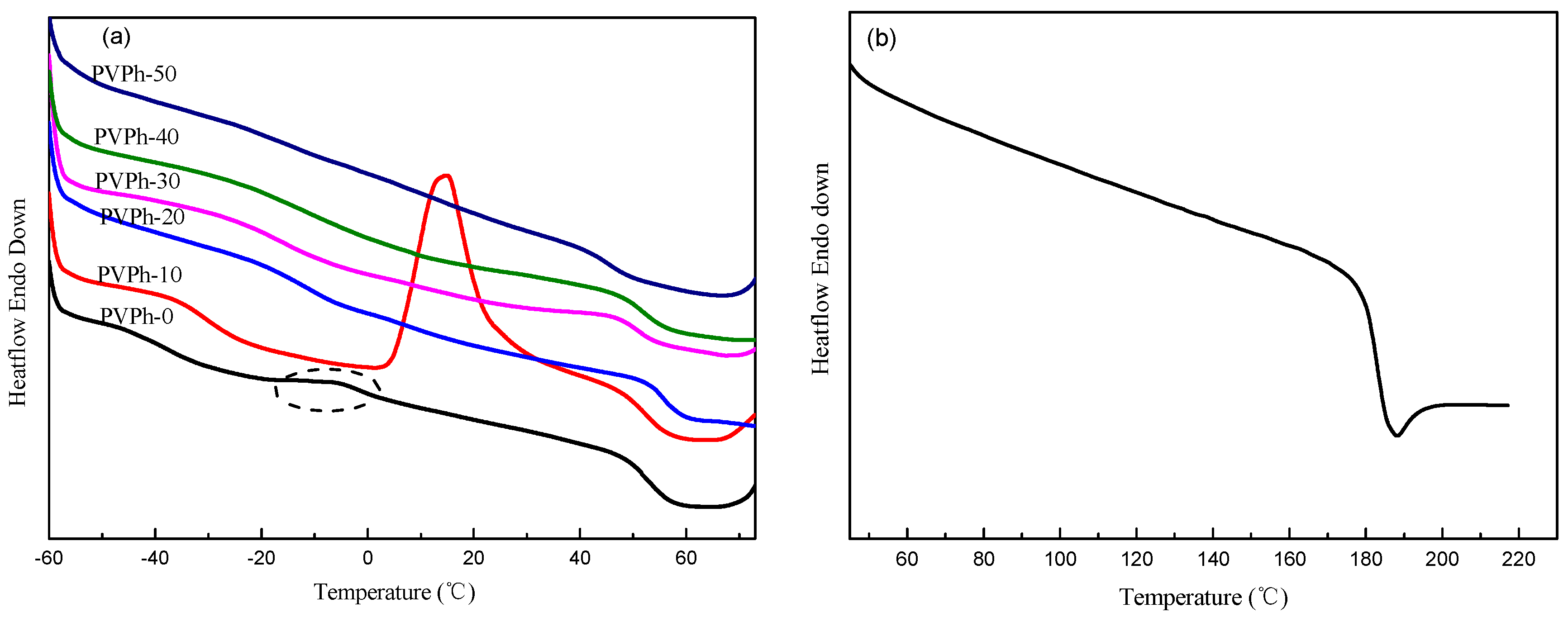
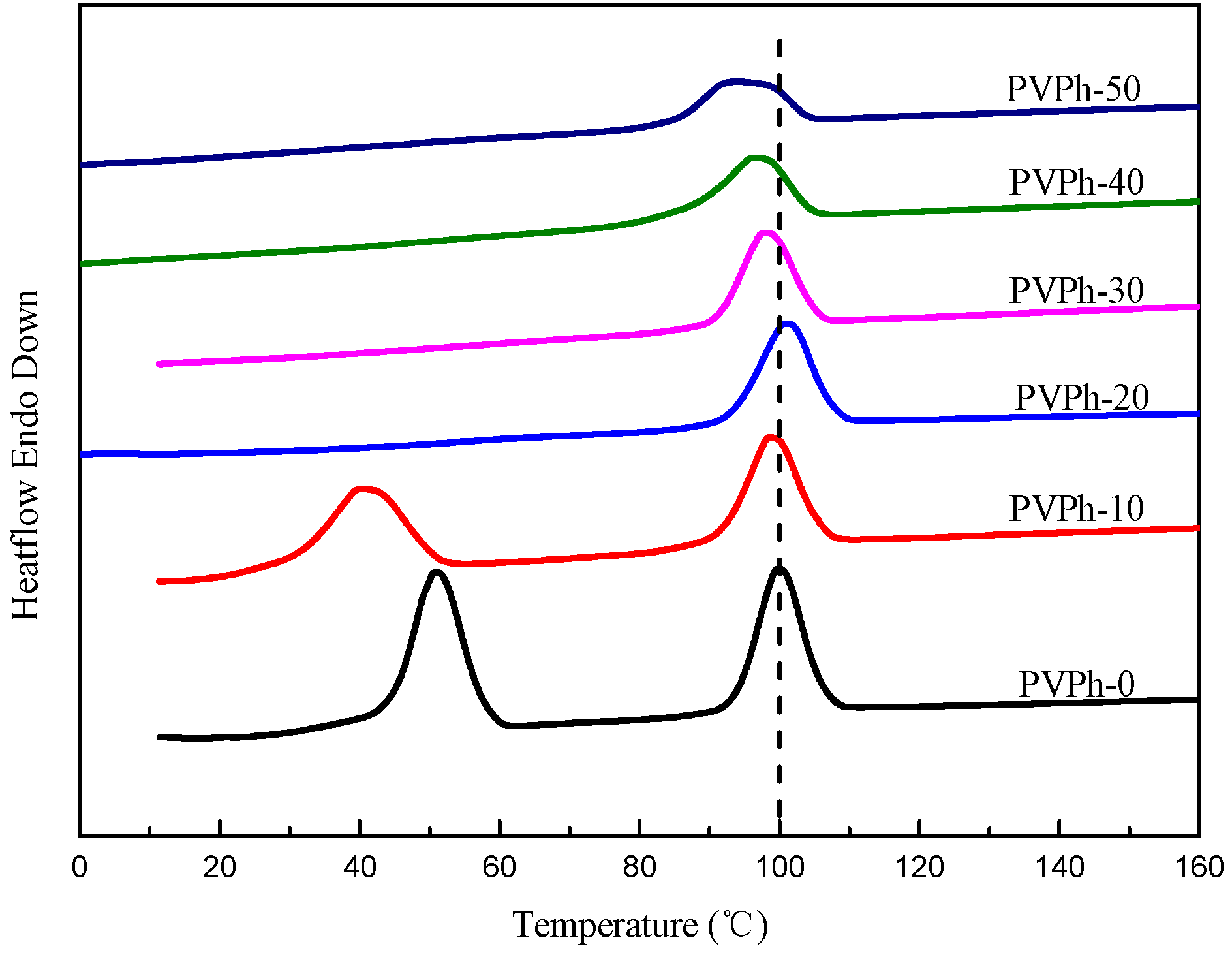
3.3. Non-Isothermal Crystallization and Melting Behavior of PBSA/PVPh/PLLA Blends
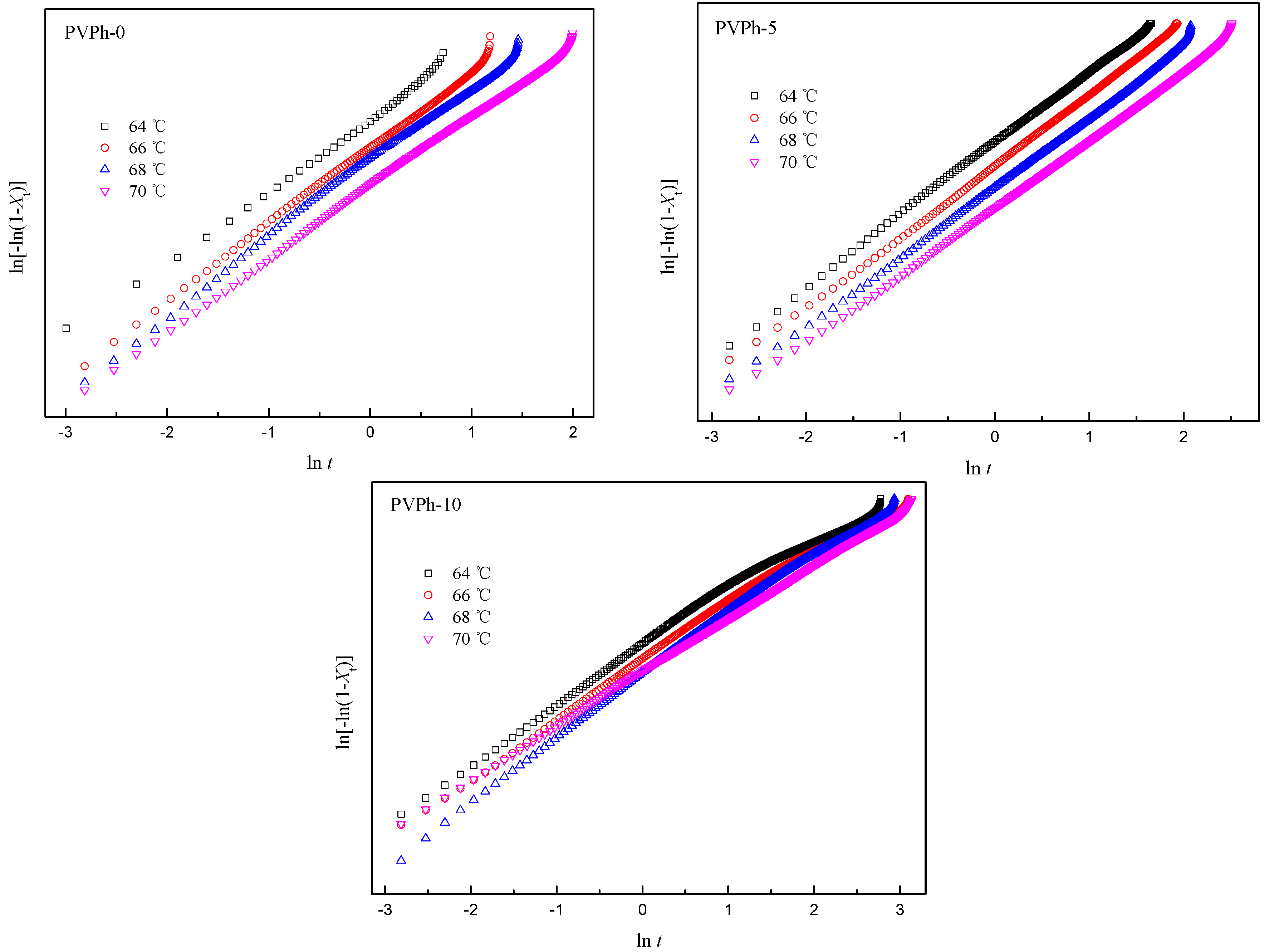
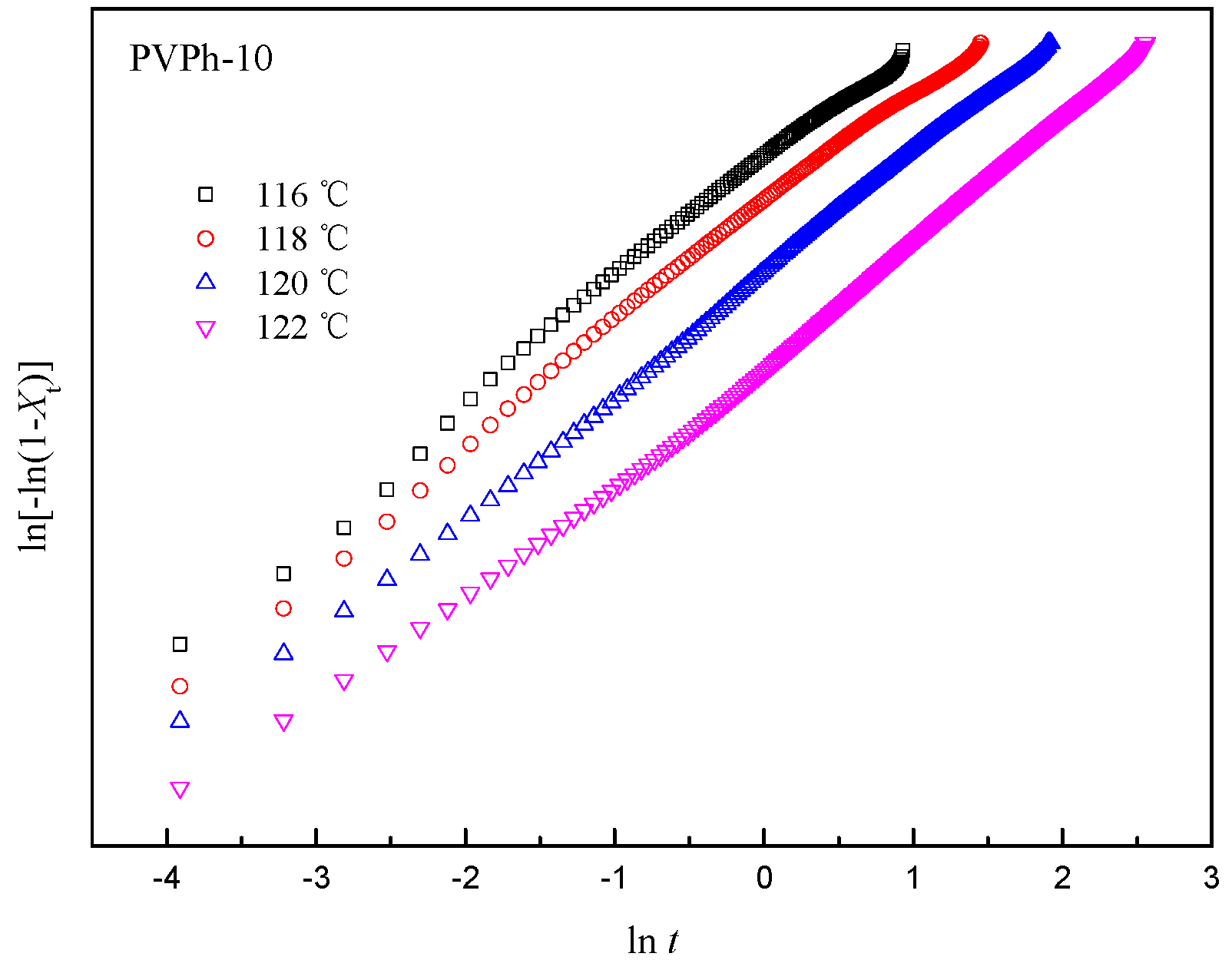
3.4. AvramiAnalyses for Isothermal Crystallization of PBSA/PVPh/PLLA Blends
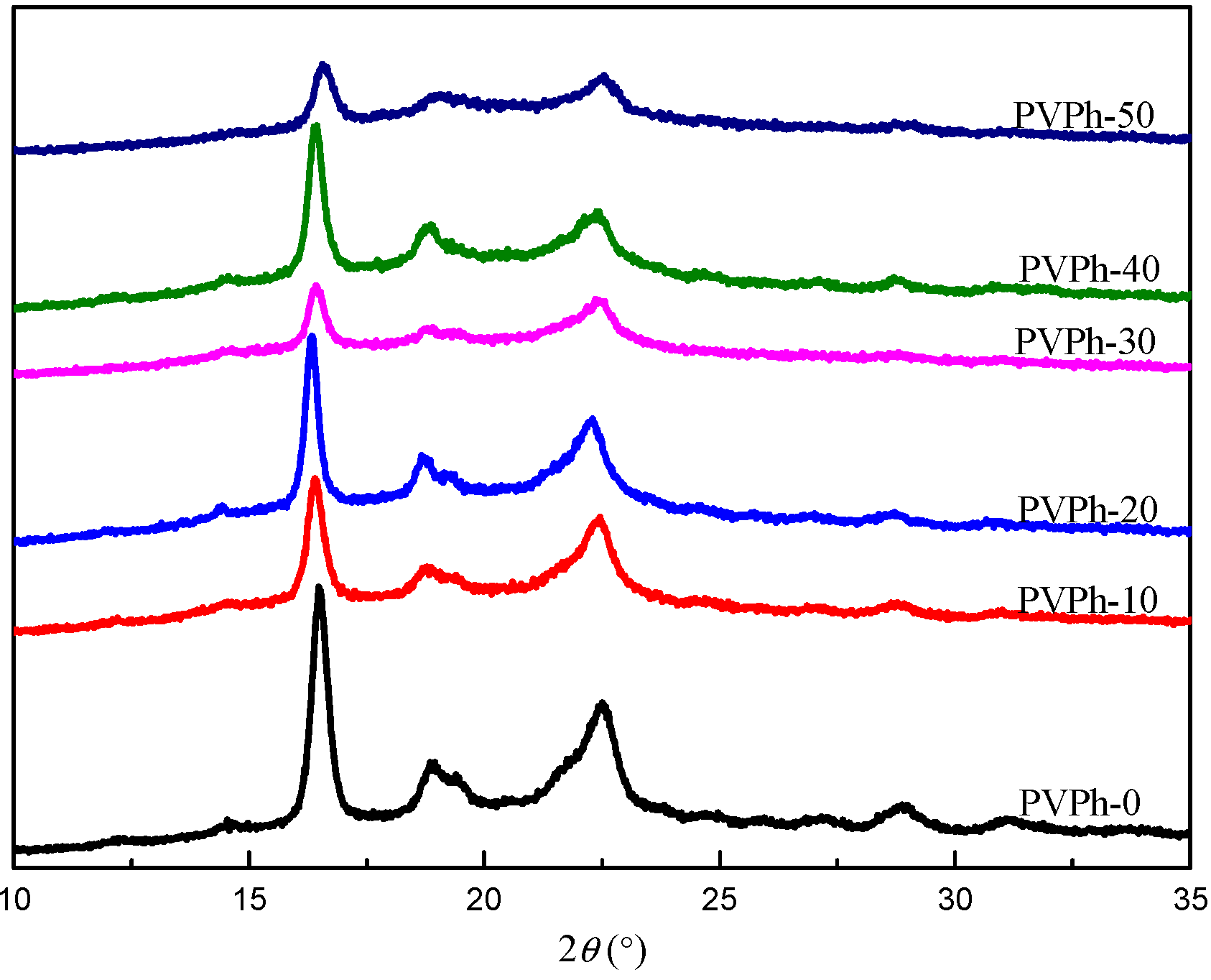
3.5. WAXD Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Shibata, M.; Inoue, Y.; Miyoshi, M. Mechanical properties, morphology, and crystallization behavior of blends of poly(l-lactide) with poly(butylene succinate-co-l-lactate) and poly(butylene succinate). Polymer 2006, 47, 3557–3564. [Google Scholar] [CrossRef]
- Cai, Y.; Yan, S.; Yin, J.; Fan, Y.; Chen, X. Crystallization behavior of biodegradable poly(l-lactic acid) filled with a powerful nucleating agent: N,N′-bis(benzoyl) suberic acid dihydrazide. J. Appl. Polym. Sci. 2011, 121, 1408–1416. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Improving mechanical performance of injection molded PLA by controlling crystallinity. J. Appl. Polym. Sci. 2008, 107, 2246–2255. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, W.; Deng, H.; Zhang, Q.; Fu, Q. Control of crystal morphology in poly(l-lactide) by adding nucleating agent. Macromolecules 2011, 44, 1233–1237. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Huang, Z.; Weng, Y. Heat resistance, crystallization behavior, and mechanical properties of polylactide/nucleating agent composites. Mater. Des. 2015, 66, 7–15. [Google Scholar] [CrossRef]
- Broz, M.E.; VanderHart, D.L.; Washburn, N.R. Structure and mechanical properties of poly(d,l-lactic acid)/poly(ε-caprolactone) blends. Biomaterials 2003, 24, 4181–4190. [Google Scholar] [CrossRef]
- Chen, C.; Chueh, J.; Tseng, H.; Huang, H.; Lee, S. Preparation and characterization of biodegradable PLA polymeric blends. Biomaterials 2003, 24, 1167–1173. [Google Scholar] [CrossRef]
- Lu, J.; Qiu, Z.; Yang, W. Fully biodegradable blends of poly(l-lactide) and poly(ethylene succinate): Miscibility, crystallization, and mechanical properties. Polymer 2007, 48, 4196–4204. [Google Scholar] [CrossRef]
- Yokohara, T.; Yamaguchi, M. Structure and properties for biomass-based polyester blends of PLA and PBS. Eur. Polym. J. 2008, 44, 677–685. [Google Scholar] [CrossRef]
- Jiao, L.; Huang, C.; Zeng, J.; Wang, Y.; Wang, X. Miscibility, crystallization and mechanical properties of biodegradable blends of poly(l-lactic acid) and poly(butylene succinate-b-ethylene succinate) multiblock copolymer. Thermochim. Acta 2012, 539, 16–22. [Google Scholar] [CrossRef]
- Chen, G.; Kim, H.; Kim, E.; Yoon, J. Compatibilization-like effect of reactive organoclay on the poly(l-lactide)/poly(butylene succinate) blends. Polymer 2005, 46, 11829–11836. [Google Scholar] [CrossRef]
- Chen, G.; Yoon, J. Thermal stability of poly(l-lactide)/poly(butylene succinate)/clay nanocomposites. Polym. Degrad. Stab. 2005, 88, 206–212. [Google Scholar] [CrossRef]
- Qiu, Z. Subsequent melting behavior of poly(butylene succinate) in its miscible blend with poly(vinyl phenol) crystallized nonisothermally from the melt. J. Appl. Polym. Sci. 2007, 104, 3637–3641. [Google Scholar] [CrossRef]
- Qiu, Z.; Komura, M.; Ikehara, T.; Nishi, T. Poly(butylene succinate)/poly(vinyl phenol) blends. Part 1. Miscibility and crystallization. Polymer 2003, 44, 8111–8117. [Google Scholar] [CrossRef]
- Yang, F.; Qiu, Z.; Yang, W. Miscibility and crystallization of biodegradable poly(butylene succinate-co-butylene adipate)/poly(vinyl phenol) blends. Polymer 2009, 50, 2328–2333. [Google Scholar] [CrossRef]
- Shirahase, T.; Komatsu, Y.; Marubayashi, H.; Tominaga, Y.; Asai, S.; Sumita, M. Miscibility and hydrolytic degradation in alkaline solution of poly(l-lactide) and poly(p-vinyl phenol) blends. Polym. Degrad. Stab. 2007, 92, 1626–1631. [Google Scholar] [CrossRef]
- Xing, P.; Dong, L.; An, Y.; Feng, Z.; Avella, M.; Martuscelli, E. Miscibility and crystallization of poly(β-hydroxybutyrate) and poly(p-vinyl phenol) blends. Macromolecules 1997, 30, 2726–2733. [Google Scholar] [CrossRef]
- Si, P.; Luo, F.; Xue, P.; Yan, D. Crystallization mechanism, microstructure and mechanical property of poly (l-lactic acid) modified by introduction of hydrogen bonding. J. Polym. Res. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Si, P.; Luo, F. Hydrogen bonding interaction and crystallization behavior of poly(butylene succinate-co-butylene adipate)/thiodiphenol complexes. Polym. Adv. Technol. 2016, 27, 1413–1421. [Google Scholar] [CrossRef]
- Si, P.; Luo, F.; Hai, M. Intermolecular interactions and crystallization and melting behavior of poly(l-lactic acid)/4,4′-thiobis phenol blends. Chem. J. Chin. Univ. 2015, 36, 188–194. [Google Scholar]
- Qiu, Z.; Komura, M.; Ikehara, T.; Nishi, T. Miscibility and crystallization behavior of biodegradable blends of two aliphatic polyesters. Poly(butylene succinate) and poly(ε-caprolactone). Polymer 2003, 44, 7749–7756. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Biodegradable aliphatic polyesters. Part I. Properties and biodegradation of poly(butylene succinate-co-butylene adipate). Polym. Degrad. Stab. 2006, 91, 367–376. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Ren, M.; Song, J.; Song, C.; Zhang, H.; Sun, X.; Chen, Q.; Zhang, H.; Mo, Z. Crystallization kinetics and morphology of poly(butylene succinate-co-adipate). J. Polym. Sci. B Polym. Phys. 2005, 43, 3231–3241. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Sakamo, K.; Ono, Y.; Kawahara, W. Melting behavior of poly(l-lactic acid): X-ray and DSC analyses of the melting process. Polymer 2008, 49, 1943–1951. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Iura, K.; Dan, Y. Melting behavior of poly(l-lactic acid): Effects of crystallization temperature and time. Polymer 2007, 48, 5398–5407. [Google Scholar] [CrossRef]
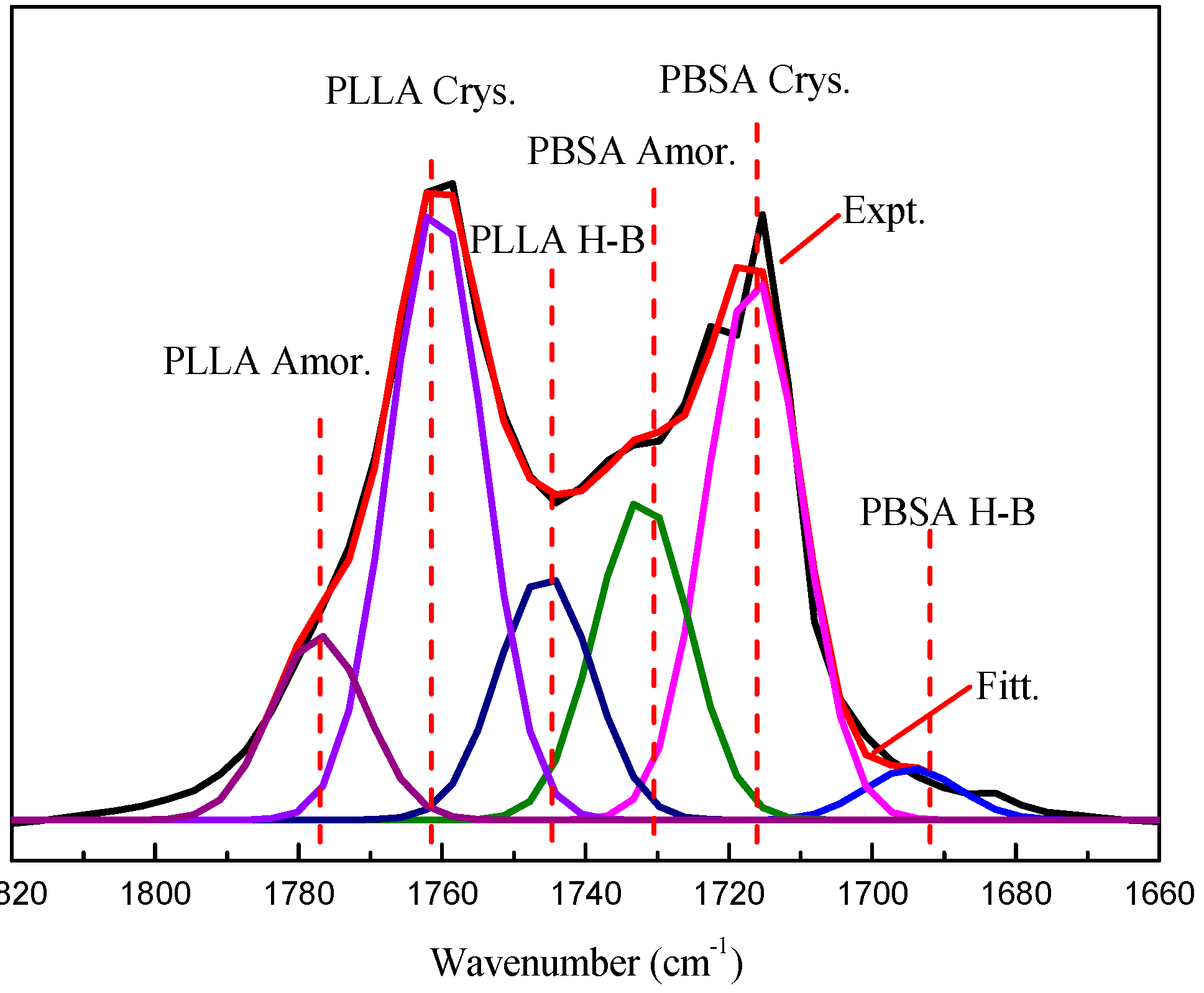

| Samples | PLLA | PBSA | ||||
|---|---|---|---|---|---|---|
| fAmor. (%) | fCrys. (%) | fH-B (%) | fAmor. (%) | fCrys. (%) | fH-B (%) | |
| PVPh-10 | 9.40 | 31.43 | 12.51 | 16.37 | 27.68 | 2.61 |
| PVPh-20 | 9.70 | 31.34 | 12.09 | 16.34 | 26.77 | 3.79 |
| PVPh-40 | 8.53 | 31.28 | 13.26 | 16.61 | 26.32 | 4.00 |
| Samples | Tc1 (°C) | ΔHc1 (J/g) | Tc2 (°C) | ΔHc2 (J/g) | Tg1 (°C) | Tg2 (°C) |
|---|---|---|---|---|---|---|
| PVPh-0 | 50.91 | 21.68 | 99.91 | 16.82 | −37.69 | 51.58 |
| PVPh-10 | 40.11 | 16.55 | 98.57 | 15.23 | −30.40 | 52.36 |
| PVPh-20 | - | - | 100.78 | 14.37 | −11.08 | 56.04 |
| PVPh-30 | - | - | 97.70 | 12.37 | −15.15 | 51.61 |
| PVPh-40 | - | - | 96.24 | 11.86 | −11.26 | 51.92 |
| PVPh-50 | - | - | 93.32 | 8.85 | 45.04 | 45.04 |
| PVPh-100 | - | - | - | - | - | 182.98 |
| Samples | Tc (°C) | n | k (min−n) | t1/2 (min) |
|---|---|---|---|---|
| PVPh-0 | 64 | 2.4 | 5.4 × 10−1 | 1.11 |
| 66 | 2.5 | 2.0 × 10−1 | 1.63 | |
| 68 | 2.5 | 0.7 × 10−2 | 2.58 | |
| 70 | 2.3 | 0.6 × 10−2 | 2.96 | |
| PVPh-5 | 64 | 2.7 | 9.6 × 10−2 | 2.07 |
| 66 | 2.7 | 4.0 × 10−2 | 2.83 | |
| 68 | 2.7 | 1.7 × 10−2 | 3.90 | |
| 70 | 2.6 | 0.8 × 10−3 | 5.56 | |
| PVPh-10 | 64 | 2.3 | 0.1 × 10−2 | 5.92 |
| 66 | 2.3 | 0.7 × 10−3 | 7.59 | |
| 68 | 2.7 | 0.3 × 10−3 | 7.66 | |
| 70 | 2.4 | 0.3 × 10−3 | 9.18 |
| Samples | Tc (°C) | n | k (min−n) | t1/2 (min) |
|---|---|---|---|---|
| PVPh-0 | 116 | 2.6 | 7.8 × 10−2 | 2.30 |
| 118 | 2.8 | 6.4 × 10−2 | 2.32 | |
| 120 | 2.8 | 1.5 × 10−2 | 3.93 | |
| 122 | 2.8 | 1.7 × 10−2 | 8.56 | |
| PVPh-5 | 116 | 2.6 | 4.8 × 10−1 | 1.15 |
| 118 | 2.7 | 1.7 × 10−1 | 1.71 | |
| 120 | 2.8 | 2.7 × 10−2 | 3.24 | |
| 122 | 2.8 | 2.7 × 10−3 | 7.37 | |
| PVPh-10 | 116 | 2.7 | 0.6 × 10−1 | 1.04 |
| 118 | 2.6 | 0.2 × 10−1 | 1.55 | |
| 120 | 2.7 | 5.1 × 10−2 | 2.62 | |
| 122 | 2.8 | 6.4 × 10−3 | 5.40 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, P.; Luo, F.; Luo, F. Miscibility, Morphology and Crystallization Behavior of Poly(Butylene Succinate-co-Butylene Adipate)/Poly(Vinyl Phenol)/Poly(l-Lactic Acid) Blends. Polymers 2016, 8, 421. https://doi.org/10.3390/polym8120421
Si P, Luo F, Luo F. Miscibility, Morphology and Crystallization Behavior of Poly(Butylene Succinate-co-Butylene Adipate)/Poly(Vinyl Phenol)/Poly(l-Lactic Acid) Blends. Polymers. 2016; 8(12):421. https://doi.org/10.3390/polym8120421
Chicago/Turabian StyleSi, Pengfei, Faliang Luo, and Fahai Luo. 2016. "Miscibility, Morphology and Crystallization Behavior of Poly(Butylene Succinate-co-Butylene Adipate)/Poly(Vinyl Phenol)/Poly(l-Lactic Acid) Blends" Polymers 8, no. 12: 421. https://doi.org/10.3390/polym8120421






