Development of PVDF Membrane Nanocomposites via Various Functionalization Approaches for Environmental Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Spongy PVDF Membrane Casting
2.3. Surface Functionalization
2.4. Iron Nanoparticle in-Situ Synthesis in Membranes
2.5. Functionalized Membrane Characterization
2.6. Stimuli-Responsive and Ion-Exchange Properties of Functionalized Membranes
2.7. TCE Dechlorination with SPVDF-PAA-Fe/Pd

3. Results and Discussion
3.1. Membrane Formation and Functionalization
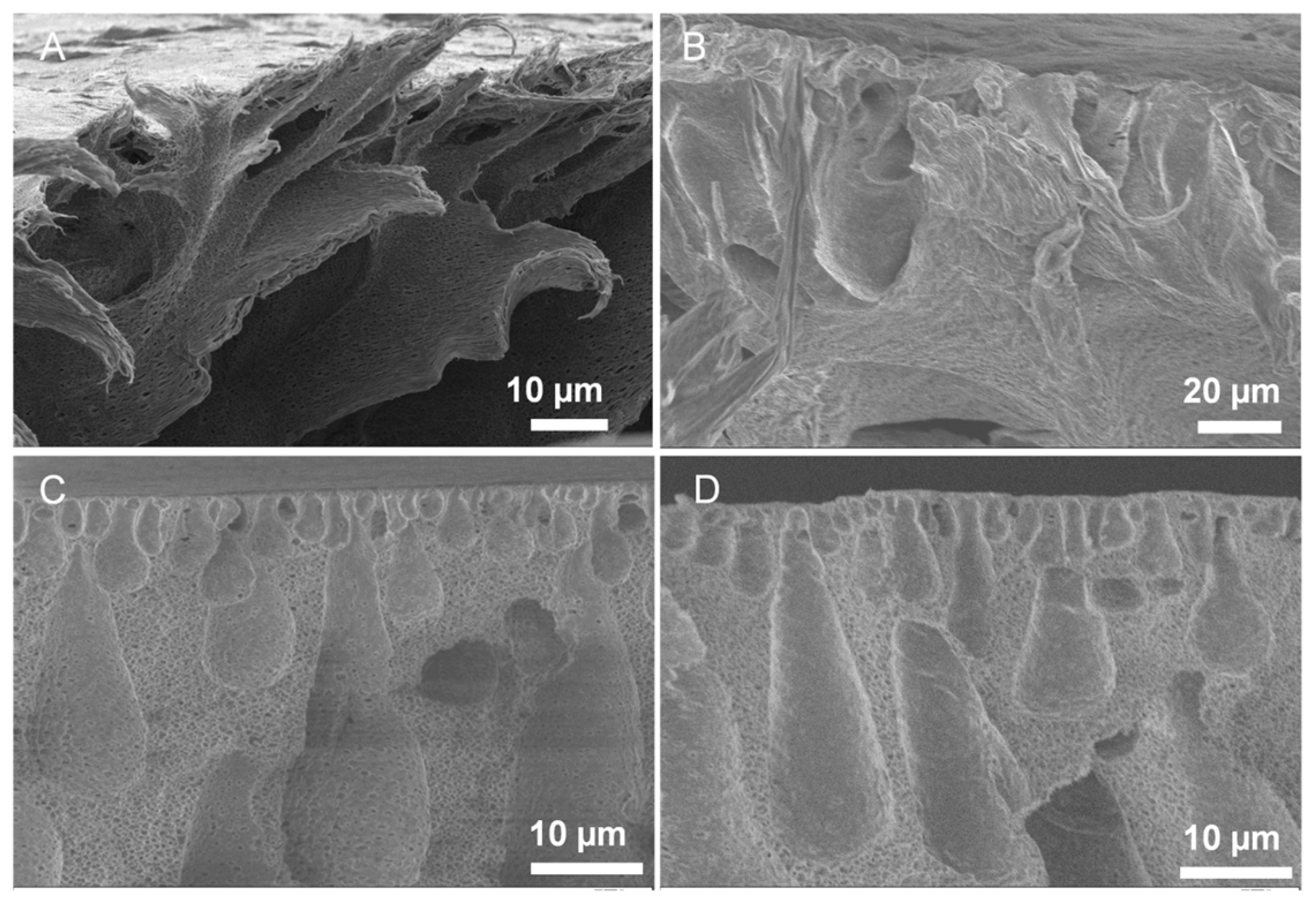
3.1.1. Spongy Membrane Casting
| Name | PVDF membrane casting scale | PVDF layer thickness (µm) | Membrane backing material (Thickness in µm) | PAA modification | Mass gain (g PAA/g PVDF × 100%) | Pure water permeability at pH 6 (LMH/Bar) |
|---|---|---|---|---|---|---|
| L-SPVDF-AA 1 | Lab-scale | 175 | Polypropylene (175) | Lab-scale | 89.5% | 13.0 |
| N-SPVDF-AA 1 | Full-scale | 175 | Polypropylene (175) | Lab-scale | 46.4% | 1.7 |
| N-PVDF-AA 2 | Full-scale | 75 | Polyester (125) | Full-scale | 23.6% | 1320 |
| N-PVDF-NaAc 2 | Full-scale | 75 | Polyester (125) | Lab-scale | 14.6% | 137 |
3.1.2. Surface Modification with Carboxyl Groups and Membrane Characterization



3.1.3. Amine Functionalization


3.2. Functionalized Membrane Properties
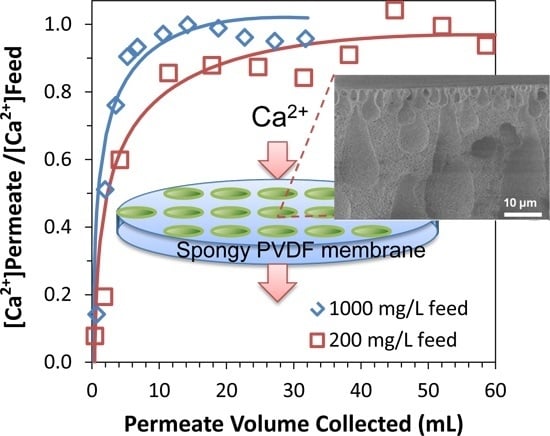
3.2.1. Equilibrium and Dynamic Binding Capacity of Functionalized Spongy Membranes




3.2.2. Stimuli-Responsive Pores


3.2.3. Mechanical Strength
| Tensile modulus (MPa) | Tensile yield strain (MPa) | |
|---|---|---|
| N-SPVDF 1 | 156 ± 14 | 18 ± 1 |
| N-SPVDF-AA | 273 ± 9 | 24 ± 4 |
| N-PVDF 2 | 310 ± 22 | 25 ± 1 |
| N-PVDF-AA | 543 ± 45 | 29 ± 4 |
3.3. TCE Dechlorination with Fe/Pd Functionalized Membranes

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Calo, V.M.; Iliev, O.; Lakdawala, Z.; Leonard, K.H.L.; Printsypar, G. Pore-scale modeling and simulation of flow, transport, and adsorptive or osmotic effects in membranes: The influence of membrane microstructure. Int. J. Adv. Eng. Sci. Appl. Math. 2015, 7, 2–13. [Google Scholar] [CrossRef]
- Shao, X.; Dong, D.H.; Parkinson, G.; Li, C.Z. Microstructure control of oxygen permeation membranes with templated microchannels. J. Mater. Chem. A 2014, 2, 410–417. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.T.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.T.; Li, K. Isothermal phase diagrams and phase-inversion behavior of poly(vinylidene fluoride)/solvents/additives/water systems. J. Appl. Polym. Sci. 2003, 90, 2150–2155. [Google Scholar] [CrossRef]
- Kinasiewicz, A.; Dudzinski, K.; Chwojnowski, A.; Werynski, A.; Kawiak, J. Three-dimensional culture of hepatocytes on spongy polyethersulfone membrane developed for cell transplantation. Transplant. Proc. 2007, 39, 2914–2916. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.L.; Shyu, S.S.; Wu, Y.B.; Lee, S.T.; Shyong, J.Y.; Huang, R.N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Mao, Y.C.; Zhao, P.; McConohy, G.; Yang, H.; Tong, Y.X.; Wang, X.D. Sponge-like piezoelectric polymer films for scalable and integratable nanogenerators and self-powered electronic systems. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Ji, G.L.; Zhu, B.K.; Cui, Z.Y.; Zhang, C.F.; Xu, Y.Y. Pvdf porous matrix with controlled microstructure prepared by tips process as polymer electrolyte for lithium ion battery. Polymer 2007, 48, 6415–6425. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Sun, L.Q.; Wang, Q.; Li, B.A.; Wang, S.C. A novel approach to fabricate interconnected sponge-like and highly permeable polyvinylidene fluoride hollow fiber membranes for direct contact membrane distillation. Eur. Polym. J. 2014, 60, 262–272. [Google Scholar] [CrossRef]
- Silva, T.L.S.; Morales-Torres, S.; Figueiredo, J.L.; Silva, A.M.T. Multi-walled carbon nanotube/PVDF blended membranes with sponge- and finger-like pores for direct contact membrane distillation. Desalination 2015, 357, 233–245. [Google Scholar] [CrossRef]
- Xiao, L.; Davenport, D.M.; Ormsbee, L.; Bhattacharyya, D. Polymerization and functionalization of membrane pores for water related applications. Ind. Eng. Chem. Res. 2015, 54, 4174–4182. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.L.; Lim, T.T.; Krantz, W.B.; Fane, A.G.; Hu, X. Potential evaluation and perspectives on using sponge-like superabsorbent cryogels for onsite water treatment in emergencies. Desalin. Water Treat. 2015, 53, 1506–1515. [Google Scholar] [CrossRef]
- Lakes, R.S. Materials with structural hierarchy. Nature 1993, 361, 511–515. [Google Scholar] [CrossRef]
- Miserez, A.; Weaver, J.C.; Thurner, P.J.; Aizenberg, J.; Dauphin, Y.; Fratzl, P.; Morse, D.E.; Zok, F.W. Effects of laminate architecture on fracture resistance of sponge biosilica: Lessons from nature. Adv. Funct. Mater. 2008, 18, 1241–1248. [Google Scholar] [CrossRef]
- Chen, Q.; Pugno, N.M. Bio-mimetic mechanisms of natural hierarchical materials: A review. J. Mech. Behav. Biomed. 2013, 19, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Qiu, C.Q.; Tang, C.Y.Y.; Wang, R.; Fane, A.G. Synthesis and characterization of flat-sheet thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
- Kotte, M.R.; Kuvarega, A.T.; Cho, M.; Mamba, B.B.; Diallo, M.S. Mixed matrix pvdf membranes with in situ synthesized pamam dendrimer-like particles: A new class of sorbents for Cu(ii) recovery from aqueous solutions by ultrafiltration. Environ. Sci. Technol. 2015, 49, 9431–9442. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Z.; Xie, W.; Li, J.Y.; Xing, T.L.; Jin, J. pH-induced non-fouling membrane for effective separation of oil-in-water emulsion. J. Membr. Sci. 2015, 477, 131–138. [Google Scholar] [CrossRef]
- Gui, M.H.; Papp, J.K.; Colburn, A.S.; Meeks, N.D.; Weaver, B.; Wilf, I.; Bhattacharyya, D. Engineered iron/iron oxide functionalized membranes for selenium and other toxic metal removal from power plant scrubber water. J. Membr. Sci. 2015, 488, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, J.T.; Tratnyek, P.G.; Sarathy, V.; Baer, D.R.; Amonette, J.E.; Pecher, K.; Wang, C.M.; Linehan, J.C.; Matson, D.W.; Penn, R.L.; et al. Characterization and properties of metallic iron nanoparticles: Spectroscopy, electrochemistry, and kinetics. Environ. Sci. Technol. 2005, 39, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Gui, M.; Ormsbee, L.E.; Bhattacharyya, D. Reactive functionalized membranes for polychlorinated biphenyl degradation. Ind. Eng. Chem. Res. 2013, 52, 10430–10440. [Google Scholar] [CrossRef] [PubMed]
- Smuleac, V.; Bachas, L.; Bhattacharyya, D. Aqueous—Phase synthesis of PAA in PVDF membrane pores for nanoparticle synthesis and dichlorobiphenyl degradation. J. Membr. Sci. 2010, 346, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Bottino, A.; Cameraroda, G.; Capannelli, G.; Munari, S. The formation of microporous polyvinylidene difluoride membranes by phase-separation. J. Membr. Sci. 1991, 57, 1–20. [Google Scholar] [CrossRef]
- Nejati, S.; Boo, C.; Osuji, C.O.; Elimelech, M. Engineering flat sheet microporous PVDF films for membrane distillation. J. Membr. Sci. 2015, 492, 355–363. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.T.; Li, K. Morphological study of poly(vinylidene fluoride) asymmetric membranes: Effects of the solvent, additive, and dope temperature. J. Appl. Polym. Sci. 2004, 92, 1782–1789. [Google Scholar] [CrossRef]
- Cheng, L.P. Effect of temperature on the formation of microporous pvdf membranes by precipitation from 1-octanol/DMF/PVDF and water/DMF/PVDF systems. Macromolecules 1999, 32, 6668–6674. [Google Scholar] [CrossRef]
- Guo, Y.F.; Feng, X.; Chen, L.; Zhao, Y.P.; Bai, J.N. Influence of the coagulation-bath temperature on the phase-separation process of poly(vinylidene fluoride)-graft-poly(n-isopropylacrylamide) solutions and membrane structures. J. Appl. Polym. Sci. 2010, 116, 1005–1009. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.L.; Yu, L.Y. Effects of mixed solvents and PVDF types on performances of PVDF microporous membranes. J. Appl. Polym. Sci. 2010, 115, 2277–2287. [Google Scholar] [CrossRef]
- Bottino, A.; Capannelli, G.; Munari, S.; Turturro, A. High-performance ultrafiltration membranes cast from licl doped solutions. Desalination 1988, 68, 167–177. [Google Scholar] [CrossRef]
- Sun, S.T.; Wang, H.N.; Wu, P.Y. Dynamic self-aggregation and disaggregation behavior of thermoresponsive hyperbranched polyethylenimine with peripheral nipam groups: An infrared spectroscopic study. Soft Matter 2013, 9, 2878–2888. [Google Scholar] [CrossRef]
- Dukhin, S.S.; Zimmermann, R.; Werner, C. Surface conductivity reveals counterion condensation within grafted polyelectrolyte layers. J. Phys. Chem. B 2007, 111, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.S. Limiting laws and counterion condensation in polyelectrolyte solutions. I. Colligative properties. J. Chem. Phys. 1969, 51, 924. [Google Scholar] [CrossRef]
- Darvishmanesh, S.; Qian, X.H.; Wickramasinghe, S.R. Responsive membranes for advanced separations. Curr. Opin. Chem. Eng. 2015, 8, 98–104. [Google Scholar] [CrossRef]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Stimuli-responsive membranes. J. Membr. Sci. 2010, 357, 6–35. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, Q.; Gorb, S.N.; Li, Z.Y. A multiscale study on the structural and mechanical properties of the luffa sponge from luffa cylindrica plant. J. Biomech. 2014, 47, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, M. Preparation and properties of flat-sheet membranes from poly(vinylidene fluoride) for membrane distillation. Desalination 1996, 104, 1–11. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.Q.; Rong, M.Z.; Ruan, W.H. Studies on the transformation process of PVDF from α to β phase by stretching. RSC Adv. 2014, 4, 3938–3943. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davenport, D.M.; Gui, M.; Ormsbee, L.R.; Bhattacharyya, D. Development of PVDF Membrane Nanocomposites via Various Functionalization Approaches for Environmental Applications. Polymers 2016, 8, 32. https://doi.org/10.3390/polym8020032
Davenport DM, Gui M, Ormsbee LR, Bhattacharyya D. Development of PVDF Membrane Nanocomposites via Various Functionalization Approaches for Environmental Applications. Polymers. 2016; 8(2):32. https://doi.org/10.3390/polym8020032
Chicago/Turabian StyleDavenport, Douglas M., Minghui Gui, Lindell R. Ormsbee, and Dibakar Bhattacharyya. 2016. "Development of PVDF Membrane Nanocomposites via Various Functionalization Approaches for Environmental Applications" Polymers 8, no. 2: 32. https://doi.org/10.3390/polym8020032