Investigations of the Ligand Electronic Effects on α-Diimine Nickel(II) Catalyzed Ethylene Polymerization
Abstract
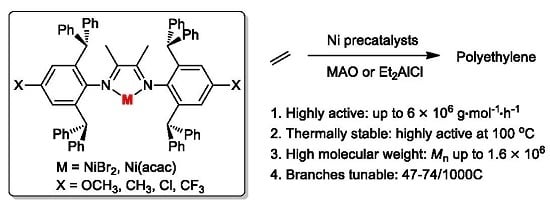
:1. Introduction

2. Experimental Section
2.1. General Information
2.2. Standard Procedure for the Synthesis of Complexes 1a–1c
2.3. Standard Procedure for the Synthesis of Complexes 2a–2d
2.4. General Procedure for Ethylene Polymerization
3. Results and Discussion
3.1. Synthesis and Characterization of the Ni(II) Complexes


3.2. Ethylene Polymerization Studies
| Entry | Cat. | Activator | T (°C) | Yield (g) | Act. b | Mn c (×10−4) | PDI c | Br d | Tm e (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | MAO | 40 | 0.48 | 0.96 | 111 | 1.19 | 48 | 62.1 |
| 2 | 1a | MAO | 60 | 0.88 | 1.76 | 126 | 1.22 | 53 | 53.7 |
| 3 | 1a | MAO | 80 | 0.89 | 1.78 | 146 | 1.21 | 56 | 49.2 |
| 4 | 1a | MAO | 100 | 1.15 | 2.30 | 125 | 1.44 | 62 | 41.4 |
| 5 | 1a | AlEt2Cl | 100 | 0.79 | 1.58 | 103 | 1.88 | 59 | 47.6 |
| 6 | 1b | MAO | 40 | 0.90 | 1.80 | 161 | 1.12 | 55 | 57.2 |
| 7 | 1b | MAO | 60 | 1.16 | 2.32 | 159 | 1.25 | 59 | 46.0 |
| 8 | 1b | MAO | 80 | 1.14 | 2.28 | 164 | 1.23 | 62 | 43.8 |
| 9 | 1b | MAO | 100 | 1.31 | 2.62 | 154 | 1.40 | 66 | 39.1 |
| 10 | 1b | AlEt2Cl | 100 | 1.24 | 2.48 | 152 | 1.55 | 62 | 42.8 |
| 11 | 1c | MAO | 40 | 0.90 | 1.80 | 143 | 1.20 | 59 | 48.3 |
| 12 | 1c | MAO | 60 | 0.81 | 1.62 | 147 | 1.25 | 65 | 39.8 |
| 13 | 1c | MAO | 80 | 0.96 | 1.92 | 129 | 1.34 | 71 | 35.1 |
| 14 | 1c | MAO | 100 | 0.94 | 1.88 | 121 | 1.57 | 74 | 34.9 |
| 15 | 1c | AlEt2Cl | 100 | 0.39 | 0.78 | 80.4 | 1.79 | 65 | 37.9 |

| Entry | Cat. | Activitor | T (°C) | Yield (g) | Act. b | Mn c (×10−4) | PDI c | Br d | Tm e (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2b | MAO | 40 | trace | − | − | − | − | |
| 2 | 2b | Al(i-Bu)3 | 40 | trace | − | − | − | − | |
| 3 | 2b | MAO | 80 | trace | − | − | − | − | |
| 4 | 2b | Al(i-Bu)3 | 80 | trace | − | − | − | − | |
| 5 | 2b | AlEtCl2 | 100 | trace | − | − | − | − | |
| 6 | 2b | AlEt2Cl | 40 | 0.53 | 1.06 | 115 | 1.26 | 47 | 70.5 |
| 7 | 2b | AlEt2Cl | 60 | 0.86 | 1.72 | 130 | 1.44 | 51 | 62.6 |
| 8 | 2b | AlEt2Cl | 80 | 1.43 | 2.86 | 145 | 1.35 | 63 | 53.5 |
| 9 | 2b | AlEt2Cl | 100 | 1.88 | 3.76 | 157 | 1.52 | 66 | 45.3 |
| 10 | 2a | AlEt2Cl | 100 | 1.07 | 2.14 | 60.6 | 1.75 | 61 | 46.0 |
| 11 | 2c | AlEt2Cl | 100 | 1.40 | 2.80 | 81.0 | 1.79 | 63 | 39.9 |
| 12 | 2d | AlEt2Cl | 100 | 3.09 | 6.18 | 57.5 | 2.14 | 62 | 41.5 |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd (II)- and Ni (II)-based catalysts for polymerization of ethylene and α-olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Killian, C.M.; Tempel, D.J.; Johnson, L.K.; Brookhart, M. Living polymerization of α-olefins using NiII-α-diimine catalysts. Synthesis of new block polymers based on α-olefins. J. Am. Chem. Soc. 1996, 118, 11664–11665. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo- and copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination—Insertion copolymerization of fundamental polar monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef] [PubMed]
- Camacho, D.H.; Guan, Z. Designing late-transition metal catalysts for olefin insertion polymerization and copolymerization. Chem. Commun. 2010, 46, 7879–7893. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Xu, L.; Dong, Z.; Xiang, P. Designing polyethylenes of complex chain architectures via Pd-diimine-catalyzed “living” ethylene polymerization. Chem. Commun. 2013, 49, 6235–6255. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Cotts, P.M.; McCord, E.F.; McLain, S.J. Chain walking: A new strategy to control polymer topology. Science 1999, 283, 2059–2061. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Luo, S.; Jordan, R.F. Multiple insertion of a silyl vinyl ether by (α-diimine)PdMe+ species. J. Am. Chem. Soc. 2008, 130, 12892–12893. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Luo, S.; Jordan, R.F. Cationic polymerization and insertion chemistry in the reactions of vinyl ethers with (α-diimine)PdMe+ species. J. Am. Chem. Soc. 2010, 132, 5273–5284. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Jordan, R.F. Palladium-catalyzed dimerization of vinyl ethers to acetals. J. Am. Chem. Soc. 2010, 132, 10254–10255. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, T.; Klimovica, K.; LaPointe, A.M.; Keresztes, I.; Lobkovsky, E.B.; Daugulis, O.; Coates, G.W. Secondary alkene insertion and precision chain-walking: A new route to semicrystalline “polyethylene” from α-olefins by combining two rare catalytic events. J. Am. Chem. Soc. 2014, 136, 7213–7216. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Takeuchi, D.; Osakada, K.; Akamatsu, N.; Shishido, A. Dipalladium catalyst for olefin polymerization: Introduction of acrylate units into the main chain of branched polyethylene. Angew. Chem. Int. Ed. 2014, 53, 9246–9250. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.K.; Sui, X.L.; Pang, W.M.; Chen, C.L. Ethylene polymerization by xanthene bridged dinuclear α-diimine Ni(II) complexes. Chem. Cat. Chem. 2015. [Google Scholar] [CrossRef]
- Guo, L.H.; Chen, C.L. (α-Diimine) palladium catalyzed ethylene polymerization and copolymerization with polar comonomers. Sci. China Chem. 2015, 58, 1663–1673. [Google Scholar] [CrossRef]
- Guo, L.H.; Dai, S.Y.; Sui, X.L.; Chen, C.L. Palladium and nickel catalyzed chain walking olefin polymerization and copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- Xie, T.Y.; Mcauley, K.B.; Hsu, J.C.C.; Bacon, D.W. Gas phase ethylene polymerization: Production processes, polymer properties, and reactor modeling. Ind. Eng. Chem. Res. 1994, 33, 449–479. [Google Scholar] [CrossRef]
- Tempel, D.J.; Johnson, L.K.; Huff, R.L.; White, P.S.; Brookhart, M. Mechanistic studies of Pd(II)-α-diimine-catalyzed olefin polymerizations. J. Am. Chem. Soc. 2000, 122, 6686–6700. [Google Scholar] [CrossRef]
- Gates, D.P.; Svejda, S.A.; Oñate, E.; Killian, C.M.; Johnson, L.K.; White, P.S.; Brookhart, M. Synthesis of branched polyethylene using (α-diimine)nickel(II) catalysts: Influence of temperature, ethylene pressure, and ligand structure on polymer properties. Macromolecules 2000, 33, 2320–2334. [Google Scholar] [CrossRef]
- Berkefeld, A.; Mecking, S. Deactivation pathways of neutral Ni(II) polymerization catalysts. J. Am. Chem. Soc. 2009, 131, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Gao, H.; Guan, Q.; Hu, H.; Deng, J.; Liu, J.; Liu, F.; Wu, Q. Substituent effects of the backbone in α-diimine palladium catalysts on homo- and copolymerization of ethylene with methyl acrylate. Organometallics 2012, 31, 6054–6062. [Google Scholar] [CrossRef]
- Ionkin, A.S.; Marshall, W.J. ortho-5-Methylfuran- and benzofuran-substituted η3-allyl(α-diimine)nickel(II) complexes: Syntheses, structural characterization, and the first polymerization results. Organometallics 2004, 23, 3276–3283. [Google Scholar] [CrossRef]
- Camacho, D.H.; Salo, E.V.; Ziller, J.W.; Guan, Z. Cyclophane-based highly active late-transition-metal catalysts for ethylene polymerization. Angew. Chem. Int. Ed. 2004, 43, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Camacho, D.H.; Guan, Z. Living polymerization of α-olefins at elevated temperatures catalyzed by a highly active and robust cyclophane-based Nickel catalyst. Macromolecules 2005, 38, 2544–2546. [Google Scholar] [CrossRef]
- Popeney, C.S.; Camacho, D.H.; Guan, Z. Efficient incorporation of polar comonomers in copolymerizations with ethylene using a cyclophane-based Pd(II) α-diimine catalyst. J. Am. Chem. Soc. 2007, 129, 10062–10063. [Google Scholar] [CrossRef] [PubMed]
- Popeney, C.S.; Guan, Z. A mechanistic investigation on copolymerization of ethylene with polar monomers using a cyclophane-based Pd(II) α-diimine catalyst. J. Am. Chem. Soc. 2009, 131, 12384–12393. [Google Scholar] [CrossRef] [PubMed]
- Popeney, C.S.; Rheingold, A.L.; Guan, Z. Nickel(II) and Palladium(II) polymerization catalysts bearing a fluorinated cyclophane ligand: Stabilization of the reactive intermediate. Organometallics 2009, 28, 4452–4463. [Google Scholar] [CrossRef]
- Liu, F.; Hu, H.; Xu, Y.; Guo, L.; Zai, S.; Song, K.; Gao, H.; Zhang, L.; Zhu, F.; Wu, Q. Thermostable α-diimine Nickel(II) catalyst for ethylene polymerization: Effects of the Substituted backbone structure on catalytic properties and branching structure of polyethylene. Macromolecules 2009, 42, 7789–7796. [Google Scholar] [CrossRef]
- Kong, S.; Guo, C.Y.; Yang, W.; Wang, L.; Sun, W.H.; Glaser, R. 2,6-Dibenzhydryl-N-(2-phenyliminoacenaphthylenylidene)-4-chloro-aniline nickel dihalides: Synthesis, characterization and ethylene polymerization for polyethylenes with high molecular weights. J. Organomet. Chem. 2013, 725, 37–45. [Google Scholar] [CrossRef]
- Kong, S.; Song, K.; Liang, T.; Guo, C.Y.; Sun, W.H.; Redshaw, C. Methylene-bridged bimetallic nickel(II) α-diimino nickel(II) complexes: Synthesis and high efficiency in ethylene polymerization. Dalton Trans. 2013, 42, 9176–9187. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, W.H. 2,6-Dibenzhydryl-N-(2-phenyliminoacenaphthylenylidene)-4-methylbenzenamine nickel dibromides: Synthesis, characterization, and ethylene polymerization. Organometallics 2011, 30, 2418–2424. [Google Scholar] [CrossRef]
- Rhinehart, J.L.; Brown, L.A.; Long, B.K. A robust Ni(II) α-diimine catalyst for high temperature ethylene polymerization. J. Am. Chem. Soc. 2013, 135, 16316–16319. [Google Scholar] [CrossRef] [PubMed]
- Rhinehart, J.L.; Mitchell, N.E.; Long, B.K. Enhancing α-diimine catalysts for high-temperature ethylene polymerization. ACS Catal. 2014, 4, 2501–2504. [Google Scholar] [CrossRef]
- Dai, S.Y.; Sui, X.L.; Chen, C.L. Highly robust Pd(II) α–diimine catalysts for slow-chain-walking polymerization of ethylene and copolymerization with methyl acrylate. Angew. Chem. Int. Ed. 2015, 54, 9948–9953. [Google Scholar] [CrossRef] [PubMed]
- Popeney, C.S.; Guan, Z. Ligand electronic effects on late transition metal polymerization catalysts. Organometallics 2005, 24, 1145–1155. [Google Scholar] [CrossRef]
- Popeney, C.S.; Guan, Z. Effect of ligand electronics on the stability and chain transfer rates of substituted Pd(II) α-diimine catalysts. Macromolecules 2010, 43, 4091–4097. [Google Scholar] [CrossRef]
- Popeney, C.S.; Levins, C.M.; Guan, Z. Systematic investigation of ligand substitution effects in cyclophane-based nickel(II) and palladium(II) olefin polymerization catalysts. Organometallics 2011, 30, 2432–2452. [Google Scholar] [CrossRef]
- Moody, L.S.; Mackenzie, P.B.; Killian, C.M.; Lavoie, G.G.; Ponasik, J.A., Jr.; Barrett, A.G.; Smith, T.W.; Pearson, J.C. Catalysts Containing N-pyrrolyl Substituted Nitrogen Donors. WO 00/50470, 2002. [Google Scholar]
- Meinhard, D.; Wegner, M.; Kipiani, G.; Hearley, A.; Reuter, P.; Fischer, S.; Marti, O.; Rieger, B. New Nickel(II) diimine complexes and the control of polyethylene microstructure by catalyst design. J. Am. Chem. Soc. 2007, 129, 9182–9191. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Dai, S.; Chen, C. Investigations of the Ligand Electronic Effects on α-Diimine Nickel(II) Catalyzed Ethylene Polymerization. Polymers 2016, 8, 37. https://doi.org/10.3390/polym8020037
Guo L, Dai S, Chen C. Investigations of the Ligand Electronic Effects on α-Diimine Nickel(II) Catalyzed Ethylene Polymerization. Polymers. 2016; 8(2):37. https://doi.org/10.3390/polym8020037
Chicago/Turabian StyleGuo, Lihua, Shengyu Dai, and Changle Chen. 2016. "Investigations of the Ligand Electronic Effects on α-Diimine Nickel(II) Catalyzed Ethylene Polymerization" Polymers 8, no. 2: 37. https://doi.org/10.3390/polym8020037
APA StyleGuo, L., Dai, S., & Chen, C. (2016). Investigations of the Ligand Electronic Effects on α-Diimine Nickel(II) Catalyzed Ethylene Polymerization. Polymers, 8(2), 37. https://doi.org/10.3390/polym8020037