New Concept of Polymethyl Methacrylate (PMMA) and Polyethylene Terephthalate (PET) Surface Coating by Chitosan
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
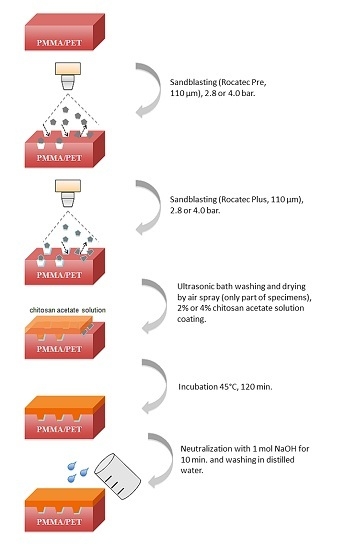
- Sandblasting of the surfaces to be CS coated at a distance of 20–30 mm with Rocatec Pre and Rocatec Plus.
- Washing in an ultrasonic bath (distilled water) for 10 min to remove loose grains from sandblasting and drying with an air spray.
- Coating with acetic CS solution of 1 mm thickness using a metal template (Figure 3).
- Drying the CS solution in an incubator (B6030, Heraeus, Hanau, Germany) for 120 min at 45 °C.
- Neutralization of the precipitated CS in 1 mol NaOH solution for 10 min.
- Washing in distilled water and storing dry at room temperature until further use.
- sandblasting with Rocatec Pre only (10 s, 2.8 bar) or
- sandblasting with Rocatec Pre (10 s, 2.8 bar) and sandblasting with Rocatec Plus (13 s, 2.8 bar) or
- sandblasting with Rocatec Pre (10 s, 2.8 bar) and sandblasting with Rocatec Plus (13 s, 4.0 bar)
- washing in an ultrasonic bath
- omitting the step of washing in an ultrasonic bath
- coating with acetic CS solution containing 2% chitosan or
- coating with acetic CS solution containing 4% chitosan
2.3. Hypotheses and Statistical Analysis
- Modifying the sandblasting procedure (using/omitting Rocatec Plus) does not influence the bond of CS to PMMA or PET
- The CS concentration in the acetic solution (2% or 4%) does not influence the bond of CS to PMMA or PET
- The blast pressure (2.8 or 4.0 bar) does not influence the bond of CS to PMMA or PET
- Omitting the step of ultrasonic cleaning does not influence the bond of CS to PMMA or PET
- PMMA and PET are equally suitable for bonding CS to their surfaces.
3. Results and Discussion
3.1. Abrasion Resistance of Chitosan (CS) on Polyethylene Terephthalate (PET) Surfaces
3.2. Abrasion Resistance of CS on Polymethyl methacrylate (PMMA) Surfaces
3.3. Discussion
4. Conclusions
- Sandblasting of the surfaces to be CS coated with Rocatec Pre and Rocatec Plus (2.8 to 4.0 bar blast pressure)
- Coating with acetic CS solution (2% to 4% CS)
- Drying of the CS solution for 120 min at 45 °C.
- Neutralization of the precipitated CS in mol NaOH solution for 10 min
- Washing in distilled water.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CS | chitosan |
| PMMA | polymethyl methacrylate |
| PET | polyethylene terephthalate |
References
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Func. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Zhao, Y.; Park, R.D.; Muzzarelli, R.A.A. Chitin deacetylases: Properties and applications. Mar. Drugs 2010, 8, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan chemistry: Relevance to the biomedical sciences. Adv. Polym. Sci. 2005, 186, 151–209. [Google Scholar]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Patel, M.P.; Patel, R.R.; Patel, J.K. Chitosan mediated targeted drug delivery system: A review. J. Pharm. Pharmaceut. Sci. 2010, 13, 536–557. [Google Scholar] [CrossRef]
- Salerno, C.; Pascale, M.; Contaldo, M.; Esposito, V.; Busciolano, M.; Milillo, L.; Guida, A.; Petruzzi, M.; Serpico, R. Candida-associated denture stomatitis. Med. Oral. Patol. Oral. Cir. Bucal 2011, 16, 139–143. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodontics 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; van Thompson, V.P. Sandblasting and silica coating of a glass-infiltrated alumina ceramic: Volume loss, morphology, and changes in the surface composition. J. Prosthet. Dent. 1994, 71, 453–461. [Google Scholar] [CrossRef]
- Robina, C.; Scherrera, S.S.; Wiskotta, H.W.A.; de Rijkb, W.G.; Belsera, U.C. Weibull parameters of composite resin bond strengths to porcelain and noble alloy using the Rocatec system. Dent. Mater. 2002, 18, 389–395. [Google Scholar] [CrossRef]
- Massouda, D.; Cotts, P. Attachment of Chitosan to Surfaces Using Rehydration Process. US Patent 20060177489 A1, 2006. [Google Scholar]
- Connell, L.S.; Romer, F.; Suarez, M.; Valliant, E.M.; Ziyu Zhang, Z.; Lee, P.D.; Smith, M.E.; Hannab, J.V.; Jones, J.R. Chemical characterisation and fabrication of chitosan–silica hybrid scaffolds with 3-glycidoxypropyltrimethoxysilane. J. Mater. Chem. B 2014, 2, 668–680. [Google Scholar] [CrossRef]
- Liua, Y.L.; Sua, Y.H.; Leeb, K.R.; Laia, J.Y. Crosslinked organic–inorganic hybrid chitosan membranes for pervaporation dehydration of isopropanol–water mixtures with a long-term stability. J. Memb. Sci. 2005, 251, 233–238. [Google Scholar] [CrossRef]
- Liu, Y.L.; Su, Y.H.; Lai, J.Y. In situ crosslinking of chitosan and formation of chitosan–silica hybrid membranes with using γ-glycidoxypropyltrimethoxysilane as a crosslinking agent. Polymer 2004, 45, 6831–6837. [Google Scholar] [CrossRef]
- Shirosaki, Y.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Lopes, M.A.; Santos, J.; Costa, M.; Fernandes, M. Physical, chemical and in vitro biological profile of chitosan hybrid membrane as a function of organosiloxane concentration. Acta Biomater. 2009, 5, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Rashidovaa, S.S.; Shakarovaa, D.S.; Ruzimuradova, O.N.; Satubaldievaa, D.T.; Zalyalievaa, S.V.; Shpigunb, O.A.; Varlamovc, V.P.; Kabulova, B.D. Bionanocompositional chitosan–silica sorbent for liquid chromatography. J. Chromatogr. B 2004, 800, 49–53. [Google Scholar] [CrossRef]
- Al-Sagheer, F.; Muslim, J. Thermal and mechanical properties of chitosan/SiO2 hybrid composites. J. Nanomater. 2010, 2010, 1–7. [Google Scholar] [CrossRef]












| Test series | Steps of basic procedure |
|---|---|
| I |
|
| II |
|
| III |
|
| IV |
|
| V |
|
| VI |
|
| VII |
|
| 1. | 82.93% Aqua dest |
| 2. | 12.50% Hydroxyethyl cellulose (4%) |
| 3. | 4.28% Sorbitol solution (70%) |
| 4. | 0.12% Potassium chloride |
| 5. | 0.08% Sodium chloride |
| 6. | 0.06% Sodium monohydrogenphosphate 12 H2O |
| 7. | 0.02% Calcium chloride 2 H2O |
| 8. | 0.01% Magnesium chloride 6 H2O |
| 9. | Preservative: Propyl 4-Hydroxybenzoate |
| Percentage of remaining chitosan after 3000 cycles of abrasion | ||||||
| Test Series | Min | 1. Quartile | Median | Mean | 3. Quartile | Max |
| I | 73.9 | 98.0 | 99.8 | 95.1 | 100.0 | 100.0 |
| II | 99.1 | 99.9 | 100.0 | 99.9 | 100.0 | 100.0 |
| III | 99.0 | 99.9 | 100.0 | 99.9 | 100.0 | 100.0 |
| IV | 98.4 | 99.5 | 99.6 | 99.8 | 100.0 | 100.0 |
| V | 93.5 | 96.3 | 96.9 | 98.6 | 99.5 | 100.0 |
| VI | 99.4 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| VII | 95.9 | 98.5 | 99.2 | 99.5 | 99.6 | 100.0 |
| Percentage of remaining chitosan after 30,000 cycles of abrasion | ||||||
| Test Series | Min | 1. Quartile | Median | Mean | 3. Quartile | Max |
| I | 23.8 | 45.3 | 68.2 | 61.8 | 79.4 | 93.3 |
| II | 88.8 | 98.2 | 99.2 | 97.4 | 100.0 | 100.0 |
| III | 97.7 | 99.6 | 100.0 | 99.6 | 100.0 | 100.0 |
| IV | 91.6 | 98.3 | 98.8 | 98.3 | 100.0 | 100.0 |
| V | 84.2 | 92.6 | 95.9 | 94.5 | 97.7 | 99.2 |
| VI | 91.8 | 99.6 | 100.0 | 98.6 | 100.0 | 100.0 |
| VII | 93.2 | 97.1 | 99.0 | 98.1 | 99.7 | 100.0 |
| 3,000 cycles of abrasion | |||
| Test series | U | p | Bonferroni-corrected significance |
| I versus II | 23.0 | 0.04516 | – |
| II versus III | 49.5 | 1.00000 | – |
| II versus IV | 36.0 | 0.30749 | – |
| IV versus V | 37.0 | 0.34471 | – |
| IV versus VI | 39.0 | 0.42736 | – |
| IV versus VII | 36.0 | 0.30749 | – |
| V versus VII | 43.0 | 0.62318 | – |
| VI versus VII | 28.0 | 0.10411 | – |
| VI versus III | 45.0 | 0.73373 | – |
| 30,000 cycles of abrasion | |||
| Test series | U | p | Bonferroni-corrected significance |
| I versus II | 2.0 | 0.00033 | * |
| II versus III | 36.0 | 0.30749 | – |
| II versus IV | 47.0 | 0.85011 | – |
| IV versus V | 14.0 | 0.00729 | * |
| IV versus VI | 36.5 | 0.32575 | – |
| IV versus VII | 45.0 | 0.73373 | – |
| V versus VII | 19.5 | 0.02334 | – |
| VI versus VII | 32.0 | 0.18588 | – |
| VI versus III | 46.5 | 0.82060 | – |
| Percentage of remaining chitosan after 3,000 cycles of abrasion | ||||||
| Test Series | Min | 1. Quartile | Median | Mean | 3. Quartile | Max |
| I | 69.0 | 79.7 | 85.5 | 85.3 | 93.2 | 97.1 |
| II | 47.1 | 80.8 | 88.2 | 85.2 | 95.7 | 100.0 |
| III | 96.9 | 99.6 | 99.9 | 99.5 | 100.0 | 100.0 |
| IV | 50.6 | 71.1 | 82.4 | 79.6 | 94.9 | 98.3 |
| V | 90.5 | 96.9 | 97.7 | 97.0 | 99.8 | 100.0 |
| VI | 92.7 | 99.6 | 99.8 | 98.7 | 99.9 | 100.0 |
| VII | 90.0 | 96.4 | 98.4 | 97.4 | 99.7 | 100.0 |
| Percentage of remaining chitosan after 30,000 cycles of abrasion | ||||||
| Test Series | Min | 1. Quartile | Median | Mean | 3. Quartile | Max |
| I | 0.0 | 48.2 | 55.2 | 51.7 | 64.5 | 68.6 |
| II | 3.6 | 56.9 | 73.1 | 66.6 | 81.4 | 95.7 |
| III | 89.1 | 96.6 | 99.0 | 97.4 | 99.1 | 99.8 |
| IV | 5.0 | 13.2 | 33.6 | 32.9 | 49.5 | 70.1 |
| V | 73.5 | 79.5 | 92.9 | 87.6 | 94.6 | 95.8 |
| VI | 71.4 | 91.9 | 98.0 | 94.1 | 99.3 | 100.0 |
| VII | 64.9 | 71.9 | 81.6 | 80.3 | 89.9 | 91.8 |
| 3,000 cycles of abrasion | |||
| Test series | U | p | Bonferroni-corrected significance |
| I versus II | 41.0 | 0.52052 | – |
| II versus III | 16.0 | 0.01133 | * |
| II versus IV | 39.0 | 0.42736 | – |
| IV versus V | 19.0 | 0.02114 | – |
| IV versus VI | 9.0 | 0.00220 | * |
| IV versus VII | 10.0 | 0.00283 | * |
| V versus VII | 37.0 | 0.34471 | – |
| VI versus VII | 48.5 | 0.93974 | – |
| VI versus III | 29.0 | 0.12123 | – |
| 30,000 cycles of abrasion | |||
| Test series | U | p | Bonferroni-corrected significance |
| I versus II | 22.0 | 0.03764 | – |
| II versus III | 4.0 | 0.00058 | * |
| II versus IV | 13.0 | 0.00580 | * |
| IV versus V | 0.0 | 0.00018 | * |
| IV versus VI | 0.0 | 0.00018 | * |
| IV versus VII | 2.0 | 0.00033 | * |
| V versus VII | 25.0 | 0.06402 | – |
| VI versus VII | 14.0 | 0.00729 | * |
| VI versus III | 36.0 | 0.30749 | – |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wieckiewicz, M.; Wolf, E.; Richter, G.; Meissner, H.; Boening, K. New Concept of Polymethyl Methacrylate (PMMA) and Polyethylene Terephthalate (PET) Surface Coating by Chitosan. Polymers 2016, 8, 132. https://doi.org/10.3390/polym8040132
Wieckiewicz M, Wolf E, Richter G, Meissner H, Boening K. New Concept of Polymethyl Methacrylate (PMMA) and Polyethylene Terephthalate (PET) Surface Coating by Chitosan. Polymers. 2016; 8(4):132. https://doi.org/10.3390/polym8040132
Chicago/Turabian StyleWieckiewicz, Mieszko, Eric Wolf, Gert Richter, Heike Meissner, and Klaus Boening. 2016. "New Concept of Polymethyl Methacrylate (PMMA) and Polyethylene Terephthalate (PET) Surface Coating by Chitosan" Polymers 8, no. 4: 132. https://doi.org/10.3390/polym8040132
APA StyleWieckiewicz, M., Wolf, E., Richter, G., Meissner, H., & Boening, K. (2016). New Concept of Polymethyl Methacrylate (PMMA) and Polyethylene Terephthalate (PET) Surface Coating by Chitosan. Polymers, 8(4), 132. https://doi.org/10.3390/polym8040132