Synthesis of Monodisperse Silica Particles Grafted with Concentrated Ionic Liquid-Type Polymer Brushes by Surface-Initiated Atom Transfer Radical Polymerization for Use as a Solid State Polymer Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Living Radical Polymerization
2.4. Preparation of Poly(DEMM-TFSI) by Radical Polymerization for GPC Analysis
2.5. PSiP/Ionic Liquid Composite Electrolyte Film Formation
3. Results and Discussion
3.1. ATRP of the Polymerizable Ionic Liquid, DEMM-TFSI
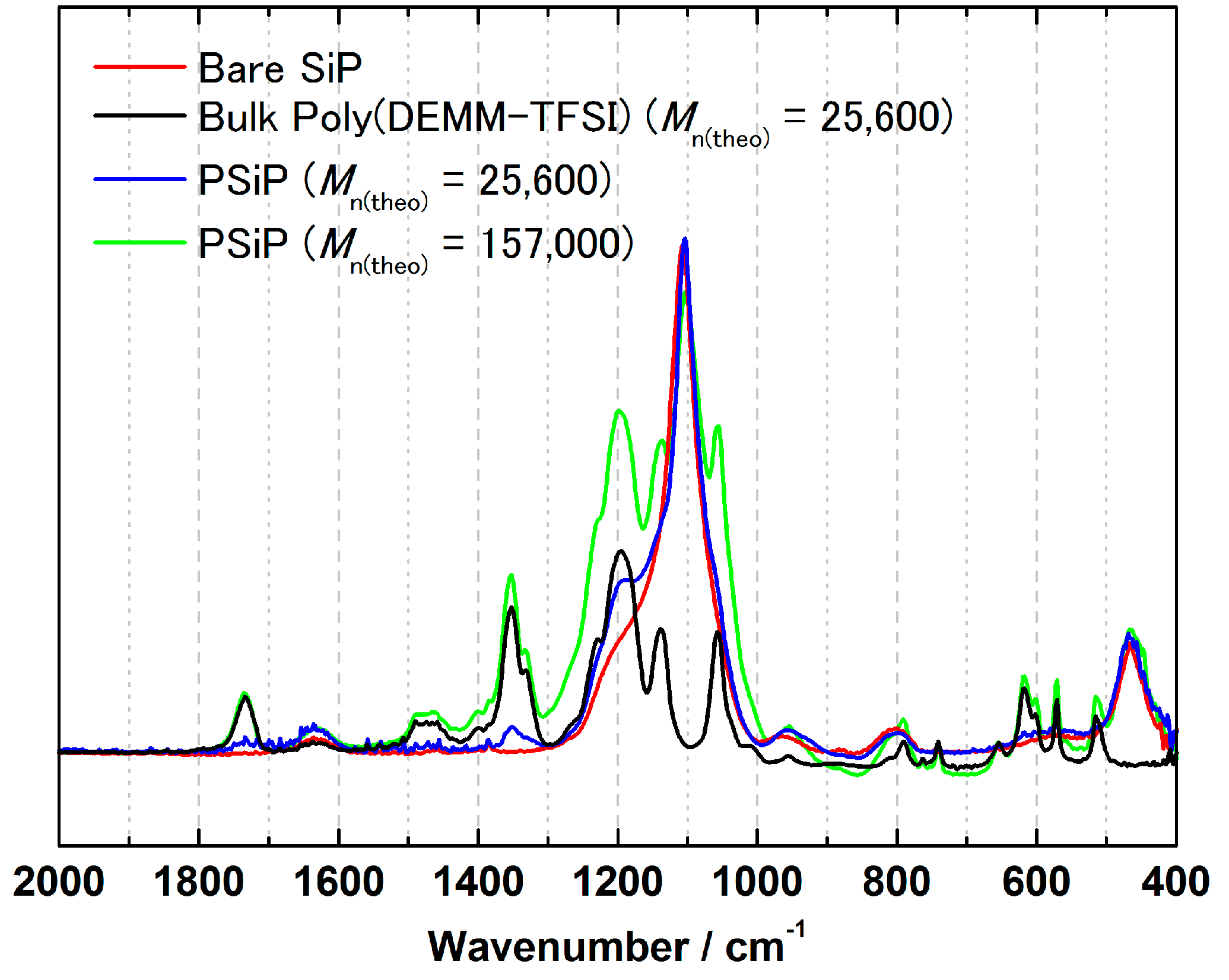
3.2. Surface-Initiated ATRP of Polymerizable Ionic Liquid, DEMM-TFSI
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DEMM-TFSI | N,N-diethyl-N-(2-methacryloylethyl)-N-methylammonium bis(trifluoromethyl-sulfonyl)imide |
| ATRP | Atom transfer radical polymerization |
| GPC | Gel permeation chromatography |
| SiP | Silica particle |
| Poly(IL) | Poly(ionic liquid) |
| IL | Ionic liquid |
| RAFT | Reversible addition-fragmentation chain-transfer |
| TERP | Organotellurium-mediated living radical polymerization |
| PSiP | Polymer/silica particle |
| CPB | Concentrated plymer brush |
| MALLS | Multi-angle laser light scattering |
| DEME-TFSI | N,N-diethyl-N-(2-methoxyethyl)-N–methyl-ammonium bis(trifl uoromethylsulfonyl)-imide (DEME-TFSI) |
| DSC | Differential scanning calorimetry |
| CTA | Charge transfer agent |
| PEG | Polyethylene glycol |
| SLS | Static light scattering |
| AIBN | Azobisisobutyronitrile |
References
- Sato, T.; Masuda, G.; Takagi, K. Electrochemical properties of novel ionic liquids for electric double layer capacitor applications. Electrochim. Acta 2004, 49, 3603–3611. [Google Scholar] [CrossRef]
- Sato, T.; Maruo, T.; Marukane, S.; Takagi, K. Ionic liquids containing carbonate solvent as electrolytes for lithium ion cells. J. Power Sources 2004, 138, 253–261. [Google Scholar] [CrossRef]
- Sato, T.; Marukane, S.; Narutomi, T.; Akao, T. High rate performance of a lithium polymer battery using a novel ionic liquid polymer composite. J. Power Sources 2006, 164, 390–396. [Google Scholar] [CrossRef]
- Shaplov, S.A.; Marcilla, R.; Mecerreyes, D. Recent advances in innovative polymer electrolytes based on poly(ionic liquid)s. Electrochim. Acta 2014, 175, 18–34. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids: Industrial Applications for Green Chemistry; ACS Symposium Series; American Chemical Society: San Diego, CA, USA, 2002. [Google Scholar]
- Ohno, H.; Yoshizawa, M.; Ogiwara, W. Development of new class of ion conductive polymers based on ionic liquid. Electrochim. Acta 2014, 50, 255–261. [Google Scholar] [CrossRef]
- Nakajima, H.; Ohno, H. Preparation of thermally stable polymer electrolytes from imidazolium-type ionic liquid derivatives. Polymer 2005, 46, 11499–11504. [Google Scholar] [CrossRef]
- Ogiwara, W.; Washiro, S.; Nakajima, H.; Ohno, H. Effect of cation structure on the electrochemical and thermal properties of ion conductive polymers obtained from polymerizable ionic liquids. Electrochim. Acta 2006, 51, 2614–2619. [Google Scholar] [CrossRef]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. J. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef]
- Susan, M.; Kaneko, T.; Noda, A.; Watanabe, M. Ion gels prepared by in situ radical polymerization of vinyl monomers in an ionic liquid and their characterization as polymer electrolytes. J. Am. Chem. Soc. 2005, 127, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Tang, H.; Radosz, M.; Shen, Y. Atom transfer radical polymerization of ionic liquid 2-(1-butylimidazolium-3-yl)ethyl methacrylate tetrafluoroborate. J. Polym. Sci. A Polym. Chem. 2004, 42, 5794–5801. [Google Scholar] [CrossRef]
- Tang, H.; Tang, J.; Ding, S.; Radosz, M.; Shen, Y. Atom transfer radical polymerization of styrenic ionic liquid monomers and carbon dioxide absorption of the polymerized ionic liquids. J. Polym. Sci. A Polym. Chem. 2005, 43, 1432–1443. [Google Scholar] [CrossRef]
- Hongkun, H.; David, L.; Hunaid, N.; Matyjaszewski, K. Synthesis of poly(ionic liquid)s by atom transfer radical polymerization with ppm of Cu catalyst. Macromolecules 2014, 47, 6601–6609. [Google Scholar]
- Hongkun, H.; Mingjiang, Z.; David, L.; Hunaid, N.; Matyjaszewski, K. Atom transfer radical polymerization of ionic liquid monomer: The influence of salt/counterion on polymerization. J. Polym. Sci. A Polym. Chem. 2014, 52, 2175–2184. [Google Scholar]
- Yuan, J.; Schlaad, H.; Giordano, C.; Antonietti, M. Double hydrophilic diblock copolymers containing a poly(ionic liquid) segment: Controlled synthesis, solution property, and application as carbon precursor. Eur. Polym. J. 2010, 47, 772–781. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nakanishi, K.; Yamago, S.; Tsujii, Y.; Takahashi, K.; Morinaga, T.; Sato, T. Controlled polymerization of protic ionic liquid monomer by ARGET-ATRP and TERP. Macromol. Rapid Commun. 2014, 35, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Morinaga, T.; Marukane, S.; Narutomi, T.; Igarashi, T.; Kawano, Y.; Ohno, K.; Fukuda, T.; Tsujii, Y. Novel solid-state polymer electrolyte of colloidal crystal decorated with ionic-liquid polymer brush. Adv. Mater. 2011, 23, 4868–4872. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Koh, K.; Tsujii, Y.; Fukuda, T. Synthesis of gold nanoparticles coated with well-defined high-density polymer brushes by surface-initiated living radical polymerization. Macromolecules 2002, 35, 8989–8993. [Google Scholar] [CrossRef]
- Matsuno, R.; Yamamoto, K.; Otsyka, H.; Takahara, A. Polystyrene- and poly(3-vinylpyridine) grafted magnetite nanoparticles prepared through surface-initiated nitroxide-mediated radical polymerization. Macromolecules 2004, 37, 2203–2209. [Google Scholar] [CrossRef]
- Kasseha, A.; Ait-Kadia, A.; Riedlb, B.; Pierson, J.F. Organic/inorganic hybrid composites prepared by polymerization compounding and controlled free radical polymerization. Polymer 2003, 44, 1367–1375. [Google Scholar] [CrossRef]
- Ohno, K.; Morinaga, T.; Koh, K.; Tsujii, Y.; Fukuda, T. Synthesis of monodisperse silica particles coated with well-defined, high-density polymer brushes by surface-initiated atom transfer radical polymerization. Macromolecules 2005, 38. [Google Scholar] [CrossRef]
- Morinaga, T.; Ohkura, M.; Ohno, K.; Tsujii, Y.; Fukuda, T. Monodisperse silica particles grafted with concentrated oxetane-carrying polymer brushes: their synthesis by surface-initiated atom transfer radical polymerization and use for fabrication of hollow spheres. Macromolecules 2007, 40, 1159–1164. [Google Scholar] [CrossRef]
- Sato, T.; Marukane, S.; Morinaga, T.; Kamijo, T.; Arafune, H.; Tsujii, Y. High voltage electric double layer capacitor using a novel solid-state polymer electrolyte. J. Power Sources 2015, 295, 108–116. [Google Scholar]
- Kubisa, P. Application of ionic liquids as solvents for polymerization processes. Prog. Polym. Sci. 2004, 29, 3–12. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Kwak, Y.; Braunecker, W.; Tsarevsky, V.N.; Coote, L.M.; Matyjaszewski, K. Understanding atom transfer radical polymerization: Effect of ligand and initiator structures on the equilibrium constants. J. Am. Chem. Soc. 2008, 130, 10702–10713. [Google Scholar] [CrossRef] [PubMed]
- Ohno, K.; Morinaga, T.; Takeno, S.; Tsujii, Y.; Fukuda, T. Suspensions of silica particles grafted with concentrated polymer brush: Effects of graft chain length on brush layer thickness and colloidal crystallization. Macromolecules 2007, 40, 9143–9150. [Google Scholar] [CrossRef]






| Entry | [AIBN]/[CTA] (by mol fraction) | Mn(MALLS) | Mw(MALLS)/Mn(MALLS) |
|---|---|---|---|
| 1 | 1/0.12 | 754,000 | 2.25 |
| 2 | 1/3 | 47,000 | 1.62 |
| 3 | 1/8 | 13,000 | 1.95 |
| Entry | Diameter of Silica Particle (nm) | Conv. (%) | Mn(theo) | Mw(PEG)/Mn(PEG) | Graft Density (Chains/nm2) |
|---|---|---|---|---|---|
| 1 | 130 | 99 | 25,600 | 1.11 | 0.15 |
| 2 | 130 | 99 | 157,000 | 1.09 | 0.21 |
| 3 | 290 | 98 | 66,000 | 1.17 | 0.15 |
| 4 | 1,550 | 90 | 285,000 | 1.17 | 0.17 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morinaga, T.; Honma, S.; Ishizuka, T.; Kamijo, T.; Sato, T.; Tsujii, Y. Synthesis of Monodisperse Silica Particles Grafted with Concentrated Ionic Liquid-Type Polymer Brushes by Surface-Initiated Atom Transfer Radical Polymerization for Use as a Solid State Polymer Electrolyte. Polymers 2016, 8, 146. https://doi.org/10.3390/polym8040146
Morinaga T, Honma S, Ishizuka T, Kamijo T, Sato T, Tsujii Y. Synthesis of Monodisperse Silica Particles Grafted with Concentrated Ionic Liquid-Type Polymer Brushes by Surface-Initiated Atom Transfer Radical Polymerization for Use as a Solid State Polymer Electrolyte. Polymers. 2016; 8(4):146. https://doi.org/10.3390/polym8040146
Chicago/Turabian StyleMorinaga, Takashi, Saika Honma, Takeo Ishizuka, Toshio Kamijo, Takaya Sato, and Yoshinobu Tsujii. 2016. "Synthesis of Monodisperse Silica Particles Grafted with Concentrated Ionic Liquid-Type Polymer Brushes by Surface-Initiated Atom Transfer Radical Polymerization for Use as a Solid State Polymer Electrolyte" Polymers 8, no. 4: 146. https://doi.org/10.3390/polym8040146
APA StyleMorinaga, T., Honma, S., Ishizuka, T., Kamijo, T., Sato, T., & Tsujii, Y. (2016). Synthesis of Monodisperse Silica Particles Grafted with Concentrated Ionic Liquid-Type Polymer Brushes by Surface-Initiated Atom Transfer Radical Polymerization for Use as a Solid State Polymer Electrolyte. Polymers, 8(4), 146. https://doi.org/10.3390/polym8040146









