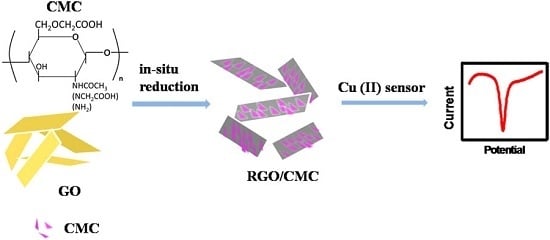
Preparation of Highly Dispersed Reduced Graphene Oxide Modified with Carboxymethyl Chitosan for Highly Sensitive Detection of Trace Cu(II) in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Instruments and Measurements
2.3. Preparation of RGO/CMC Composite
2.4. RGO/CMC/Nafion Modified GCE
2.4.1. Fabrication of Cu(II) Sensor by RGO/CMC/Nafion
2.4.2. Electrochemical Analysis
3. Results and Discussion
3.1. Characterizations of RGO/CMC
3.2. Electrochemical Detection of Cu(II)
3.3. Optimization of Detection Conditions
3.4. Anti-Interference of RGO/CMC/Nafion Modified GCE
3.5. Detection Limit of Cu(II) with RGO/CMC/Nafion Modified GCE
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
References
- Zhang, J.; Li, B.; Zhang, L.M.; Jiang, H. An optical sensor for Cu (II) detection with upconverting luminescent nanoparticles as an excitation source. Chem. Commun. 2012, 48, 4860–4862. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Li, Q.; Yue, Y.; Shao, S.J. Selective electrochemical determination of trace level copper using a salicylaldehyde azine/MWCNTs/Nafion modified pyrolytic graphite electrode by the anodic stripping voltammetric method. RSC Adv. 2015, 5, 3232–3238. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singh, L.P.; Singh, R.; Upadhyay, N.; Kaur, S.P.; Sethi, B. A novel copper (II) selective sensor based on dimethyl 4,4’(o-phenylene) bis (3-thioallophanate) in PVC matrix. J. Mol. Liq. 2012, 174, 11–16. [Google Scholar] [CrossRef]
- Ding, R.; Luo, Z.M.; Ma, X.L.; Fan, X.P.; Xue, L.Q.; Lin, X.Z.; Chen, S. High sensitive sensor fabricated by reduced graphene oxide/polyvinyl butyral nanofibers for detecting Cu (II) in water. Int. J. Anal. Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.Q.; Pu, S.Z.; Dai, Y.F. A novel colorimetric sensor based on a diarylethene derivative for selective detection of Cu (II). Anal. Methods 2015, 7, 3593–3599. [Google Scholar] [CrossRef]
- Park, G.J.; Hwang, I.H.; Song, E.J.; Kim, H.; Kim, C. A colorimetric and fluorescent sensor for sequential detection of copper ion and cyanide. Tetrahedron 2014, 70, 2822–2828. [Google Scholar] [CrossRef]
- Janegitz, B.C.; Marcolino-Junior, L.H.; Campana-Filho, S.P.; Faria, R.C.; Fatibello-Filho, O. Anodic stripping voltammetric determination of copper (II) using a functionalized carbon nanotubes paste electrode modified with crosslinked chitosan. Sens. Actuators B 2009, 142, 260–266. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, B.B.R. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Lin, H.; Li, M.X.; Mihailovič, D. Simultaneous determination of copper, lead, and cadmium ions at a Mo6S9-xIx nanowires modified glassy carbon electrode using differential pulse anodic stripping voltammetry. Electrochim. Acta 2015, 154, 184–189. [Google Scholar] [CrossRef]
- Afkhami, A.; Khoshsafar, H.; Madrakian, T.; Shirzadmehr, A. A new nano-composite electrode as a copper (II) selective potentiometric sensor. J. Iran. Chem. Soc. 2014, 11, 1373–1380. [Google Scholar] [CrossRef]
- Cantalapiedra, A.; Gismera, M.J.; Procopio, J.R.; Sevilla, M.T. Electrochemical sensor based on polystyrene sulfonate–carbon nanopowders composite for Cu (II) determination. Talanta 2015, 139, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gou, H.L.; Al-Ogaidi, I.; Wu, N.Q. Nanostructured sensors for detection of heavy metals: A review. ACS Sustain. Chem. Eng. 2013, 1, 713–723. [Google Scholar] [CrossRef]
- Afkhami, A.; Soltani-Felehgari, F.; Madrakian, T.; Ghaedi, T.; Rezaeivala, M. Fabrication and application of a new modified electrochemical sensor using nano-silica and a newly synthesized Schiff base for simultaneous determination of Cd2+, Cu2+ and Hg2+ ions in water and some foodstuff samples. Anal. Chim. Acta 2013, 771, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.H.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y.H. A graphene-based electrochemical sensor for sensitive detection of paracetamol. Talanta 2010, 81, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Gao, C.; Meng, F.L.; Li, H.H.; Wang, L.; Liu, J.H.; Huang, X.J. SnO2/reduced graphene oxide nanocomposite for the simultaneous electrochemical detection of cadmium(II), lead(II), copper(II), and mercury(II): An interesting favorable mutual interference. J. Phys. Chem. C 2011, 116, 1034–1041. [Google Scholar] [CrossRef]
- Le, T.X.H.; Bechelany, M.; Champavert, J.; Cretin, M. A highly active based graphene cathode for the electro-fenton reaction. RSC Adv. 2015, 5, 42536–42539. [Google Scholar] [CrossRef]
- Kong, N.; Liu, J.Q.; Kong, Q.S.; Wang, R.; Barrow, C.J.; Yang, W.R. Graphene modified gold electrode via π–π stacking interaction for analysis of Cu2+ and Pb2+. Sens. Actuators B 2013, 178, 426–433. [Google Scholar] [CrossRef]
- Wonsawat, W.; Chuanuwatanakul, S.; Dungchai, W.; Punrat, E.; Motomizu, S.; Chailapakul, O. Graphene-carbon paste electrode for cadmium and lead ion monitoring in a flow-based system. Talanta 2012, 100, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.H.; Cao, J.T.; Li, L.L.; Rana, R.K.; Zhu, J.J. Carboxymethyl chitosan-functionalized graphene for label-free electrochemical cytosensing. Carbon 2013, 51, 124–133. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, W.H.; Chang, Y.H. Preparation and properties of chitosan/carbon nanotube nanocomposites using poly(styrene sulfonic acid)-modified CNTs. Carbohydr. Polym. 2009, 76, 232–238. [Google Scholar] [CrossRef]
- Afkhami, A.; Moosavi, R.; Madrakian, T.; Keypour, H.; Ramezani-Aktij, A.; Mirzaei-Monsef, M. Construction and application of an electrochemical sensor for simultaneous determination of Cd(II), Cu(II) and Hg(II) in water and foodstuff samples. Electroanalysis 2014, 26, 786–795. [Google Scholar] [CrossRef]
- Bao, Q.L.; Zhang, H.; Yang, J.X.; Wang, S.; Tang, D.Y.; Jose, R.; Ramakrishna, S.; Lim, C.T.; Loh, K.P. Graphene-polymer nanofiber membrane for ultrafast photonics. Adv. Funct. Mater. 2010, 20, 782–791. [Google Scholar] [CrossRef]
- Le, T.X.H.; Bechelany, M.; Lacour, S.; Oturan, N.; Oturan, M.A.; Cretin, M. High removal efficiency of dye pollutants by electron-Fenton process using a graphene based cathode. Carbon 2015, 94, 1003–1011. [Google Scholar] [CrossRef]
- Sun, T.; Xu, P.X.; Liu, Q.; Xue, J.; Xie, W.M. Graft copolymerization of methacrylic acid onto carboxymethyl chitosan. Eur. Polym. J. 2003, 39, 189–192. [Google Scholar] [CrossRef]
- Tung, V.C.; Allen, M.J.; Yang, Y.; Kaner, R.B. High-throughput solution processing of large-scale graphene. Nat. Nanotechnol. 2009, 4, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.K.; Wang, X.R.; Liu, Y.; Wu, T.; Liu, Y.; Guo, Y.Q.; Li, R.Q.; Sun, X.Y.; Wu, F.; Li, C.B.; et al. Reduction of graphene oxide with l-lysine to prepare reduced graphene oxide stabilized with polysaccharide polyelectrolyte. J. Mater. Chem. A 2013, 1, 2192–2201. [Google Scholar] [CrossRef]
- Li, D.; Mueller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Villar-Rodil, S.; Paredes, J.I.; Martínez-Alonso, A.; Tascon, J.M.D. Preparation of graphene dispersions and graphene-polymer composites in organic media. J. Mater. Chem. 2009, 19, 3591–3593. [Google Scholar] [CrossRef]
- Wang, X.; Zhi, L.J.; Tsao, N.; Tomovic, Z.; Li, J.L.; Mullen, K. Transparent carbon films as electrodes in organic solar cells. Angew. Chem. 2008, 120, 3032–3034. [Google Scholar] [CrossRef]
- Dubin, S.; Gilje, S.; Wang, K.; Tung, V.C.; Cha, K.; Hall, A.S.; Farrar, J.; Varshneya, R.; Yang, Y.; Kaner, R.B. A one-step, solvothermal reduction method for producing reduced graphene oxide dispersions in organic solvents. ACS Nano 2010, 4, 3845–3852. [Google Scholar] [CrossRef] [PubMed]
- Paredes, J.I.; Villar-Rodil, S.; Solis-Fernandez, P.; Martinez-Alonso, A.; Tascon, J.M.D. Atomic force and scanning tunneling microscopy imaging of graphene nanosheets derived from graphite oxide. Langmuir 2009, 25, 5957–5968. [Google Scholar] [CrossRef] [PubMed]
- Han, D.X.; Han, T.T.; Shan, C.S.; Ivaska, A.; Niu, L. Simultaneous determination of ascorbic acid, dopamine and uric acid with chitosan-graphene modified electrode. Electroanalysis 2010, 22, 2001–2008. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Konkena, B.; Vasudevan, S. Covalently linked, water-dispersible, cyclodextrin: Reduced-graphene oxide sheets. Langmuir 2012, 28, 12432–12437. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, B.; Aparna, Y.; Sairam, M.; Hebalkar, N. Preparation and characterization of HAP/carboxymethyl chitosan nanocomposites. J. Appl. Polym. Sci. 2007, 105, 928–934. [Google Scholar] [CrossRef]
- Jin, M.H.; Kim, T.H.; Lim, S.C.; Duong, D.L.; Shin, H.J.; Jo, J.W.; Jeong, H.K.; Chang, J.; Xie, S.S.; Lee, Y.H. Facile physical route to highly crystalline graphene. Adv. Funct. Mater. 2011, 21, 3496–3501. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Functionalization of graphite oxide with magnetic chitosan for the preparation of a nanocomposite dye adsorbent. Langmuir 2013, 29, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Khomyakov, P.A.; Giovannetti, G.; Rusu, P.C.; Brocks, G.; Brink, J.V.D.; Kelly, P.J. First-principles study of the interaction and charge transfer between graphene and metals. Phys. Rev. B 2009, 79, 195425. [Google Scholar] [CrossRef]
- Sun, W.; Guo, Y.Q.; Ju, X.M.; Zhang, Y.Y.; Wang, X.Z.; Sun, Z.F. Direct electrochemistry of hemoglobin on graphene and titanium dioxide nanorods composite modified electrode and itselectrocatalysis. Biosens. Bioelectron. 2013, 42, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Guo, H.W.; Liu, E.; Yang, G.C.; Khun, N.W. Bismuth/polyaniline/glassy carbon electrodes prepared with different protocols for stripping voltammetric determination of trace Cd and Pb in solutions having surfactants. Electroanalysis 2010, 22, 209–215. [Google Scholar] [CrossRef]
- Lin, M.; Hu, X.; Ma, Z.; Chen, L. Functionalized polypyrrole nanotube arrays as electrochemical biosensor for the determination of copper ions. Anal. Chim. Acta 2012, 746, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.J.; Chai, Y.Q.; Yuan, R.; Zhao, Q.; Yang, C.L. Functionalized graphene oxide-based carbon paste electrode for potentiometric detection of copper ion(II). Anal. Methods 2012, 4, 3332–3337. [Google Scholar] [CrossRef]
- Qiu, S.Y.; Xie, L.D.; Gao, S.; Liu, Q.D.; Lin, Z.Y.; Qiu, B.; Chen, G.N. Determination of copper(II) in the dairy product by an electrochemical sensor based on click chemistry. Anal. Chim. Acta 2011, 707, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.H.; Dong, S.Y.; Gu, G.Z.; Huang, T.L. Simultaneous determination of Cd2+, Pb2+, Cu2+ and Hg2+ at a carbon paste electrode modified with ionic liquid-functionalized ordered mesoporous silica. Bull. Korean Chem. Soc. 2010, 31, 2949–2954. [Google Scholar] [CrossRef]
- Etienne, M.; Bessiere, J.; Walcarius, A. Voltammetric detection of copper(II) at a carbon paste electrode containing an organically modified silica. Sens. Actuators B 2001, 76, 531–538. [Google Scholar] [CrossRef]







| Supporting Electrolyte | Stripping Peak Current (μA) |
|---|---|
| KCl (0.1 mol·L−1) | 0.3670 |
| NaOH (0.1 mol·L−1) | No response |
| H2SO4 (0.1 mol·L−1) | 0.9271 |
| HCl (0.1 mol·L−1) | 1.056 |
| NaAc–HAc buffer solution | 1.568 |
| N | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| ip (μA) | 0.723 | 0.711 | 0.718 | 0.714 | 0.719 | 0.719 | 0.722 | 0.716 | 0.721 | 0.723 |
| Concentration (mol·L−1) | Species | Interference (%) |
|---|---|---|
| 5.0 × 10−4 | Na+ | −3.5 |
| K+ | −2.9 | |
| Ca2+ | −4.8 | |
| Mg2+ | −1.5 | |
| 1.0 × 10−4 | Mn2+ | −3.1 |
| Cd2+ | −4.6 | |
| 2.0 × 10−5 | Pb2+ | −4.9 |
| Zn2+ | −4.4 |
| Modifier | Electrode | Method | Detection Range (μmol·L−1) | Detection Limit (nmol·L−1) | Ref. |
|---|---|---|---|---|---|
| Tripeptide (Gly-Gly-His) | GCE | DPSV | 0.1–30 | 46 | [41] |
| AMT-g-NGO | CPE | SWASV | 0.1–1.0 × 105 | 40 | [42] |
| Graphene | Gold electrode | OSWV | 1.5 × 10−3–0.02 | 1.5 ± 0.2 | [17] |
| Propargyl-functionalized ferrocene | Gold electrode | DPV | 1.0 × 10−8–1.0 × 10−3 | 3.4 × 10−6 | [43] |
| Ionic liquid-functionalized orderd mesoporous silica SBA-15 | CPE | DPASV | 0.3–100 | 10 | [44] |
| Crosslinked chitosan | CNPE | LSASV | 0.079–16 | 10 | [7] |
| Silica | CPE | DPSV | 0.05–0.2 | 3 | [45] |
| RGO/CMC | GCE | DPASV | 0.02–1.2 | 3.25 | Present work |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Ding, R.; Ma, X.; Xue, L.; Lin, X.; Fan, X.; Luo, Z. Preparation of Highly Dispersed Reduced Graphene Oxide Modified with Carboxymethyl Chitosan for Highly Sensitive Detection of Trace Cu(II) in Water. Polymers 2016, 8, 78. https://doi.org/10.3390/polym8040078
Chen S, Ding R, Ma X, Xue L, Lin X, Fan X, Luo Z. Preparation of Highly Dispersed Reduced Graphene Oxide Modified with Carboxymethyl Chitosan for Highly Sensitive Detection of Trace Cu(II) in Water. Polymers. 2016; 8(4):78. https://doi.org/10.3390/polym8040078
Chicago/Turabian StyleChen, Sheng, Rui Ding, Xiuling Ma, Liqun Xue, Xiuzhu Lin, Xiaoping Fan, and Zhimin Luo. 2016. "Preparation of Highly Dispersed Reduced Graphene Oxide Modified with Carboxymethyl Chitosan for Highly Sensitive Detection of Trace Cu(II) in Water" Polymers 8, no. 4: 78. https://doi.org/10.3390/polym8040078
APA StyleChen, S., Ding, R., Ma, X., Xue, L., Lin, X., Fan, X., & Luo, Z. (2016). Preparation of Highly Dispersed Reduced Graphene Oxide Modified with Carboxymethyl Chitosan for Highly Sensitive Detection of Trace Cu(II) in Water. Polymers, 8(4), 78. https://doi.org/10.3390/polym8040078