Photothermal Effects and Applications of Polydimethylsiloxane Membranes with Carbon Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photothermal Effects in Polymer–Carbon Nanoparticle Composites
2.2. PDMS/CNP Membranes: Materials and Preparation
- (a)
- The required base material of the elastomer (silicone oil) is weighted inside of a glass beaker.
- (b)
- CNP is added in a concentration of 0.1% in weight compared to the total PDMS amount. For the PDMS+CNP mixture, the nanopowder is simply poured into the beaker; alternatively, chloroform is also added in a proportion of 0.75 mL per g of PDMS in order to obtain the mixture for the PDMS+CNP+CHCl3 samples.
- (c)
- The materials are mixed to evenly distribute the carbon nanopowder in the silicone oil. For the PDMS+CNP, mixing of the materials is achieved with the dispersing device operating for 5 min at a speed of 2800 rpm, and subsequently increasing the speed to 3600 rpm for 8 additional minutes. The second mixture, PDMS+CNP+CHCl3, yields a liquid solution due to the addition of chloroform; this is mixed using a magnetic bar activated by a hot plate with magnetic stirrer while heated at 150 °C until full evaporation of the CHCl3.
- (d)
- The curing agent is added with a 1:10 ratio compared to the base material and both parts are mixed by hand during two minutes.
- (e)
- Air captured during the mixing process is removed thru a degassing process.
- (f)
- The PDMS/CNP mixture is poured into a glass mold; this was fabricated from glass substrates and height spacers of approximately 420 μm. The excess of material is then removed using the doctor blade technique.
- (g)
- Solidification of the composites is achieved upon heating at 80 °C during 2 h.
- (h)
- After cooling at room temperature, the resulting PDMS/CNP composite is finally removed from the mold.
3. Results and Discussion
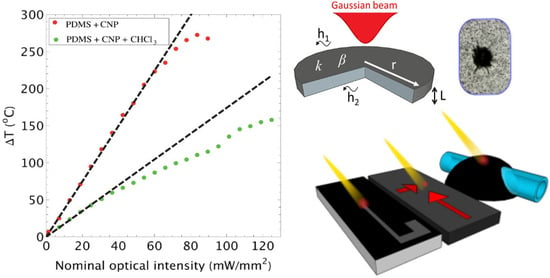
3.1. Photothermal Effects: Heat Generation and Transfer in the Composites
3.2. Structural and Mechanical Characterization of the Composites
3.3. Photomechanical Effects: Light Induced Stress
3.4. Applications
3.4.1. Coatings for Capillaries
3.4.2. Coating for Fiber Optic Devices
3.4.3. Laser Micropatterning
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, J.; van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide particles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Hu, L.; Gao, S.; Ding, X.; Wang, D.; Jiang, J.; Jin, J.; Jiang, L. Photothermo-responsive single-walled carbon nanotube-based ultrathin membranes for on/off switchable separation of oil-in-water nanoemulsions. ACS Nano 2015, 9, 4835–4842. [Google Scholar] [CrossRef] [PubMed]
- Nicoletta, F.P.; Cupelli, D.; Formoso, P.; De Filpo, G.; Colella, V.; Gugliuzza, A. Light responsive polymer membranes: A review. Membranes 2012, 2, 134–197. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Fleischman, A.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Alrifail, A.; Lindahl, O.A.; Ramser, K. Polymer-based microfluidic devices for pharmacy, biology and tissue engineering. Polymers 2012, 4, 1349–1398. [Google Scholar] [CrossRef]
- Seethapathy, S.; Górecki, T. Applications of polydimethylsiloxane in analytical chemistry: A review. Anal. Chim. Acta 2012, 750, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Vélez-Cordero, J.R.; Hernández-Cordero, J. Heat generation and conduction in PDMS-carbon nanoparticle membranes irradiated with optical fibers. Int. J. Therm. Sci. 2015, 96, 12–22. [Google Scholar] [CrossRef]
- Miyako, E.; Nagata, H.; Hirano, K.; Hirotsu, T. Carbon nanotube–polymer composite for light-driven microthermal control. Angew. Chem. Int. Ed. 2008, 47, 3610–3613. [Google Scholar] [CrossRef] [PubMed]
- Miyako, E.; Nagata, H.; Hirano, K.; Hirotsu, T. Laser-triggered carbon nanotube microdevice for remote control of biocatalytic reactions. Lab Chip 2009, 9, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Hautefeuille, M.; Cabriales, L.; Pimentel-Domínguez, R.; Velázquez, V.; Hernández-Cordero, J.; Oropeza-Ramos, L.; Rivera, M.; Carreón-Castro, M.P.; Grether, M.; López-Moreno, E. New perspectives for direct PDMS microfabrication using a CD-DVD laser. Lab Chip 2013, 13, 4848–4854. [Google Scholar] [CrossRef] [PubMed]
- Ahir, S.V.; Terentjev, E.M. Photomechanical actuation in polymer-nanotube composites. Nat. Mater. 2005, 4, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Arévalo, F.M.; Garnica-Palafox, I.M.; Jagdale, P.; Hernández-Cordero, J.; Rodil, S.; Okonkwo, A.; Robles Hernandez, F.; Tagliaferro, A. Photomechanical response of composites based on PDMS and carbon soot nanoparticles under IR laser irradiation. Opt. Mater. Express 2015, 5, 1792–1805. [Google Scholar] [CrossRef]
- Velázquez-Benítez, A.M.; Reyes-Medrano, M.; Vélez-Cordero, J.R.; Hernández-Cordero, J. Controlled deposition of polymer coatings on cylindrical photonic devices. J. Lightwave Technol. 2015, 33, 176–182. [Google Scholar] [CrossRef]
- Vélez-Cordero, J.R.; Velázquez-Benítez, A.M.; Hernández-Cordero, J. Thermocapillary flow in glass tubes coated with photoresponsive layers. Langmuir 2014, 30, 5326–5336. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Cordero, J.R.; Pérez Zúñiga, M.G.; Hernández-Cordero, J. An optopneumatic piston for microfluidics. Lab Chip 2015, 15, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Miyako, E.; Nagata, H.; Hirano, K.; Hirotsu, T. Micropatterned carbon nanotube-gel composite as photothermal material. Adv. Mater. 2009, 21, 2819–2823. [Google Scholar] [CrossRef]
- Tjahjono, I.K.; Bayazitoglu, Y. Near-infrared light heating of a slab by embedded nanoparticles. Int. J. Heat Mass Transfer 2008, 51, 1505–1515. [Google Scholar] [CrossRef]
- Modest, M.F. Radiative Heat Transfer, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Wang, J.; Chen, Y.; Li, R.; Dong, H.; Zhang, L.; Lotya, M.; Coleman, J.N.; Blau, W.J. Nonlinear optical properties of graphene and carbon nanotube composites. In Carbon Nanotubes—Synthesis, Characterization, Applications; Yellampalli, S., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Lax, M. Temperature rise induced by a laser beam. J. Appl. Phys. 1977, 48, 3919–3924. [Google Scholar] [CrossRef]
- Han, C.-W.; Birringer, R.; Clarke, D.R.; Gleiter, H. Effective thermal conductivity of particulate composites with interfacial thermal resistance. J. Appl. Phys. 1997, 81, 6692–6699. [Google Scholar]
- Hong, J.; Lee, J.; Hong, C.-K.; Shim, S.-E. Effect of dispersion state of carbon nanotube on the thermal conductivity of poly(dimethyl siloxane) composites. Curr. Appl. Phys. 2010, 10, 359–363. [Google Scholar] [CrossRef]
- Majeed, S.; Filiz, V.; Shishatskiy, S.; Wind, J.; Abetz, C.; Abetz, V. Pyrene-POSS nanohybrid as a dispersant for carbon nanotubes in solvents of various polarities: Its synthesis and application in the preparation of a composite membrane. Nanoscale Res. Lett. 2012, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, X.; Sun, X.; Lin, J.; Ji, W.; Tan, K.L. Electronic structure and optical limiting behavior of carbon nanotubes. Phys. Rev. Lett. 1999, 82, 2548–2551. [Google Scholar] [CrossRef]
- Kuo, A.C.M. Poly(dimethylsiloxane). In Polymer Data Handbook; Mark, J.E., Ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Camino, G.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 2001, 42, 2395. [Google Scholar] [CrossRef]
- Chenoweth, K.; Cheung, S.; van Duin, A.C.T.; Goddard III, W.A.; Kober, E.M. Simulations on the thermal decomposition of a poly(dimethylsiloxane) polymer using the reaxff reactive force field. J. Am. Chem. Soc. 2005, 127, 7192–7202. [Google Scholar] [CrossRef] [PubMed]
- Camino, G.; Lomakin, S.M.; Lageard, M. Thermal polydimethylsiloxane degradation. Part 2. The degradation mechanisms. Polymer 2002, 43, 2011. [Google Scholar] [CrossRef]
- Briones-Herrera, J.C.; Cuando-Espitia, N.; Sánchez-Arévalo, F.M.; Hernández-Cordero, J. Evaluation of mechanical behavior of soft tissue by means of random laser emission. Rev. Sci. Instrum. 2013, 84, 104301. [Google Scholar] [CrossRef] [PubMed]
- Loomis, J.; King, B.; Burkhead, T.; Xu, P.; Bessler, N.; Terentjev, E.; Panchapakesan, B. Graphene-nanoplatelet-based photomechanical actuators. Nanotechnology 2012, 23, 045501. [Google Scholar] [CrossRef] [PubMed]
- Loomis, J.; Fan, X.; Khosravi, F.; Xu, P.; Fletcher, M.; Cohn, R.W.; Panchapakesan, B. Graphene/elastomer composite-based photo-thermal nanopositioners. Sci. Rep. 2013, 3, 1900. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, J.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, J.M.; Stroock, A.D. Flexible methods for microfluidics. Phys. Today 2001, 54, 42–48. [Google Scholar] [CrossRef]
- Szczukiewicz, S.; Magnini, M.; Thome, J.R. Proposed models, ongoing experiments, and latest numerical simulations of microchannel two-phase flow boiling. Int. J. Multiph. Flow 2014, 59, 84–101. [Google Scholar] [CrossRef]
- Colchester, R.J.; Zhang, E.Z.; Mosse, C.A.; Beard, P.C.; Papakonstantinou, I.; Desjardins, A.E. Broadband miniature optical ultrasound probe for high resolution vascular tissue imaging. Biomed. Opt. Express. 2015, 6, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Colchester, R.J.; Mosse, C.A.; Bhachu, D.S.; Bear, J.C.; Carmalt, C.J.; Parkin, I.P.; Treeby, B.E.; Papakonstantinou, I.; Desjardins, A.E. Laser-generated ultrasound with optical fibres using functionalized carbon nanotube composite coatings. Appl. Phys. Lett. 2014, 104, 173502. [Google Scholar] [CrossRef]
- Velazquez-Benitez, A.M.; Hernández-Cordero, J. Optically controlled wavelength tunable fused fiber coupler. In Proceeding of the Workshop on Specialty Optical Fibers and their Applications 2013, Sigtuna, Sweden, 28–30 August 2013.
- Hill, K.O.; Meltz, G. Fiber Bragg grating technology fundamentals and overview. J. Lightwave Technol. 1997, 15, 1263–1276. [Google Scholar] [CrossRef]
- Cabriales, L.; Hautefeuille, M.; Fernández, G.; Velázquez, V.; Grether, M.; López-Moreno, E. Rapid fabrication of on-demand high-resolution optical masks with a CD–DVD pickup unit. Appl. Opt. 2014, 53, 1802–1807. [Google Scholar]










| Parameter | PDMS+CNP | PDMS+CNP+CHCl3 | Notes |
|---|---|---|---|
| L (µm) | 480 | 480 | As determined from direct measurements. |
| A (mm2) | 0.1465 | 0.1465 | Estimated using the fiber tip-membrane separation distance. |
| β (mm−1) | 4.4 | 2.8 | Determined from experiments (see below). |
| h1,2 (W/(m2·K)) | 28 | 28 | Typical value of heat convection in air. |
| k (W/(m·K)) | 0.26 | 0.6 | Typical value for pristine PDMS is 0.1678 at 14.7 °C and 12,500 cst [24]. |
| α (mm2/s) | 0.18 | 0.42 | Evaluated as α = k/ρ Cp, where Cp is the specific heat capacity and ρ is the materials density. |
| 0.04 | 0.04 | Estimated considering a refractive index of 1.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimentel-Domínguez, R.; Velázquez-Benítez, A.M.; Vélez-Cordero, J.R.; Hautefeuille, M.; Sánchez-Arévalo, F.; Hernández-Cordero, J. Photothermal Effects and Applications of Polydimethylsiloxane Membranes with Carbon Nanoparticles. Polymers 2016, 8, 84. https://doi.org/10.3390/polym8040084
Pimentel-Domínguez R, Velázquez-Benítez AM, Vélez-Cordero JR, Hautefeuille M, Sánchez-Arévalo F, Hernández-Cordero J. Photothermal Effects and Applications of Polydimethylsiloxane Membranes with Carbon Nanoparticles. Polymers. 2016; 8(4):84. https://doi.org/10.3390/polym8040084
Chicago/Turabian StylePimentel-Domínguez, Reinher, Amado M. Velázquez-Benítez, J. Rodrigo Vélez-Cordero, Mathieu Hautefeuille, Francisco Sánchez-Arévalo, and Juan Hernández-Cordero. 2016. "Photothermal Effects and Applications of Polydimethylsiloxane Membranes with Carbon Nanoparticles" Polymers 8, no. 4: 84. https://doi.org/10.3390/polym8040084