Comparison of the Physical and Mechanical Properties of Resin Matrix with Two Photoinitiator Systems in Dental Adhesives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Resin Components
2.2. Degree of Conversion (DC)
2.3. Three Point Bending Test
2.4. Microhardness
2.5. Ultimate Tensile Strength
2.6. Adhesive Resins with Selected Photoinitiators
2.7. Statistical Analysis
3. Results
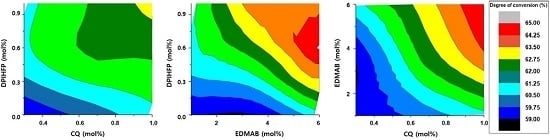
3.1. Degree of Conversion
3.2. Three Point Bending Test
3.3. Microhardness
3.4. Ultimate Tensile Strength
3.5. Contribution of Each Photoinitiator to the DC in the Selected Group
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, X.; Peng, Z.; Spencer, P.; Wang, Y. Effect of initiator on photopolymerization of acidic, aqueous dental model adhesives. J. Biomed. Mater. Res. A 2009, 90, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Greener, E.H. Effect of photoinitiator on degree of conversion of unfilled light-cured resin. J. Dent. 1994, 22, 296–299. [Google Scholar] [CrossRef]
- Harorli, O.T.; Bayindir, Y.Z.; Altunkaynak, Z.; Tatar, A. Cytotoxic effects of TEGDMA on THP-1 cells in vitro. Med. Oral Patol. Oral Cir. Bucal 2009, 14, e489–e493. [Google Scholar] [PubMed]
- Emmler, J.; Seiss, M.; Kreppel, H.; Reichl, F.X.; Hickel, R.; Kehe, K. Cytotoxicity of the dental composite component TEGDMA and selected metabolic by-products in human pulmonary cells. Dent. Mater. 2008, 24, 1670–1675. [Google Scholar] [PubMed]
- Stansbury, J.W.; Dickens, S.H. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent. Mater. 2001, 17, 71–79. [Google Scholar] [PubMed]
- Baroudi, K.; Saleh, A.M.; Silikas, N.; Watts, D.C. Shrinkage behaviour of flowable resin-composites related to conversion and filler-fraction. J. Dent. 2007, 35, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M. In vitro and in vivo studies on the toxicity of dental resin components: A review. Clin. Oral Investig. 2008, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; King, N.M.; Suh, B.I.; Pashley, D.H. Effect of delayed activation of light-cured resin composites on bonding of all-in-one adhesives. J. Adhes. Dent. 2001, 3, 207–225. [Google Scholar] [PubMed]
- Pfeifer, C.S.; Ferracane, J.L.; Sakaguchi, R.L.; Braga, R.R. Photoinitiator content in restorative composites: Influence on degree of conversion, reaction kinetics, volumetric shrinkage and polymerization stress. Am. J. Dent. 2009, 22, 206–210. [Google Scholar] [PubMed]
- Shin, D.H.; Rawls, H.R. Degree of conversion and color stability of the light curing resin with new photoinitiator systems. Dent. Mater. 2009, 25, 1030–1038. [Google Scholar] [PubMed]
- Schneider, L.F.; Consani, S.; Sakaguchi, R.L.; Ferracane, J.L. Alternative photoinitiator system reduces the rate of stress development without compromising the final properties of the dental composite. Dent. Mater. 2009, 25, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Musanje, L.; Ferracane, J.L.; Sakaguchi, R.L. Determination of the optimal photoinitiator concentration in dental composites based on essential material properties. Dent. Mater. 2009, 25, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.; Morisbak, E.; Tonnesen, H.H.; Bruzell, E.M. In vitro photosensitization initiated by camphorquinone and phenyl propanedione in dental polymeric materials. J. Photochem. Photobiol. B 2010, 100, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Chae, K.H.; Rawls, H.R. Development of a new photoinitiation system for dental light-cure composite resins. Dent. Mater. 1999, 15, 120–127. [Google Scholar] [CrossRef]
- Padon, K.S.; Scranton, A.B. Mechanistic investigation of a three-component radical photoinitiator system comprising methylene blue, N-methyldiethanolamine, and diphenyliodonium chloride. J. Polym. Sci. A Polym. Chem. 2000, 38, 2057–2066. [Google Scholar] [CrossRef]
- Lin, Y.; Stansbury, J.W. Kinetics studies of hybrid structure formation by controlled photopolymerization. Polymer 2003, 44, 4781–4789. [Google Scholar] [CrossRef]
- Leal, F.B.; Lima, G.S.; Collares, F.M.; Samuel, S.M.; Petzhold, C.L.; Piva, E.; Ogliari, F.A. Iodonium salt improves the dentin bonding performance in an experimental dental adhesive resin. Int. J. Adhes. Adhes. 2012, 38, 1–4. [Google Scholar] [CrossRef]
- Park, J.; Ye, Q.; Topp, E.M.; Misra, A.; Kieweg, S.L.; Spencer, P. Effect of photoinitiator system and water content on dynamic mechanical properties of a light-cured bisGMA/HEMA dental resin. J. Biomed. Mater. Res. A 2010, 93, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Alessi, S.; Calderaro, E.; Parlato, A.; Fuochi, P.; Lavalle, M.; Corda, U.; Dispenza, C.; Spadaro, G. Ionizing radiation induced curing of epoxy resin for advanced composites matrices. Nuclear Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2005, 236, 55–60. [Google Scholar] [CrossRef]
- Schroeder, W.F.; Cook, W.D.; Vallo, C.I. Photopolymerization of N,N-dimethylaminobenzyl alcohol as amine co-initiator for light-cured dental resins. Dent. Mater. 2008, 24, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.J.; Chae, K.H. Properties of 2,3-butanedione and 1-phenyl-1,2-propanedione as new photosensitizers for visible light cured dental resin composites. Polymer 2000, 41, 6205–6212. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevée, J.; Morlet-Savary, F.; Blanchard, N.; Fries, C.; Graff, B.; Allonas, X.; Louërat, F.; Fouassier, J.P. Near UV–visible light induced cationic photopolymerization reactions: A three component photoinitiating system based on acridinedione/silane/iodonium salt. Eur. Polym. J. 2010, 46, 2138–2144. [Google Scholar] [CrossRef]
- Cook, W.D.; Chen, F. Enhanced photopolymerization of dimethacrylates with ketones, amines, and iodonium salts: The CQ system. J. Polym. Sci. A Polym. Chem. 2011, 49, 5030–5041. [Google Scholar] [CrossRef]
- Cook, W.D.; Standish, P.M. Polymerization kinetics of resin-based restorative materials. J. Biomed. Mater. Res. 1983, 17, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, W.F.; Vallo, C.I. Effect of different photoinitiator systems on conversion profiles of a model unfilled light-cured resin. Dent. Mater. 2007, 23, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, F.; Xie, H.; Gu, N. Nanoparticle-reinforced resin-based dental composites. J. Dent. 2008, 36, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, F.C.; Daronch, M.; Rueggeberg, F.A.; Braga, R.R. Degree of conversion and mechanical properties of a BisGMA:TEGDMA composite as a function of the applied radiant exposure. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Beun, S.; Glorieux, T.; Devaux, J.; Vreven, J.; Leloup, G. Characterization of nanofilled compared to universal and microfilled composites. Dent. Mater. 2007, 23, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Yamauti, M.; Sauro, S.; Watson, T.F.; Toledano, M. Experimental resin cements containing bioactive fillers reduce matrix metalloproteinase-mediated dentin collagen degradation. J. Endod. 2012, 38, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Weir, M.D.; Giuseppetti, A.A.; Chow, L.C.; Antonucci, J.M.; Xu, H.H. Long-term mechanical durability of dental nanocomposites containing amorphous calcium phosphate nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Giannini, M.; Liberti, M.S.; Arrais, C.A.; Reis, A.F.; Mettenburg, D.; Rueggeberg, F.A. Influence of filler addition, storage medium and evaluation time on biaxial flexure strength and modulus of adhesive systems. Acta Odontol. Scand. 2012, 70, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Saku, S.; Kotake, H.; Scougall-Vilchis, R.J.; Ohashi, S.; Hotta, M.; Horiuchi, S.; Hamada, K.; Asaoka, K.; Tanaka, E.; Yamamoto, K. Antibacterial activity of composite resin with glass-ionomer filler particles. Dent. Mater. J. 2010, 29, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Carvalho, C.A.; Goracci, C.; Antoniolli, F.; Mazzoni, A.; Mazzotti, G.; Cadenaro, M.; Breschi, L. Influence of luting material filler content on post cementation. J. Dent. Res. 2009, 88, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Cho, B.H.; Lee, I.B.; Um, C.M.; Lim, B.S.; Oh, M.H.; Chang, C.G.; Son, H.H. Effect of the hydrophilic nanofiller loading on the mechanical properties and the microtensile bond strength of an ethanol-based one-bottle dentin adhesive. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, R.H.; Erickson, R.L.; Davidson, C.L. The effect of filler and silane content on conversion of resin-based composite. Dent. Mater. 2003, 19, 327–333. [Google Scholar] [CrossRef]
- Rodrigues, S.A., Jr.; Scherrer, S.S.; Ferracane, J.L.; Della Bona, A. Microstructural characterization and fracture behavior of a microhybrid and a nanofill composite. Dent. Mater. 2008, 24, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Turssi, C.P.; Ferracane, J.L.; Vogel, K. Filler features and their effects on wear and degree of conversion of particulate dental resin composites. Biomaterials 2005, 26, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Ogliari, F.A.; Ely, C.; Petzhold, C.L.; Demarco, F.F.; Piva, E. Onium salt improves the polymerization kinetics in an experimental dental adhesive resin. J. Dent. 2007, 35, 583–587. [Google Scholar] [CrossRef] [PubMed]






| CQ (mol%) | DMAEMA (mol%) | EDMAB (mol%) | DPIHFP (mol%) | |
|---|---|---|---|---|
| Group 1 | 1.0 | 1.0 | - | - |
| Group 2 | 1.0 | - | 1.0 | - |
| Group 3 | 1.0 | - | - | 1.0 |
| Group 4 | 1.0 | 1.0 | - | 1.0 |
| Group 5 | 1.0 | - | 1.0 | 1.0 |
| CQ (mol%) | EDMAB (mol%) | DPIHFP (mol%) | |
|---|---|---|---|
| I | 0.30 | 1.00 | 0.00 |
| II | 0.30 | 1.00 | 0.50 |
| III | 0.30 | 1.00 | 1.00 |
| IV | 0.30 | 3.50 | 0.00 |
| V | 0.30 | 3.50 | 0.50 |
| VI | 0.30 | 3.50 | 1.00 |
| VII | 0.30 | 6.00 | 0.00 |
| VIII | 0.30 | 6.00 | 0.50 |
| IX | 0.30 | 6.00 | 1.00 |
| X | 0.65 | 1.00 | 0.00 |
| XI | 0.65 | 1.00 | 0.50 |
| XII | 0.65 | 1.00 | 1.00 |
| XIII | 0.65 | 3.50 | 0.00 |
| XIV | 0.65 | 3.50 | 0.50 |
| XV | 0.65 | 3.50 | 1.00 |
| XVI | 0.65 | 6.00 | 0.00 |
| XVII | 0.65 | 6.00 | 0.50 |
| XVIII | 0.65 | 6.00 | 1.00 |
| XIX | 1.00 | 1.00 | 0.00 |
| XX | 1.00 | 1.00 | 0.50 |
| XXI | 1.00 | 1.00 | 1.00 |
| XXII | 1.00 | 3.50 | 0.00 |
| XXIII | 1.00 | 3.50 | 0.50 |
| XXIV | 1.00 | 3.50 | 1.00 |
| XXV | 1.00 | 6.00 | 0.00 |
| XXVI | 1.00 | 6.00 | 0.50 |
| XXVII | 1.00 | 6.00 | 1.00 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Suh, B.-I.; Shin, D.; Kim, K.-M. Comparison of the Physical and Mechanical Properties of Resin Matrix with Two Photoinitiator Systems in Dental Adhesives. Polymers 2016, 8, 250. https://doi.org/10.3390/polym8070250
Kim M, Suh B-I, Shin D, Kim K-M. Comparison of the Physical and Mechanical Properties of Resin Matrix with Two Photoinitiator Systems in Dental Adhesives. Polymers. 2016; 8(7):250. https://doi.org/10.3390/polym8070250
Chicago/Turabian StyleKim, Mijoo, Byoung-In Suh, Daehwan Shin, and Kwang-Mahn Kim. 2016. "Comparison of the Physical and Mechanical Properties of Resin Matrix with Two Photoinitiator Systems in Dental Adhesives" Polymers 8, no. 7: 250. https://doi.org/10.3390/polym8070250