Study of Water-Based Lithium Titanate Electrode Processing: The Role of pH and Binder Molecular Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrode Processing
2.2. Electrode Characterization
2.3. Electrochemical Characterization
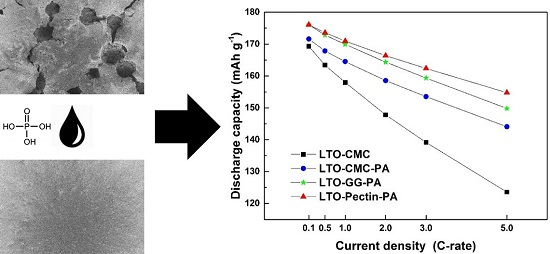
3. Results and Discussion
3.1. Thermal Stability
3.2. Electrode Surface Characterization
3.3. Adhesion Strength
3.4. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Chou, S.; Pan, Y.; Wang, J.; Liu, H.; Dou, S. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, N.; Bresser, D.; Passerini, S. Secondary Lithium-Ion Battery Anodes: From First Commercial Batteries to Recent Research Activities. Johnson Matthey Technol. Rev. 2015, 59, 34–44. [Google Scholar] [CrossRef]
- Kim, G.-T.; Löffler, N.; Doberdo, I.; Laszczynski, N.; Bresser, D.; Passerini, S. Method for Producing an Electrode for a Lithium-Ion Battery. U.S. Patent No. WO 2015/062985 A1, 7 May 2015. [Google Scholar]
- Loeffler, N.; Kim, G.-T.; Mueller, F.; Diemant, T.; Kim, J.-K.; Behm, R.J.; Passerini, S. In Situ Coating of Li[Ni0.33Mn0.33Co0.33]O2 Particles to Enable Aqueous Electrode Processing. ChemSusChem 2016, 9, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, N.; Ramos, A.; Cameán, I.; Antuna, C.; García, A.B. Hydrocolloids as binders for graphite anodes of lithium-ion batteries. Electrochim. Acta 2015, 155, 140–147. [Google Scholar] [CrossRef]
- Kuruba, R.; Kanchan, M.; Damodaran, K.; Jampani, P.H.; Gattu, B.; Patel, P.P.; Shanthi, P.M.; Damle, S.; Kumta, P.N. Guar gum: Structural and electrochemical characterization of natural polymer based binder for silicon e carbon composite rechargeable Li-ion battery anodes. J. Power Sources 2015, 298, 331–340. [Google Scholar] [CrossRef]
- Dufficy, M.K.; Khan, S.A.; Fedkiw, P.S. Galactomannan binding agents for silicon anodes in Li-ion batteries. J. Mater. Chem. A 2015, 3, 12023–12030. [Google Scholar] [CrossRef]
- Lee, B.-R.; Kim, S.-J.; Oh, E.-S. Bio-Derivative Galactomannan Gum Binders for Li4Ti5O12 Negative Electrodes in Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A2128–A2132. [Google Scholar] [CrossRef]
- Yoon, D.; Hwang, C.; Kang, N.; Lee, U.; Ahn, D.; Kim, J.; Song, H. Dependency of electrochemical performances of silicon lithium ion batteries on glycosidic linkages of polysaccharide binders. ACS Appl. Mater. Interfaces 2016, 8, 4042–4047. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Preparation and physicochemical evaluation of chitosan/poly(vinyl alcohol)/pectin ternary film for food-packaging applications. Carbohydr. Polym. 2010, 79, 711–716. [Google Scholar] [CrossRef]
- Loeffler, N.; Von Zamory, J.; Laszczynski, N.; Doberdo, I.; Kim, G.T.; Passerini, S. Performance of LiNi1/3Mn1/3Co1/3O2/graphite batteries based on aqueous binder. J. Power Sources 2014, 248, 915–922. [Google Scholar] [CrossRef]
- Carvalho, D.V.; Loeffler, N.; Kim, G.T.; Passerini, S. High temperature stable separator for lithium batteries based on SiO2 and hydroxypropyl guar gum. Membranes 2015, 5, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Lee, J.T.; Tung, Y.L.; Yang, C.R. Effects of pH on the dispersion and cell performance of LiCoO2 cathodes based on the aqueous process. J. Mater. Sci. 2007, 42, 5773–5777. [Google Scholar] [CrossRef]
- Haselrieder, W.; Westphal, B.; Bockholt, H.; Diener, A.; Höft, S.; Kwade, A. Measuring the coating adhesion strength of electrodes for lithium-ion batteries. Int. J. Adhes. Adhes. 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Zhang, T.; Li, J.-T.; Huang, L.; Sun, S.-G. A Robust Ion-Conductive Biopolymer as a Binder for Si Anodes of Lithium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 3599–3605. [Google Scholar] [CrossRef]
- Sudhakar, Y.N.; Selvakumar, M.; Bhat, D.K. Tubular array, dielectric, conductivity and electrochemical properties of biodegradable gel polymer electrolyte. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2014, 180, 12–19. [Google Scholar] [CrossRef]





© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, D.V.; Loeffler, N.; Kim, G.-T.; Marinaro, M.; Wohlfahrt-Mehrens, M.; Passerini, S. Study of Water-Based Lithium Titanate Electrode Processing: The Role of pH and Binder Molecular Structure. Polymers 2016, 8, 276. https://doi.org/10.3390/polym8080276
Carvalho DV, Loeffler N, Kim G-T, Marinaro M, Wohlfahrt-Mehrens M, Passerini S. Study of Water-Based Lithium Titanate Electrode Processing: The Role of pH and Binder Molecular Structure. Polymers. 2016; 8(8):276. https://doi.org/10.3390/polym8080276
Chicago/Turabian StyleCarvalho, Diogo Vieira, Nicholas Loeffler, Guk-Tae Kim, Mario Marinaro, Margret Wohlfahrt-Mehrens, and Stefano Passerini. 2016. "Study of Water-Based Lithium Titanate Electrode Processing: The Role of pH and Binder Molecular Structure" Polymers 8, no. 8: 276. https://doi.org/10.3390/polym8080276
APA StyleCarvalho, D. V., Loeffler, N., Kim, G. -T., Marinaro, M., Wohlfahrt-Mehrens, M., & Passerini, S. (2016). Study of Water-Based Lithium Titanate Electrode Processing: The Role of pH and Binder Molecular Structure. Polymers, 8(8), 276. https://doi.org/10.3390/polym8080276