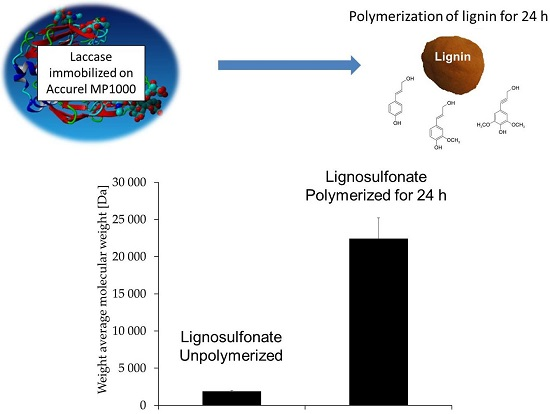
Polymerization of Various Lignins via Immobilized Myceliophthora thermophila Laccase (MtL)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzyme Activity
2.3. Protein Concentration
2.4. Immobilization of Laccases on Polymeric Supports
2.5. Polymerization of Lignin with Immobilized Enzyme
2.6. Fluorescence Intensity and Phenol Content Measurements
2.7. Size Exclusion Chromatography
3. Results and Discussion
3.1. Laccase Immobilization
3.2. Lignin Polymerization
Fluorescence Intensity and Phenol Content
3.3. Oxygen Consumption during Lignin Polymerization
3.4. Molecular Weight Changes during Polymerization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MTL | Myceliophthora thermophila laccase |
| PP | polypropylene |
| RFU | relative fluorescence units |
| SEC | size exclusion chromatography |
| FC | Folin–Cioulteau |
| NaPi | sodium phosphate |
| BCA | bicinchoninic acid |
| kat | katal |
| ABTS | 2,2′-Azino-di-(3-ethylbenzthiazolin-6-sulfonsäure) |
| KL | Kraft lignin |
| LS | lignosulfonate |
| MtL–PP | Myceliophthora thermophila laccase immobilized on polypropylene beads |
References
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Crestini, C.; Crucianelli, M.; Orlandi, M.; Saladino, R. Oxidative strategies in lignin chemistry: A new environmental friendly approach for the functionalisation of lignin and lignocellulosic fibers. Catal. Today 2010, 156, 8–22. [Google Scholar] [CrossRef] [Green Version]
- Areskogh, D.; Li, J.; Gellerstedt, G.; Henriksson, G. Investigation of the Molecular Weight Increase of Commercial Lignosulfonates by Laccase Catalysis. Biomacromolecules 2010, 11, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, M.; Mollah, A.; Palta, P.; Hess, T.R.; Vempati, R.K.; Cocke, D.L. Chemical and physical effects of sodium lignosulfonate superplasticizer on the hydration of portland cement and solidification/stabilization consequences. Cem. Concr. Res. 1995, 25, 671–682. [Google Scholar] [CrossRef]
- Nugroho Prasetyo, E.; Kudanga, T.; Østergaard, L.; Rencoret, J.; Gutiérrez, A.; del Río, J.C.; Ignacio Santos, J.; Nieto, L.; Jiménez-Barbero, J.; Martínez, A.T.; et al. Polymerization of lignosulfonates by the laccase-HBT (1-hydroxybenzotriazole) system improves dispersibility. Bioresour. Technol. 2010, 101, 5054–5062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouveia, S.; Fernández-Costas, C.; Sanromán, M.A.; Moldes, D. Enzymatic polymerisation and effect of fractionation of dissolved lignin from Eucalyptus globulus Kraft liquor. Bioresour. Technol. 2012, 121, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ortner, A.; Huber, D.; Haske-Cornelius, O.; Weber, H.K.; Hofer, K.; Bauer, W.; Nyanhongo, G.S.; Guebitz, G.M. Laccase mediated oxidation of industrial lignins: Is oxygen limiting? Process Biochem. 2015, 50, 1277–1283. [Google Scholar] [CrossRef]
- Huber, D.; Ortner, A.; Daxbacher, A.; Nyanhongo, G.S.; Bauer, W.; Guebitz, G.M. Influence of oxygen and mediators on laccase catalyzed polymerization of lignosulfonate. ACS Sustain. Chem. Eng. 2016. [Google Scholar] [CrossRef]
- Fiţigău, I.F.; Peter, F.; Boeriu, C.G. Oxidative polymerization of lignins by laccase in water-acetone mixture. Acta Biochim. Pol. 2013, 60, 817–822. [Google Scholar] [PubMed]
- Durán, N.; Rosa, M.A.; D’Annibale, A.; Gianfreda, L. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: A review. Enzym. Microb. Technol. 2002, 31, 907–931. [Google Scholar] [CrossRef]
- Misra, N.; Kumar, V.; Goel, N.K.; Varshney, L. Laccase immobilization on radiation synthesized epoxy functionalized polyethersulfone beads and their application for degradation of acid dye. Polymer 2014, 55, 6017–6024. [Google Scholar] [CrossRef]
- Hublik, G.; Schinner, F. Characterization and immobilization of the laccase from Pleurotus ostreatus and its use for the continuous elimination of phenolic pollutants. Enzym. Microb. Technol. 2000, 27, 330–336. [Google Scholar] [CrossRef]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanefeld, U.; Gardossi, L.; Magner, E. Understanding enzyme immobilisation. Chem. Soc. Rev. 2009, 38, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Pellis, A.; Ferrario, V.; Zartl, B.; Brandauer, M.; Gamerith, C.; Herrero Acero, E.; Ebert, C.; Gardossi, L.; Guebitz, G.M. Enlarging the tools for efficient enzymatic polycondensation: Structural and catalytic features of cutinase 1 from Thermobifida cellulosilytica. Catal. Sci. Technol. 2016, 6, 3430–3442. [Google Scholar] [CrossRef] [Green Version]
- Greimel, K.J.; Perz, V.; Koren, K.; Feola, R.; Temel, A.; Sohar, C.; Acero, E.H.; Klimant, I.; Guebitz, G.M. Banning toxic heavy-metal catalysts from paints: Enzymatic cross-linking of alkyd resins. Green Chem. 2013, 15, 381–388. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; de Mello, J.C.P. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.A.; Radotic, K. Fluorescence lifetime imaging of lignin autofluorescence in normal and compression wood. J. Microsc. 2013, 251, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Silva, C.J.; Zille, A.; Guebitz, G.M.; Cavaco-Paulo, A. Laccase immobilization on enzymatically functionalized polyamide 6,6 fibres. Enzym. Microb. Technol. 2007, 41, 867–875. [Google Scholar] [CrossRef] [Green Version]
- Yinghui, D.; Qiuling, W.; Shiyu, F. Laccase stabilization by covalent binding immobilization on activated polyvinyl alcohol carrier. Lett. Appl. Microbiol. 2002, 35, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.S.; Kalia, V.C.; Choi, J.-H.; Haw, J.-R.; Kim, I.-W.; Lee, J.K. Immobilization of laccase on SiO nanocarriers improves its stability and reusability. J. Microbiol. Biotechnol. 2014, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Crestini, C.; Perazzini, R.; Saladino, R. Oxidative functionalisation of lignin by layer-by-layer immobilised laccases and laccase microcapsules. Appl. Catal. A Gen. 2010, 372, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Albinsson, B.; Li, S.; Lundquist, K.; Stomberg, R. The origin of lignin fluorescence. J. Mol. Struct. 1999, 508, 19–27. [Google Scholar] [CrossRef]
- Kudanga, T.; Prasetyo, E.N.; Sipilä, J.; Guebitz, G.M.; Nyanhongo, G.S. Reactivity of long chain alkylamines to lignin moieties: Implications on hydrophobicity of lignocellulose materials. J. Biotechnol. 2010, 149, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, V.; Lele, S.S. Laccase: Properties and Applications. Bioresources 2009, 4, 1694–1717. [Google Scholar]
- Bourbonnais, R.; Paice, M.; Reid, I.; Lanthier, P.; Yaguchi, M. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl. Environ. Microbiol. 1995, 61, 1876–1880. [Google Scholar] [PubMed]
- Berka, R.; Schneider, P.; Golightly, E.; Brown, S.; Madden, M.; Brown, K.; Halkier, T.; Mondorf, K.; Xu, F. Characterization of the gene encoding an extracellular laccase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl. Envir. Microbiol. 1997, 63, 3151–3157. [Google Scholar]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Gierer, J. Chemical aspects of kraft pulping. Wood Sci. Technol. 1980, 14, 241–266. [Google Scholar] [CrossRef]
- Bento, I.; Silva, C.S.; Chen, Z.; Martins, L.O.; Lindley, P.F.; Soares, C.M. Mechanisms underlying dioxygen reduction in laccases. Structural and modelling studies focusing on proton transfer. BMC Struct. Biol. 2010, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Crestini, C.; Argyropoulos, D.S. The early oxidative biodegradation steps of residual kraft lignin models with laccase. Bioorg. Med. Chem. 1998, 6, 2161–2169. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Ferrario, V.; Ribitsch, D.; Guebitz, G.M.; Gardossi, L. The Closure of the Cycle: Enzymatic Synthesis and Functionalization of Bio-Based Polyesters. Trends Biotechnol. 2016, 34, 316–328. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huber, D.; Pellis, A.; Daxbacher, A.; Nyanhongo, G.S.; Guebitz, G.M. Polymerization of Various Lignins via Immobilized Myceliophthora thermophila Laccase (MtL). Polymers 2016, 8, 280. https://doi.org/10.3390/polym8080280
Huber D, Pellis A, Daxbacher A, Nyanhongo GS, Guebitz GM. Polymerization of Various Lignins via Immobilized Myceliophthora thermophila Laccase (MtL). Polymers. 2016; 8(8):280. https://doi.org/10.3390/polym8080280
Chicago/Turabian StyleHuber, Daniela, Alessandro Pellis, Andreas Daxbacher, Gibson S. Nyanhongo, and Georg M. Guebitz. 2016. "Polymerization of Various Lignins via Immobilized Myceliophthora thermophila Laccase (MtL)" Polymers 8, no. 8: 280. https://doi.org/10.3390/polym8080280