Additive Manufacture of Three Dimensional Nanocomposite Based Objects through Multiphoton Fabrication
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Approach
2.2. Multiphoton Fabrication
2.3. Material Preparation
3. Results
3.1. Fabrication of 2D and 3D Polymeric Objects
3.2. The Effect of Adding Gold Salt on the Integrity of Structures
3.3. The Effect of Adding a Two Photon Sensitive Dye on the Integrity of Structures
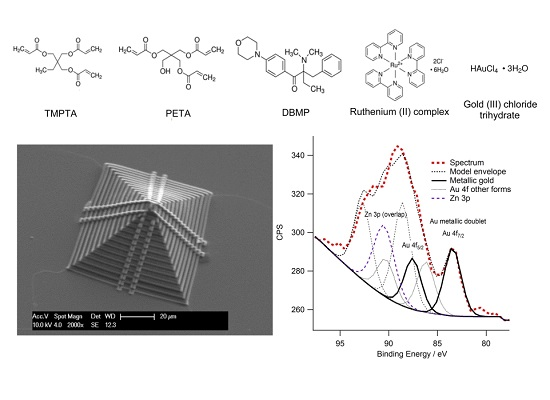
3.4. Characterisation of the Presence of Gold within Gold-Polymer Nanocomposites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2D | 2-dimensional |
| 3D | 3-dimensional |
| AM | Additive Manufacturing |
| BSE | Backscattered electron |
| DBMP | 2-benzyl-2-(dimethylamino)-4′-morpholinobutyrophenone |
| EDX | Energy-dispersive X-ray |
| MF | Multiphoton fabrication |
| PETA | Pentaerythritol triacrylate |
| PGMEA | Propylene glycol monomethyl ether acetate |
| PVA | Polyvinyl alcohol |
| SEM | Scanning electron microscopy |
| TMPTA | Trimethylopropane triacrylate |
| TPL | Two-photon lithography |
| XPS | X-ray photon spectroscopy |
References
- Hopkinson, N.; Hague, R.; Dickens, P. Rapid Manufacturing: An Industrial Revolution for the Digital Age; John Wiley & Sons: West Sussex, UK, 2006; pp. 1–5. [Google Scholar]
- Kaneko, K.; Sun, H.-B.; Duan, X.-M.; Kawata, S. Two-photon photoreduction of metallic nanoparticle gratings in a polymer matrix. Appl. Phys. Lett. 2003, 83, 1426–1428. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, R.H.; Yang, D.-Y.; Park, S.H. Advances in 3D nano/microfabrication using two-photon initiated polymerization. Prog. Polym. Sci. 2008, 33, 631–681. [Google Scholar] [CrossRef]
- Shukla, S.; Furlani, E.P.; Vidal, X.; Swihart, M.T.; Prasad, P.N. Two-photon lithography of sub-wavelength metallic structures in a polymer matrix. Adv. Mater. 2010, 22, 3695–3699. [Google Scholar] [CrossRef] [PubMed]
- Formanek, F.; Takeyasu, N.; Tanaka, T.; Chiyoda, K.; Ishikawa, A.; Kawata, S. Selective electroless plating to fabricate complex three-dimensional metallic micro/nanostructures. Appl. Phys. Lett. 2006, 88, 0831101–0831103. [Google Scholar] [CrossRef]
- Maruo, S.; Nakamura, O.; Kawata, S. Three-dimensional microfabrication with two-photon-absorbed photopolymerization. Opt. Lett. 1997, 22, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Farsari, M.; Vamvakaki, M.; Chichkov, B.N. Multiphoton polymerization of hybrid materials. J. Opt. 2010, 12, 1–16. [Google Scholar] [CrossRef]
- Xianzi, D.; Zhensheng, Z.; Xuanming, D. Improving spatial resolution and reducing aspect ratio in multiphoton polymerization nanofabrication. Appl. Phys. Lett. 2008, 92, 0911131–0911133. [Google Scholar]
- Deubel, M.; Wegener, M.; Linden, S.; von Freymann, G.; John, S. 3D-2D-3D photonic crystal heterostructures fabricated by direct laser writing. Opt. Lett. 2006, 31, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Galajda, P.; Ormos, P. Complex micromachines produced and driven by light. Appl. Phys. Lett. 2001, 78, 249–251. [Google Scholar] [CrossRef]
- Park, S.H.; Yang, D.Y.; Lee, K.S. Two-photon stereolithography for realizing ultraprecise three-dimensional nano/microdevices. Laser Photon. Rev. 2009, 3, 1–11. [Google Scholar] [CrossRef]
- Tanaka, T.; Ishikawa, A.; Kawata, S. Two-photon-induced reduction of metal ions for fabricating three-dimensional electrically conductive metallic microstructure. Appl. Phys. Lett. 2006, 88, 0811071–0811073. [Google Scholar] [CrossRef]
- Albota, M.; Beljonne, D.; Brédas, J.-L.; Ehrlich, J.E.; Fu, J.-Y.; Heikal, A.A.; Hess, S.E.; Kogej, T.; Levin, M.D.; Marder, S.R.; et al. Design of organic molecules with large two-photon absorption cross sections. Science 1998, 281, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, B.H.; Ananthavel, S.P.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E.; Erskine, L.L.; Heikal, A.A.; Kuebler, S.M.; Lee, I.Y.S.; McCord-Maughon, D.; et al. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 398, 51–54. [Google Scholar]
- Ishikawa, A.; Tanaka, T.; Kawata, S. Improvement in the reduction of silver ions in aqueous solution using two-photon sensitive dye. Appl. Phys. Lett. 2006, 89, 1131021–1131023. [Google Scholar] [CrossRef]
- Vurth, L.; Baldeck, P.; Stéphan, O.; Vitrant, G. Two-photon induced fabrication of gold microstructures in polystyrene sulfonate thin films using a ruthenium(II) dye as photoinitiator. Appl. Phys. Lett. 2008, 92, 1711031–1711033. [Google Scholar] [CrossRef]
- Stellacci, F.; Bauer, C.A.; Meyer-Friedrichsen, T.; Wenseleers, W.; Alain, V.; Kuebler, S.M.; Pond, S.J.; Zhang, Y.; Marder, S.R.; Perry, J.W. Laser and electron-beam induced growth of nanoparticles for 2D and 3D metal patterning. Adv. Mater. 2002, 14, 194–198. [Google Scholar] [CrossRef]
- Sun, H.-B.; Tanaka, T.; Takada, K.; Kawata, S. Two-photon photopolymerization and diagnosis of three-dimensional microstructures containing fluorescent dyes. Appl. Phys. Lett. 2001, 79, 1411–1413. [Google Scholar] [CrossRef]
- Hossain, M.M.; Gu, M. Fabrication methods of 3D periodic metallic nano/microstructures for photonics applications. Laser Photon. Rev. 2014, 8, 233–249. [Google Scholar] [CrossRef]
- He, Y.; Wildman, R.D.; Tuck, C.J.; Christie, S.D.R.; Edmondson, S. An investigation of the behavior of solvent based polycaprolactone ink for material jetting. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunasekera, D.H.A.T.; Kuek, S.; Hasanaj, D.; He, Y.; Tuck, C.; Croft, A.; Wildman, R.D. Three dimensional ink-jet printing of biomaterials using ionic liquids and co-solvents. Faraday Discuss. 2016, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.R.; Li, S.; Sturgess, C.; Wildman, R.; Jones, J.R.; Hayes, W. 3D printing of biocompatible supramolecular polymers and their composites. ACS Appl. Mater. Interfaces 2016, 8, 3115–3122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tuck, C.; Hague, R.; He, Y.; Saleh, E.; Li, Y.; Sturgess, C.; Wildman, R. Inkjet printing of polyimide insulators for the 3D printing of dielectric materials for microelectronic applications. J. Appl. Polym. Sci. 2016, 133, 1–11. [Google Scholar] [CrossRef]
- Panesar, A.; Brackett, D.; Ashcroft, I.; Wildman, R.; Hague, R. Design framework for multifunctional additive manufacturing: Placement and routing of three-dimensional printed circuit volumes. J. Mech. Des. 2015, 137, 11141401–11141410. [Google Scholar] [CrossRef]
- Shukla, S.; Vidal, X.; Furlani, E.P.; Swihart, M.T.; Kim, K.T.; Yoon, Y.K.; Urbas, A.; Prasad, P.N. Subwavelength direct laser patterning of conductive gold nanostructures by simultaneous photopolymerization and photoreduction. ACS Nano 2011, 5, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Rill, M.S.; Plet, C.; Thiel, M.; Staude, I.; von Freymann, G.; Linden, S.; Wegener, M. Photonic metamaterials by direct laser writing and silver chemical vapour deposition. Nat. Mater. 2008, 7, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Blasco, E.; Müller, J.; Müller, P.; Trouillet, V.; Schön, M.; Scherer, T.; Barner-Kowollik, C.; Wegener, M. Fabrication of conductive 3D gold-containing microstructures via direct laser writing. Adv. Mater. 2016, 3592–3595. [Google Scholar] [CrossRef] [PubMed]
- Coe, B.J.; Samoc, M.; Samoc, A.; Zhu, L.; Yi, Y.; Shuai, Z. Two-photon absorption properties of iron(II) and ruthenium(II) trischelate complexes of 2,2′:4,4′ ′:4′,4′ ′′-quaterpyridinium ligands. J. Phys. Chem. A 2007, 111, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Lemercier, G.; Bonne, A.; Four, M.; Lawson-Daku, L.M. 3mlct excited states in ru(II) complexes: Reactivity and related two-photon absorption applications in the near-infrared spectral range. C. R. Chim. 2008, 11, 709–715. [Google Scholar] [CrossRef]
- Jiang, L.J.; Zhou, Y.S.; Xiong, W.; Gao, Y.; Huang, X.; Jiang, L.; Baldacchini, T.; Silvain, J.-F.; Lu, Y.F. Two-photon polymerization: Investigation of chemical and mechanical properties of resins using raman microspectroscopy. Opt. Lett. 2014, 39, 3034–3037. [Google Scholar] [CrossRef] [PubMed]
- Seah, M.; Gilmore, I.; Beamson, G. Xps: Binding energy calibration of electron spectrometers 5—re-evaluation of the reference energies. Surf. Interface Anal. 1998, 26, 642–649. [Google Scholar] [CrossRef]
- Huo, Z.; Tsung, C.-K.; Huang, W.; Zhang, X.; Yang, P. Sub-two nanometer single crystal Au nanowires. Nano Lett. 2008, 8, 2041–2044. [Google Scholar] [CrossRef] [PubMed]











| Monomer | Gold Chloride Hydrate wt % | DBMP wt % | Ru(II) wt % | |
|---|---|---|---|---|
| 1 | TMPTA | 0 | 3 | 0 |
| 2 | TMPTA | 3 | 3 | 0 |
| 3 | TMPTA | 3 | 3 | 0.1 |
| 4 | PETA | 0 | 3 | 0 |
| 5 | PETA | 3 | 3 | 0 |
| 6 | PETA | 3 | 3 | 0.1 |
| Without Degassing | With Degassing | |
|---|---|---|
| Formulation 2 | 25.5 | 22.5 |
| Formulation 3 | 22.0 | 22.0 |
| Formulation 5 | 24.0 | 21.5 |
| Formulation 6 | 20.0 | 20.0 |
| Formulation | Degree of Conversion, Δ% |
|---|---|
| 2 | 41.6 |
| 3 | 44.5 |
| 5 | 49.6 |
| 6 | 49.1 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hu, Q.; Zhang, F.; Tuck, C.; Irvine, D.; Hague, R.; He, Y.; Simonelli, M.; Rance, G.A.; Smith, E.F.; et al. Additive Manufacture of Three Dimensional Nanocomposite Based Objects through Multiphoton Fabrication. Polymers 2016, 8, 325. https://doi.org/10.3390/polym8090325
Liu Y, Hu Q, Zhang F, Tuck C, Irvine D, Hague R, He Y, Simonelli M, Rance GA, Smith EF, et al. Additive Manufacture of Three Dimensional Nanocomposite Based Objects through Multiphoton Fabrication. Polymers. 2016; 8(9):325. https://doi.org/10.3390/polym8090325
Chicago/Turabian StyleLiu, Yaan, Qin Hu, Fan Zhang, Christopher Tuck, Derek Irvine, Richard Hague, Yinfeng He, Marco Simonelli, Graham A. Rance, Emily F. Smith, and et al. 2016. "Additive Manufacture of Three Dimensional Nanocomposite Based Objects through Multiphoton Fabrication" Polymers 8, no. 9: 325. https://doi.org/10.3390/polym8090325