1. Introduction
A stent is a tiny mesh tube used to treat arterial occlusive diseases, with an angioplasty procedure performed to partially open up the blocked vessel. After being positioned in a stenosis segment, the stent is expanded radially under the action of balloon expansion. Once the balloon is deflated and removed, the expanded stent retains its diameter and provides a scaffoldlike support structure to maintain the patency of the vessel, thereby promoting blood flow. There are three development stages of stents: bare-metal stents (BMS), drug-eluting stents (DES), and bioresorbable stents. Currently, bioresorbable stents, which can address the concerns raised by permanent metallic stents (e.g., long-term safety), is considered to be the fourth revolution in interventional cardiology [
1]. Among them, biodegradable stents made of poly-
l-lactic acid (PLLA) have a promising prospect thanks to high biocompatibility and a favorable biodegradation period.
Although the previous studies have shown encouraging development prospects of biodegradable polymer stents, the low stiffness of polymers and the lack of a precise and efficient method of manufacture potentially limit the development of polymeric stents. Due to the low stiffness of polymers, polymeric stents have low radial strengths compared to metallic stents with similar dimensions [
2]. The deficiency of mechanical strength of polymeric stents can result in radial recoil after the deflation of balloon, which can reduce the luminal area of the vessel and, therefore, influence blood flow. In order to increase the stiffness of the polymeric stents, all currently known polymeric stents have thicker and wider struts than metallic stents. For example, the bioresorbable vascular scaffold (BVS)-B stent has a strut thickness of 0.15 mm, and both the Igaki-Tamai stent and ART18Z stent have the same thickness of 0.17 mm. However, a wider strut results in a larger surface area of stent, which may lead to higher rates of restenosis [
2,
3]. Moreover, stents with thicker struts can reduce stent flexibility and significantly occupy the lumen of the artery. This results in a difficult delivery of the stent and a reduction of blood flow through the lumen [
2]. Furthermore, there is a significant foreshortening in the process of stent expansion. This not only affects the position of stent, but also causes injury to the vessel wall. Therefore, it is necessary to design the structure of stent to improve its mechanical properties before manufacture.
Currently, the design of polymeric stents has learnt from the design of permanent metallic stents. Among them, there are numbers of computational-based studies of parametric comparison. For example, the expansion behavior of several stents with different geometries was compared in terms of dogboning, foreshortening, elastic recoil, and so on. Migliavacca et al. [
4,
5] and Beule et al. [
6] assessed the mechanical properties and behavior of balloon expandable stents to determine how the finite element analysis (FEA) method could be used to optimize stent designs. It is easy to perform and study the effective factors using these types of study. Although the results obtained are helpful to stent design, it is difficult to find the globally optimal design of a stent in design space.
In terms of processing methods of a polymeric stent, the traditional method of making a stent is laser-cutting, except for some coil stents with special structures. In 2008, Clarke et al. [
7] proposed a method of making a polymeric stent by a microinjection molding process with a central core pin and a plurality of slides. Using the microinjection molding process to make a stent has many advantages, such as less expensive tooling, reduced part cost, more accurate parts, very fast cycles, and obtaining parts with complex geometries. However, the injection molding process has not been used commonly in stent manufacturing because of the difficulties that appear during the process of microinjecting polymeric materials, such as difficult flow, mold cavity filling conditions, as well as some difficulties in their extraction from the cavity. In addition, the warpage induced during the injection molding process has an important influence on the quality of injection molded products [
8,
9]. It is obvious that the injection molding parameter—including clamping force, shot size, injection velocity, packing pressure, and temperature—will affect the quality of molded parts. Therefore, the optimization of injection molding parameters will help to improve the quality of the stent.
For the optimizations, whether structural optimization of the stent or process optimization of stent microinjection, the functional relationships between design variables and design objectives are complex, nonlinear, implicit, and multimodal. Moreover, the number of function evaluations is severely limited by time. It is hard to find a global optimal design using traditional optimization algorithms, such as gradient-based algorithms. Fortunately, surrogate modeling, predominantly involving the method of Kriging [
10], can address such engineering problems effectively. It can construct an approximate functional relationship between design objectives (the output) and design variables (the input), thereby replacing complex engineering computation to greatly reduce computational cost. Timmins et al. [
11] adopted Lagrange interpolating polynomials (LIPs) to optimize the stent; Shen et al. [
12] improved the stent’s resistance against compression and decreased internal pressure in an expanding stent by employing the artificial neural networks (ANN). Li et al. [
13,
14] proposed an adaptive optimization method based on Kriging surrogate model to (1) optimize the stent structure to eliminate the dogboning phenomenon during the stent expansion process and (2) optimize stent coating to prolong the effective period of drug release. Kriging surrogate model, a semi-parameter interpolation technique, is more precise and flexible compared to Lagrange interpolating polynomials and ANN, and thus widely used in multidisciplinary design optimization (MDO).
In the present paper, both the structure optimization of a biodegradable polymer stent and process optimization of polymeric stent microinjection molding were sequentially studied using an adaptive optimization method based on Kriging surrogate modeling. A multi-objective optimization was proposed to optimize the geometries of the biodegradable polymer stent so as to improve stent expansion performance. Then, microinjection molding process optimization of the optimal stent obtained from structure optimization was proposed to optimize the parameters of injection molding in order to reduce the deformation of stent, so as to improve the stent quality. The Kriging model was used to build the relationship between measures of stent expansion performance and stent geometries in the structure optimization, as well as the relationship between deformation of the stent and injection molding parameters in the microinjection molding process optimization, thereby replacing the expensive finite element reanalysis of the stent expansion and injection molding process. The optimization iterations are based on the approximate relationships for reducing the high computational cost. Optimal Latin hypercube sampling method [
15] (Optimal LHS) was used to generate the initial training sample points. In the adaptive optimization process, the expected improvement (EI) function was adopted to balance local and global searches, as it tends to find the find the global optimal design, even with a small sample size. Finite element method (FEM) was used to simulate the stent expansion and stent injection molding. The numerical results and design optimization method of this work could potentially be useful in further optimization studies and development of biodegradable polymer stents, as well as development of the method of making a polymeric stent by means of the microinjection molding process.
3. Results
3.1. Results of Stent Structure Optimization
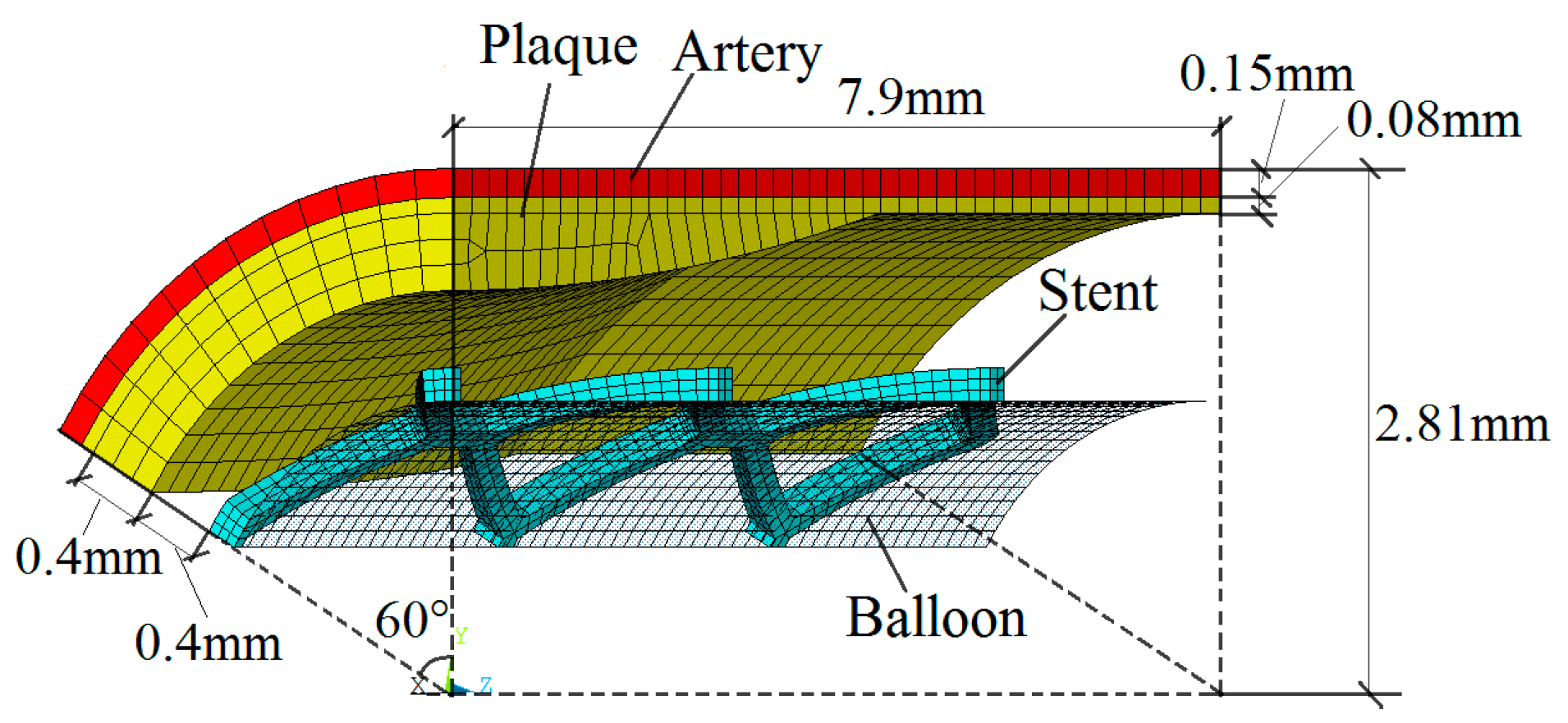
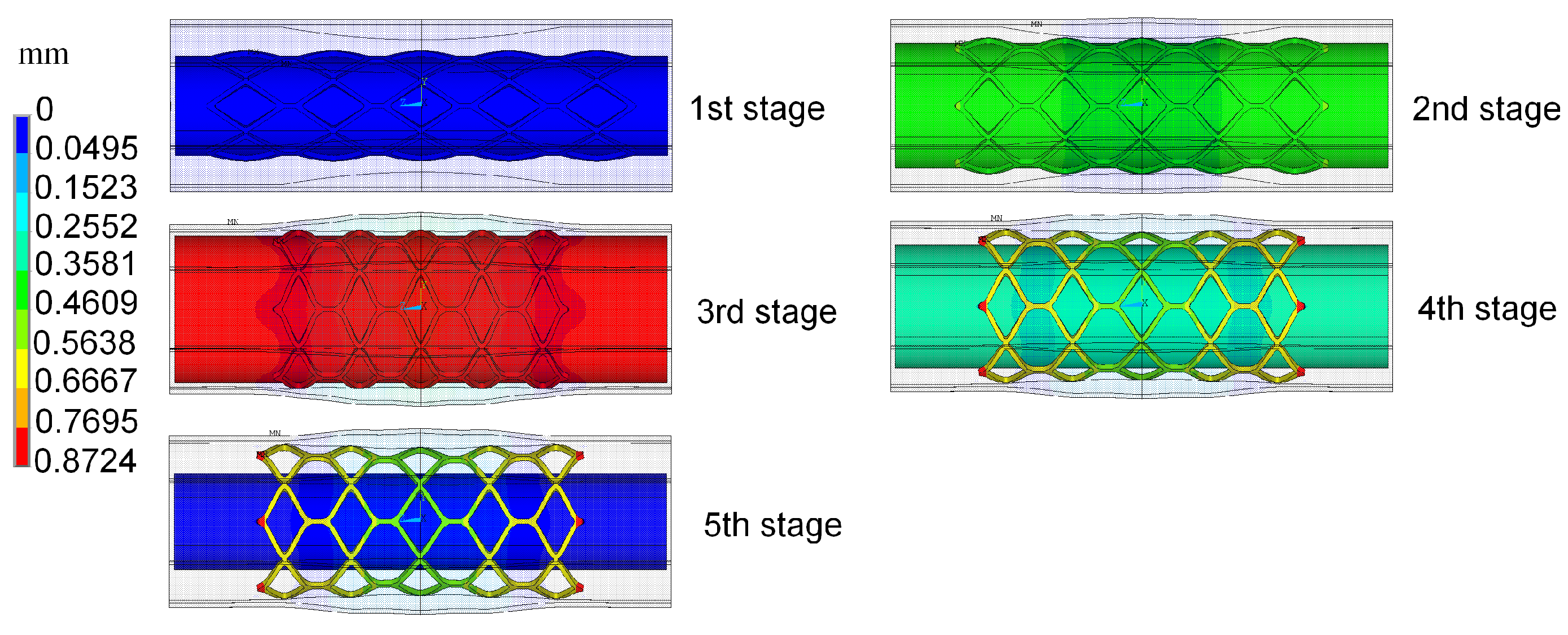
The radial recoil and foreshortening based on ART18Z stent platform was minimized by the proposed method, with the constraint that coverage is less than 20%. The initial trial samples, including an initial experience design and another 30 samples generated by optimal LHS, were selected for constructing Kriging surrogate model. The optimization iteration is based on the constructed surrogate model. The polymeric stent expansion processes in the stenotic artery for all trial samples are simulated by ANSYS software. EI function was employed to balance local and global search in the design space. The optimization process started from the initial point with the minimum value of optimization function among all the sample points. Twenty-one iterations were needed to obtain the optimal solution.
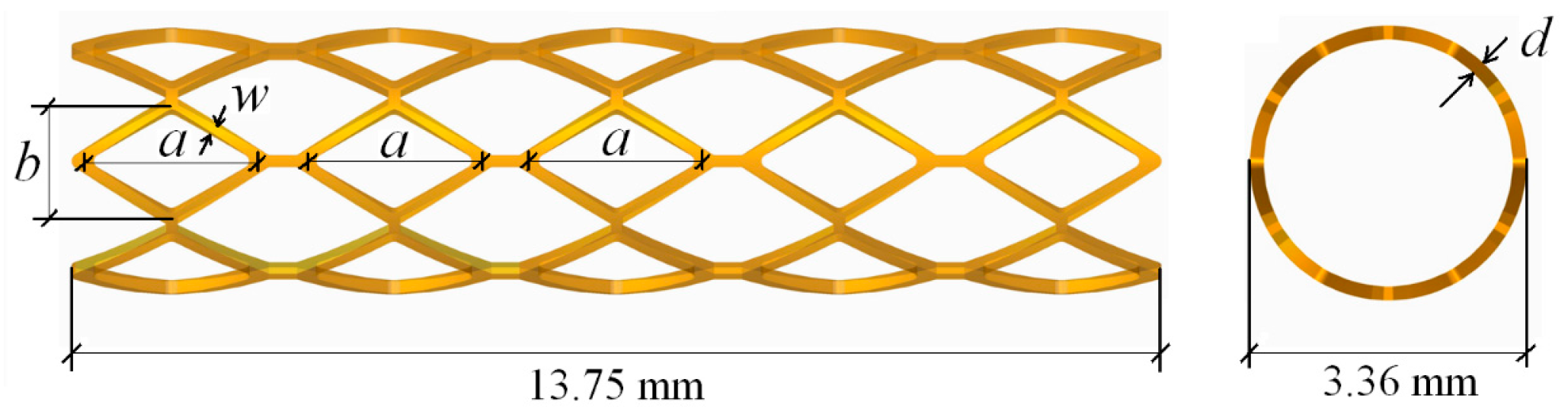
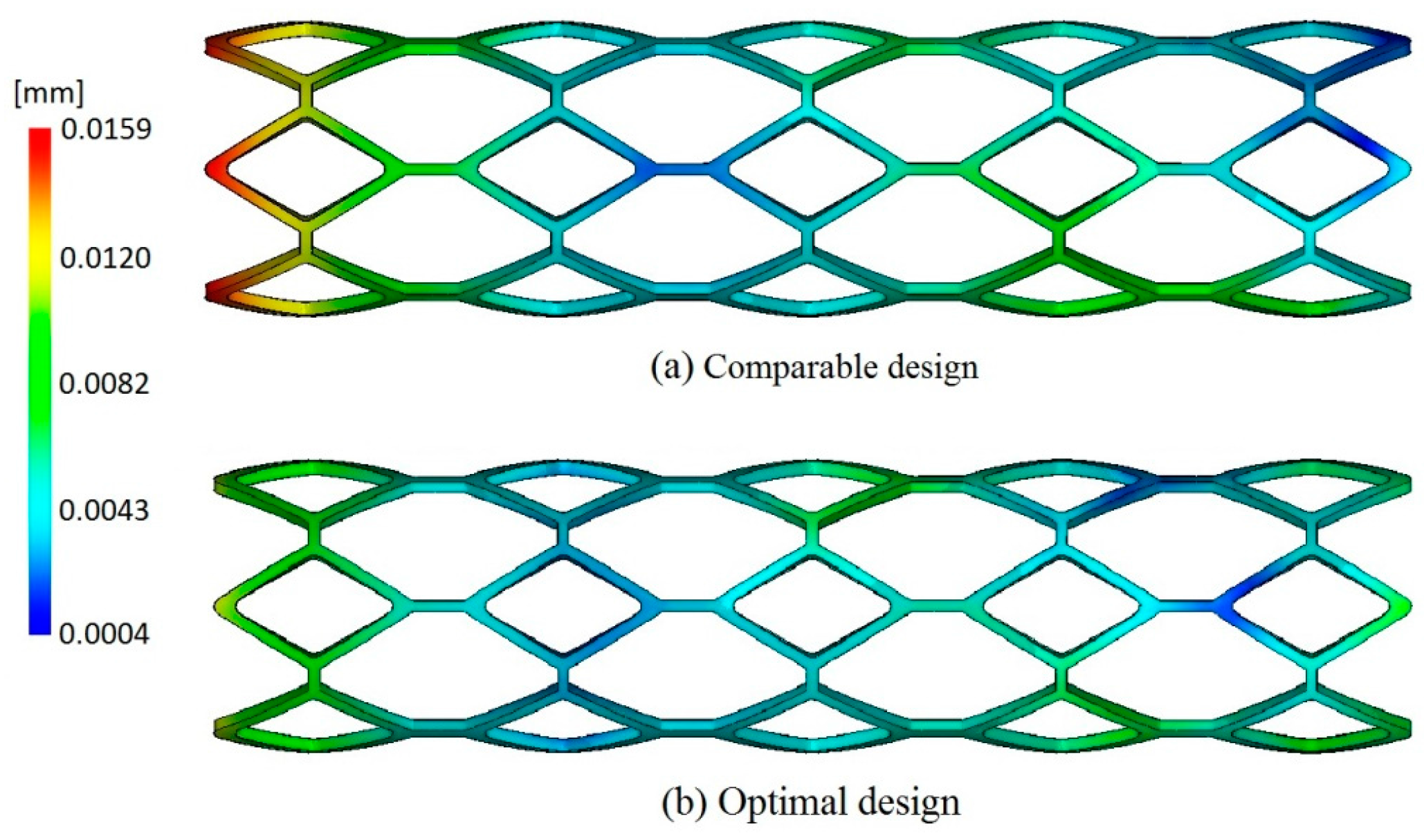
The optimization result was compared to the original design, as well as to the comparable stent, which has the same geometries as the original stent except for the width and thickness of struts, as shown in
Table 1. With the comparison to the original stent, the optimal stent has similar mechanical properties as the original stent, even though both the width and thickness of the struts of the optimal stent were decreased by 0.02 mm. The reduction of the strut width of the optimal stent is conducive to the smaller neointimal hyperplasia and better stent coverage, which can reduce the risk of in-stent restenosis. Moreover, although the radial recoil of the optimal stent is slightly larger than that of the original stent (due to the smaller thickness of the optimal stent), it has a larger lumen area than the original stent. When compared to the stent having the same geometrics as the original stent except for the width and thickness of struts (named “comparable stent” in this study), the optimal stent has lower radial recoil and larger lumen area, although both of them have the same width and thickness of struts.
The radial recoil and foreshortening are two conflicting objectives. Generally, the foreshortening is greater when the stent diameter expands to a larger degree. However, a larger deformation of the polymeric stent results in a larger region of greater plastic deformation, thereby the elastic deformation and the region in which it occurred are relatively small. This results in smaller radial elastic recoil caused by the elastic deformation of stent. Furthermore, because both the radial recoil and foreshortening are not only related to the stent structure, but also connected with the materials and expansion process of the polymeric stent, it is hard, even impossible, to eliminate them.
In order to study the effect of the strut dimensions on the expansion performance of stent, the effect of each design variable on the radial recoil and foreshortening were studied independently, with other variables fixed at their optimal values, as shown in
Figure 8. These results demonstrated that radial recoil and foreshortening are two conflicting measures of stent performance. The stent radial recoil decreased with the strut width and strut thickness, because the stent mechanical strength increased with the increase of these two design variables. Meanwhile, the foreshortening increased with these two design variables, because the foreshortening was larger when the stent was expanded to a greater degree. There was an optimal value of the diamond-shaped hole length to minimize the stent radial recoil in the design range, while the diamond-shaped hole width had a value that corresponded to the maximum of radial recoil. This was because the stent studied consists of diamonds and hexagons, which had common edges. The geometry of the diamond affected the geometrical structure of the hexagon, which also had an impact on the stent radial recoil. The foreshortening of stent decreased with the length of the diamond-shaped hole, and increased with the width of the diamond-shaped hole.
3.2. Results of Stent Injection Molding Process
16 initial trial samples generated by orthogonal LHS were used for constructing Kriging surrogate model to build the approximate relationship between stent warpage and design variables including temperature of the melt and the mold (
and
), flow rate (
), packing pressure (
), and packing time (
). Twenty-one iterations were needed to obtain the optimal solution. The optimization result was compared to the best design point, which corresponding to the minimum value of optimization function among all the initial trial sample points, as shown in
Table 2. The warpage of the optimal design was reduced by 28.3%, which was helpful to improve the molding quality of stent. The distribution of warpage of the comparable design corresponding to the best design among trial samples and optimal design are shown in
Figure 9, from which it can be observed that the optimal design has smaller deformation than the comparable design.
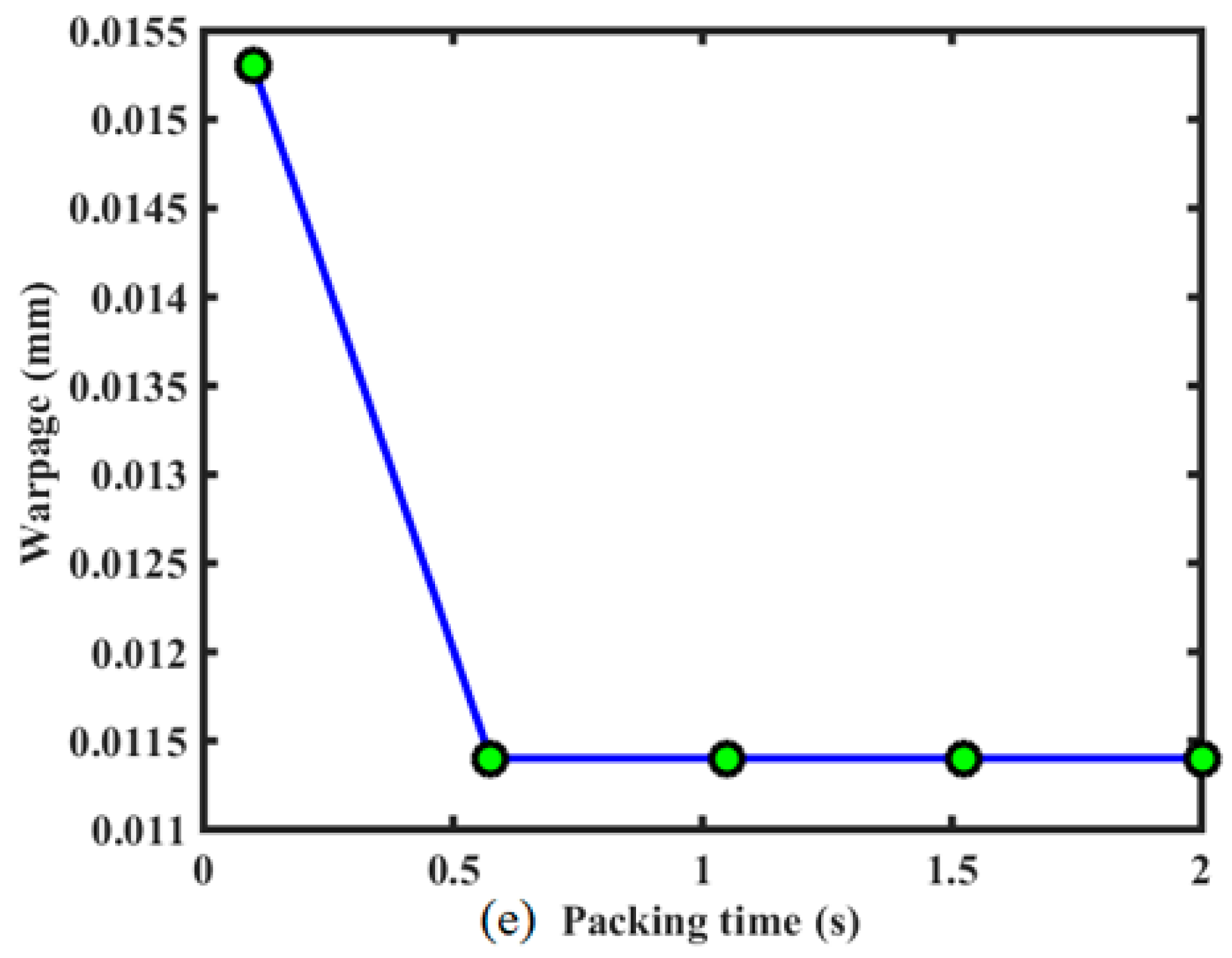
In order to study the effect of injection molding process parameters on the warpage of the stent, the computation of the single factor analysis is performed by fixing other factors in the optimal solution, the results of which are plotted in
Figure 10. It reveals that, in the given design domain, these injection process parameters do not contribute equally to the warpage of the stent. The flow rate and packing pressure have the most substantial impact, followed by mold temperature, melt temperature, and packing time.
In
Figure 10, the warpage decreases as the mold temperature increases. For one thing, the lower mold temperature usually causes larger shear stress near the wall, which subsequently evolves into the larger flow-induced residual stress in the part. This will result in greater warpage after ejection, finally. Besides, the higher mold temperature can increase the cooling time. If the packing process is not reasonable, it will cause greater shrinkage in the part, which results in larger warpage.
Figure 10 presents the phenomenon of warpage decreasing when mold temperature changes from the lowest value to the reasonable value for the given range.
The warpage increases with the melt temperature. The effect of melt temperature is similar to that of mold temperature. Increasing melt temperature can increase the cooling time as well. Then, the shrinkage of the part relies on the packing process. If packing pressure is not large enough or packing time is not long enough, there is greater shrinkage in the part, which leads to the greater warpage.
There is a nonlinear relationship between the warpage and the flow rate. Lower flow rate will increase the viscosity due to more heat lost at the flow front, which can make shear stress increase. However, higher flow rate can avoid heat loss, but increase the shear rate, which can cause higher shear stress as well. These usually result in higher residual stress in the part, and then cause serious warpage. Actually, low flow rate means long filling time, while, on the contrary, high flow rate represents short filling time. These process conditions require high injecting pressure, which is not expected during manufacturing since it indicates more energy consumption. Therefore, the reasonable value of flow rate should be determined carefully.
The warpage decreases when the packing pressure increases. This is because the higher packing pressure could ensure injection of a certain material in order to compensate for the shrinkage during cooling. However, packing pressure cannot be too high, since it can induce over-packing, which is not beneficial to prevention of warpage. Therefore, an intermediate packing pressure is preferred.
The warpage decreased with the packing time, and then had little effect on the stent warpage, as shown in
Figure 10. Because the sizes of stent and the injecting gate are both very small, the cooling time in the process is not too long. Although packing is proceeding due to hot runner and gate, no melt material can be injected into the cavity. A short packing time is sufficient, and the effect of long packing time on warpage is the same as that of this short packing time. Therefore, there exists the platform on the curve in
Figure 10.
4. Discussion
For the biodegradable polymeric stent, the low stiffness of polymers and the lack of a precise and efficient method of manufacture are the potential limitations of the development of polymeric stents. Due to the low stiffness of polymers, polymeric stents have low radial support capacity. Generally, the polymeric stents have been designed with thicker and wider struts in order to increase their stiffness. However, the thicker struts can cause a reduction of stent flexibility, as well as a reduction of the area of blood flow through the artery lumen. A wider strut also increases the level of injury to the vessel. In the present paper, in order to decrease the thickness and width of the struts of polymeric stents, the range of the thickness and width of polymeric stents studied here were set to be [0.1, 0.15] and [0.1, 0.13]. Obviously, reducing the thickness and width of struts will decrease the stent radial stiffness, and this will cause radial recoil after the deflation of balloon. Consequently, the optimization design of stent structure has an objective to minimize the radial recoil of the stent so as to provide sufficient support for the stenotic artery, as well as an objective to minimize the foreshortening so as to reduce the level of injury to the vessel. After optimization, with both the width and thickness of the struts having been reduced by 0.02 mm, the optimal stent had similar expansion performance as the original stent.
It should be acknowledged that when the stent was implanted into a stenotic artery with different diameters, the stent expansion performance (including the radial recoil and foreshortening) will differ from that in this study, and this will lead to a different optimal result of stent structure. Similarly, the injection molding process optimization result would be different if a different stent was investigated. However, as an example, this study illustrated how to use surrogate modeling to optimize stent structure and stent injection molding process, with the aim of demonstrating that a superior structure of stent can be designed and refined computationally.
Currently, metallic stents are approaching a mature stage of evolution, while degradable polymeric stents are still in the development stage. It is urgent that polymeric stent designs learn from the design of permanent metallic stents. There have been many parametric studies of metallic stents by their comparative tests. Dumoulin and Cochelin [
24] evaluated and characterized the mechanical properties and behaviors of a balloon expandable stent. In terms of stent design, Migliavacca et al. [
4,
5] and Beule et al. [
6] assessed the mechanical properties and behavior of balloon expandable stents to determine how the FEA method could be used to optimize stent designs. These studies are beneficial to aid stent design, and it is easy to analyze the effective factors. However, it is difficult to find the globally optimal solution in the design space because the functional relationship between the geometrical parameters and dilation performance of stent is complex, nonlinear, and implicit, in addition to the complicating factors of time and cost.
In contrast to the expansive computational simulations employed in the comparison test studies, the surrogate modeling approach uses surrogate models to represent the relationship between design objectives and design variables [
10]. Harewood et al. [
24] optimized the radial stiffness of a single ring. Li et al. [
12,
14] optimized stent dogboning and drug release, respectively. Grogan et al. [
25] performed an optimization for maximum radial strength. When considering multiple objectives, Pant et al. [
26] constructed the Pareto fronts generated by treating each objective separately. Bressloff [
2] recast the multi-objective optimization as a constrained problem. Although there have been some previous studies about surrogate model-based optimization of stents, and the potential for future studies have been demonstrated, the surrogate models, especially the Kriging surrogate model, as well as the optimization tools associated with Kriging, have not been used as efficiently as they could have been. Currently, the existing research of multi-objective optimization of the stent just focuses on a single or a few objectives. However, the multi-objective optimization of stents design involves a large number of design objectives, especially for the biodegradable stents, in which the degradation should be considered. Therefore, in future research, the optimization of biodegradable stents should consider more design objectives using the surrogate model-based optimization method combined with effective optimization tools.
In terms of stent manufacture, as a new method of making a polymeric stent, microinjection molding has demonstrated its potential for the future, although it has not been widely used in production. Because the stent has a complex spatial structure and tiny struts, it is difficult to make it by injection molding. The parametric study of the stent injection molding process can provide guidance for the manufacturing of it. Moreover, the wall shear stress of the stent in the injection molding process results in the safety of stent. In addition, the residual stress of the stent in the injection molding has an influence on the service performance of stent. Furthermore, the strength of weld lines, as well as the overall polymer orientation, will play a major role in the mechanical strength of the stent. However, there is quite a lack of research in these areas. Hence, there are potential challenging studies of the stent injection molding process in future.