Reversible Addition-Fragmentation Chain Transfer Polymerization of Acrylonitrile under Irradiation of Blue LED Light
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterizations
2.3. Typical Procedures for Photoinduced RAFT Polymerization of AN
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chand, S. Carbon fibers for composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Ogale, A.A.; Zhang, M.; Jin, J. Recent advances in carbon fibers derived from biobased precursors. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Newcomb, B.A.; Giannuzzi, L.A.; Lyons, K.M.; Gulgunje, P.V.; Gupta, K.; Liu, Y.D.; Kamath, M.; McDonald, K.; Moon, J.; Feng, B.; et al. High resolution transmission electron microscopy study on polyacrylonitrile/carbon nanotube based carbon fibers and the effect of structure development on the thermal and electrical conductivities. Carbon 2015, 93, 502–514. [Google Scholar] [CrossRef]
- Thunga, M.; Chen, K.; Grewell, D.; Kessler, M.R. Bio-renewable precursor fibers from lignin/polylactide blends for conversion to carbon fibers. Carbon 2014, 68, 159–166. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J.; Millington, K.; Smith, S. Producing high-quality precursor polymer and fibers to achieve theoretical strength in carbon fibers: A review. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Chae, H.G.; Newcomb, B.A.; Gulgunje, P.V.; Liu, Y.D.; Gupta, K.K.; Kamath, M.G.; Lyons, K.M.; Ghoshal, S.; Pramanik, C.; Giannuzzi, L.; et al. High strength and high modulus carbon fibers. Carbon 2015, 93, 81–87. [Google Scholar] [CrossRef]
- Xia, K.Q.; Ouyang, Q.; Chen, Y.S.; Wang, X.F.; Qian, X.; Wang, L. Preparation and characterization of lignosulfonate-acrylonitrile copolymer as a novel carbon fiber precursor. ACS Sustain. Chem. Eng. 2016, 4, 159–168. [Google Scholar] [CrossRef]
- Otsu, T.; Yoshida, M.; Tazaki, T. A model for living radical polymerization. Macromol. Chem. Rapid Commun. 1982, 3, 133–140. [Google Scholar] [CrossRef]
- Otsu, T.; Yoshida, M.; Kuriyama, A. Living radical polymerizations in homogeneous solution by using organic sulfides as photoiniferters. Polym. Bull. 1982, 7, 45–50. [Google Scholar] [CrossRef]
- Wang, J.S.; Matyjaszewski, K. Controlled living radical polymerization—Atom-transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of methyl-methacrylate with the carbon-tetrachloride dichlorotris(triphenylphosphine)ruthenium(Ii) methylaluminum bis(2,6-Di-Tert-butylphenoxide) initiating system—Possibility of living radical polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J.H. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, M.; Terashima, T.; Sawamoto, M. Transition metal-catalyzed living radical polymerization: Toward perfection in catalysis and precision polymer synthesis. Chem. Rev. 2009, 109, 4963–5050. [Google Scholar] [CrossRef] [PubMed]
- He, W.W.; Jiang, H.J.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Atom transfer radical polymerization of hydrophilic monomers and its applications. Polym. Chem. 2013, 4, 2919–2938. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living free-radical polymerization by reversible addition-fragmentation chain transfer: The RAFT process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Jiang, X.W.; Wu, J.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Highly active ppm level organic copper catalyzed photo-induced ICAR ATRP of methyl methacrylate. Macromol. Rapid Commun. 2014, 35, 1879–1885. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Wu, L.; Zhang, Z.H.; Xu, T.W. Atom transfer radical polymerization (ATRP): A versatile and forceful tool for functional membranes. Prog. Polym. Sci. 2014, 39, 124–144. [Google Scholar] [CrossRef]
- Liu, X.D.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Metal-free photoinduced electron transfer-atom transfer radical polymerization (PET-ATRP) via a visible light organic photocatalyst. Polym. Chem. 2016, 7, 689–700. [Google Scholar] [CrossRef]
- Bai, L.J.; Wang, W.X.; Wang, M.H.; Sun, J.M.; Chen, H. Triphenylphosphine as reducing agent for copper(II)-catalyzed AGET ATRP. Chin. J. Polym. Sci. 2015, 33, 1260–1270. [Google Scholar] [CrossRef]
- Bai, L.J.; Huang, S.Q.; Wang, W.X.; Xu, H.; Chen, H.; Niu, Y.Z.; Wang, M.H. Iron-mediated activators generated by electron transfer for atom-transfer radical polymerization of methyl methacrylate using ionic liquid as ligand and Fe(0) wire as reducing agent. Polym. Int. 2015, 64, 1754–1761. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Iron-mediated homogeneous ICAR ATRP of methyl methacrylate under ppm level organometallic catalyst iron(III) acetylacetonate. Polymers 2016, 8, 29. [Google Scholar] [CrossRef]
- Zhang, B.J.; Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Fe(III)-mediated ICAR ATRP in a p-xylene/PEG-200 biphasic system: Facile and highly efficient separation and recycling of an iron catalyst. Polym. Chem. 2015, 6, 6616–6622. [Google Scholar] [CrossRef]
- Wu, J.J.; Jiang, H.J.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Synthesis of amphiphilic nanoparticles and multi-block hydrophilic copolymers by a facile and effective “living” radical polymerization in water. Polym. Chem. 2016, 7, 2486–2491. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Pintauer, T.; Matyjaszewski, K. Deactivation efficiency and degree of control over polymerization in ATRP in protic solvents. Macromolecules 2004, 37, 9768–9778. [Google Scholar] [CrossRef]
- Liu, X.H.; Wang, J.; Yang, J.S.; An, S.L.; Ren, Y.L.; Yu, Y.H.; Chen, P. Fast copper catalyzed living radical polymerization of acrylonitrile utilizing a high concentration of radical initiator. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1933–1940. [Google Scholar] [CrossRef]
- Huang, Z.C.; Chen, J.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. ICAR ATRP of acrylonitrile under ambient and high pressure. Polymers 2016, 8, 59. [Google Scholar] [CrossRef]
- Dong, H.C.; Tang, W.; Matyjaszewski, K. Well-defined high-molecular-weight polyacrylonitrile via activators regenerated by electron transfer ATRP. Macromolecules 2007, 40, 2974–2977. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Sun, J.M.; Chen, H.; Bai, L.J.; Tao, Q.; Yu, L.L.; Wang, Y.T. Synthesis of polyacrylonitrile mediated by manganese(III) acetylacetonate (Mn(acac)(3)) and 2-Cyanoprop-2-yl dithionaphthalenoate. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1305–1309. [Google Scholar] [CrossRef]
- Tang, C.B.; Kowalewski, T.; Matyjaszewski, K. RAFT polymerization of acrylonitrile and preparation of block copolymers using 2-cyanoethyl dithiobenzoate as the transfer agent. Macromolecules 2003, 36, 8587–8589. [Google Scholar] [CrossRef]
- Niu, S.G.; Zhang, L.F.; Zhu, J.; Zhang, W.; Cheng, Z.P.; Zhu, X.L. Synthesis of high molecular weight and narrow molecular weight distribution poly(acrylonitrile) via RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1197–1204. [Google Scholar] [CrossRef]
- Moskowitz, J.D.; Abel, B.A.; McCormick, C.L.; Wiggins, J.S. High molecular weight and low dispersity polyacrylonitrile by low temperature RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 553–562. [Google Scholar] [CrossRef]
- Kopec, M.; Krys, P.; Yuan, R.; Matyjaszewski, K. Aqueous RAFT polymerization of acrylonitrile. Macromolecules 2016, 49, 5877–5883. [Google Scholar] [CrossRef]
- Zou, J.T.; Wang, Y.S.; Pang, W.M.; Shi, L.; Lu, F. Radiation-induced inclusion polymerization of acrylonitrile in urea canals: Toward synthesis of completely isotactic polyacrylonitrile with controlled molecular weight. Macromolecules 2013, 46, 1765–1771. [Google Scholar] [CrossRef]
- Liu, H.Z.; Yu, M.; Deng, B.; Li, L.F.; Jiang, H.Q.; Li, J.Y. Pre-irradiation induced emulsion graft polymerization of acrylonitrile onto polyethylene nonwoven fabric. Radiat. Phys. Chem. 2012, 81, 93–96. [Google Scholar] [CrossRef]
- Hanh, T.T.; Huy, H.T.; Hien, N.Q. Pre-irradiation grafting of acrylonitrile onto chitin for adsorption of arsenic in water. Radiat. Phys. Chem. 2015, 106, 235–241. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevee, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, M.J.; Johnson, J.A. Light-controlled radical polymerization: Mechanisms, methods, and applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.T.; Shanmugam, S.; Boyer, C. Organic electron donor-acceptor photoredox catalysts: Enhanced catalytic efficiency toward controlled radical polymerization. ACS Macro Lett. 2015, 4, 926–932. [Google Scholar] [CrossRef]
- Jiang, X.W.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Highly efficient and facile photocatalytic recycling system suitable for ICAR ATRP of hydrophilic monomers. Macromol. Rapid Commun. 2016, 37, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Straightforward catalyst/solvent-free iodine-mediated living radical polymerization of functional monomers driven by visible light irradiation. Chem. Commun. 2016, 52, 10850–10853. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Catalyst-free iodine-mediated living radical polymerization under irradiation over a wide visible-light spectral scope. Polym. Chem. 2016, 7, 3576–3588. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced electron transfer reactions for macromolecular syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gu, Y.; Liu, X.; Zhang, L.; Cheng, Z.; Zhu, X. Metal-free atom transfer radical polymerization of methyl methacrylate with ppm level of organic photocatalyst. Macromol. Rapid. Commun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Wang, W.X.; Xia, H.D.; Zhu, J.; Zhang, W.; Zhu, X.L. Single-electron transfer living radical polymerization (SET-LRP) of methyl methacrylate (MMA) with a typical RAFT agent as an initiator. Macromolecules 2009, 42, 7360–7366. [Google Scholar] [CrossRef]
- Subramanian, S.H.; Babu, R.P.; Dhamodharan, R. Ambient temperature polymerization of styrene by single electron transfer initiation, followed by reversible addition fragmentation chain transfer control. Macromolecules 2008, 41, 262–265. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, J. Donor-acceptor fluorophores for visible-light-promoted organic synthesis: Photoredox/Ni dual catalytic C(sp(3))-C(sp(2)) cross-coupling. Acs Catal. 2016, 6, 873–877. [Google Scholar] [CrossRef]
- Kreft, T.; Reed, W. Direct monitoring of the cross-over from diffusion-controlled to decomposition-controlled initiation in free radical polymerization. Macromol. Chem. Phys. 2008, 209, 2463–2474. [Google Scholar] [CrossRef]
- Yan, Y.F.; Zhang, W.; Qiu, Y.S.; Zhang, Z.B.; Zhu, J.A.; Cheng, Z.P.; Zhang, W.D.; Zhu, X.L. Universal xanthate-mediated controlled free radical polymerizations of the “less activated” vinyl monomers. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5206–5214. [Google Scholar] [CrossRef]
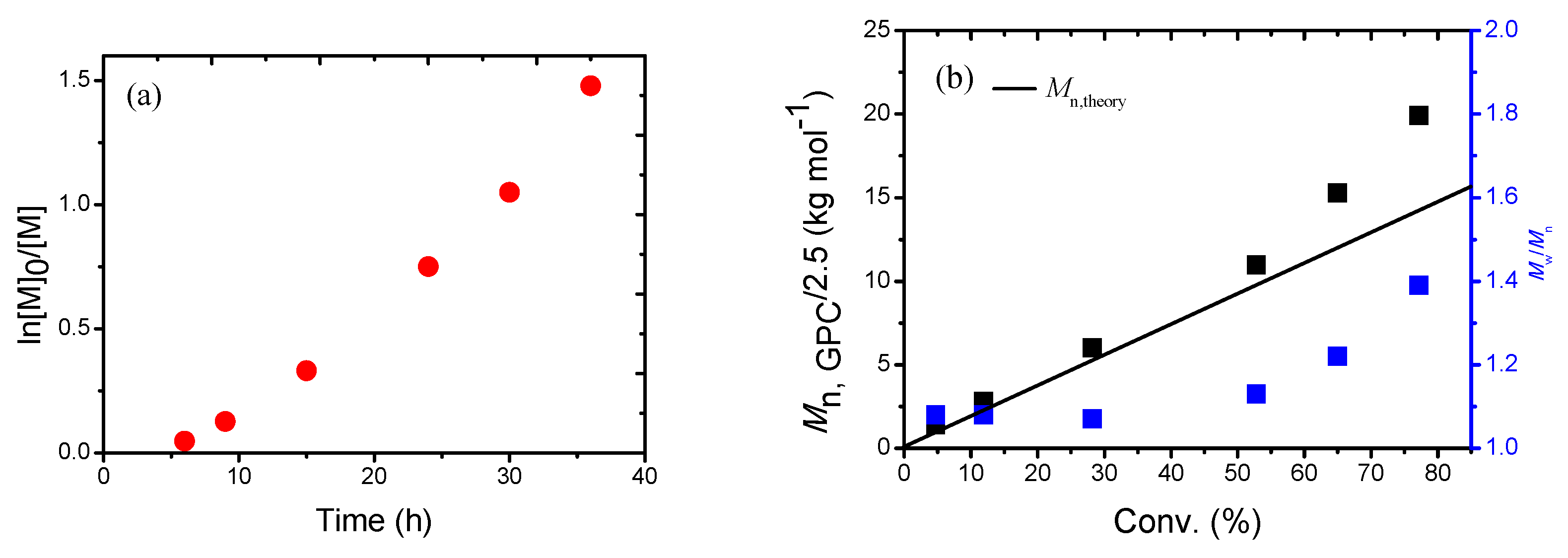
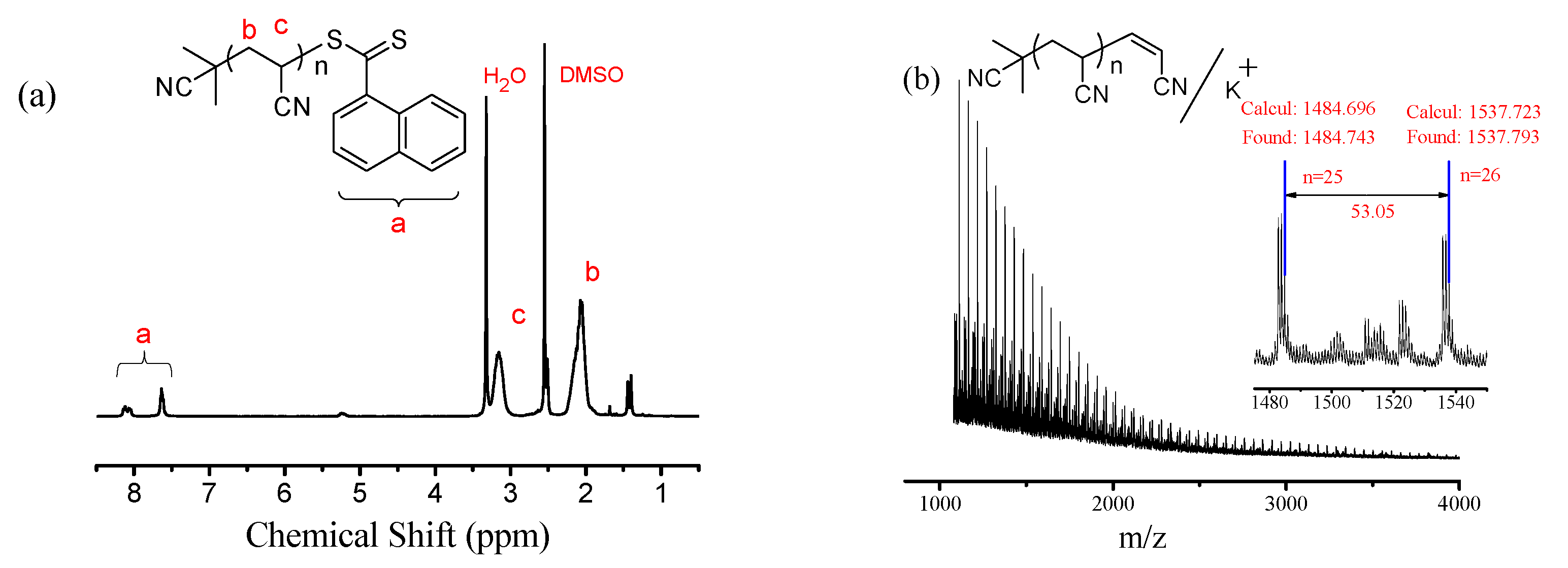
| Entry | R | Time (h) | Conv. b (%) | Mn,GPC c (g/mol) | Mn,GPC/2.5 d (g/mol) | Mn,th e (g/mol) | Mw/Mn c |
|---|---|---|---|---|---|---|---|
| 1 | [AN]0/[CPDN]0 = 400/1 | 24 | N.A. | ||||
| 2 | [AN]0/[4CzIPN]0 = 400/0.01 | 3.5 | 55.1 | 155,700 | - | - | 2.26 |
| 3 f | [AN]0/[CPDN]0/[4CzIPN]0 = 400/1/0.01 | 24 | N.A. | ||||
| 4 | [AN]0/[CPDN]0/[4CzIPN]0 = 400/1/0.01 | 24 | 54.7 | 25,200 | 10,100 | 11,600 | 1.12 |
| 5 g | [AN]0/[CPDN]0/[TPO]0 = 400/1/0.01 | 24 | 25.7 | 14,000 | 5600 | 5400 | 1.07 |
| 6 h | [AN]0/[CPDN]0/[AIBN]0 = 400/1/0.01 | 24 | 70.3 | 37,400 | 15,000 | 15,200 | 1.11 |
| 7 | [AN]0/[CPDN]0/[4CzIPN]0 = 400/1/0.01 | 22 | 65.5 | 45,200 | 18,100 | 21,100 | 1.29 |
| 8 | [AN]0/[CPDN]0/[4CzIPN]0 = 800/1/0.02 | 9 | 44.4 | 32,400 | 13,000 | 19,100 | 1.20 |
| 9 | [AN]0/[CPDN]0/[4CzIPN]0 = 1,000/1/0.025 | 9 | 47.9 | 45,500 | 18,200 | 25,700 | 1.32 |
| 10 | [AN]0/[CPDN]0/[4CzIPN]0 = 1,500/1/0.075 | 4 | 58.7 | 75,200 | 30,100 | 46,900 | 1.46 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Zhang, L.; Cheng, Z.; Zhu, X. Reversible Addition-Fragmentation Chain Transfer Polymerization of Acrylonitrile under Irradiation of Blue LED Light. Polymers 2017, 9, 4. https://doi.org/10.3390/polym9010004
Huang Z, Zhang L, Cheng Z, Zhu X. Reversible Addition-Fragmentation Chain Transfer Polymerization of Acrylonitrile under Irradiation of Blue LED Light. Polymers. 2017; 9(1):4. https://doi.org/10.3390/polym9010004
Chicago/Turabian StyleHuang, Zhicheng, Lifen Zhang, Zhenping Cheng, and Xiulin Zhu. 2017. "Reversible Addition-Fragmentation Chain Transfer Polymerization of Acrylonitrile under Irradiation of Blue LED Light" Polymers 9, no. 1: 4. https://doi.org/10.3390/polym9010004
APA StyleHuang, Z., Zhang, L., Cheng, Z., & Zhu, X. (2017). Reversible Addition-Fragmentation Chain Transfer Polymerization of Acrylonitrile under Irradiation of Blue LED Light. Polymers, 9(1), 4. https://doi.org/10.3390/polym9010004







