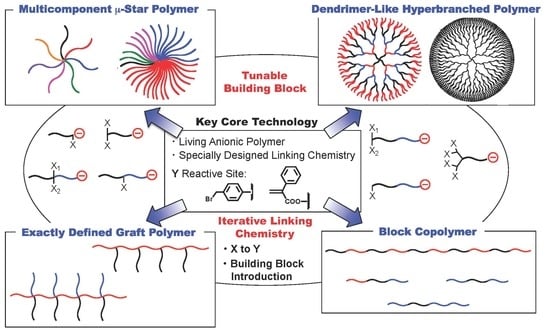
2.1. Star Polymers ((SP)s)
The first successful macromolecular architecture synthesized by the all-around iterative methodology is a series of multicomponent (SP)s. Mixed arm (SP)s with a composition asymmetry (hereinafter, called “μ-star polymers (μ-SP)”), have recently attracted interest due to their unique morphologies on the basis of star-branched architecture [
5,
8,
10,
11,
12,
13,
21,
22,
23,
24,
25,
26,
27,
28,
29]. However, the synthesis of well-defined (μ-SP)s with more than three different compositions is very difficult by two requirements: First, multistep selective reactions in a number that corresponds to all the different arms are required. Secondly, several reaction sites with different reactivities selectively operating for different arm introduction are required. For these requirements, the already reported methodologies using living anionic polymerization allow access only to the synthesis of two components A
xB
y and several ABC and ABCD (μ-SP)s [
17,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45]. Although a 5-arm ABCDE (μ-SP) was recently synthesized by the combination of living polymerization with azide-alkyne cycloaddition reaction [
46], multicomponent (μ-SP) synthesis is quite limited even now.
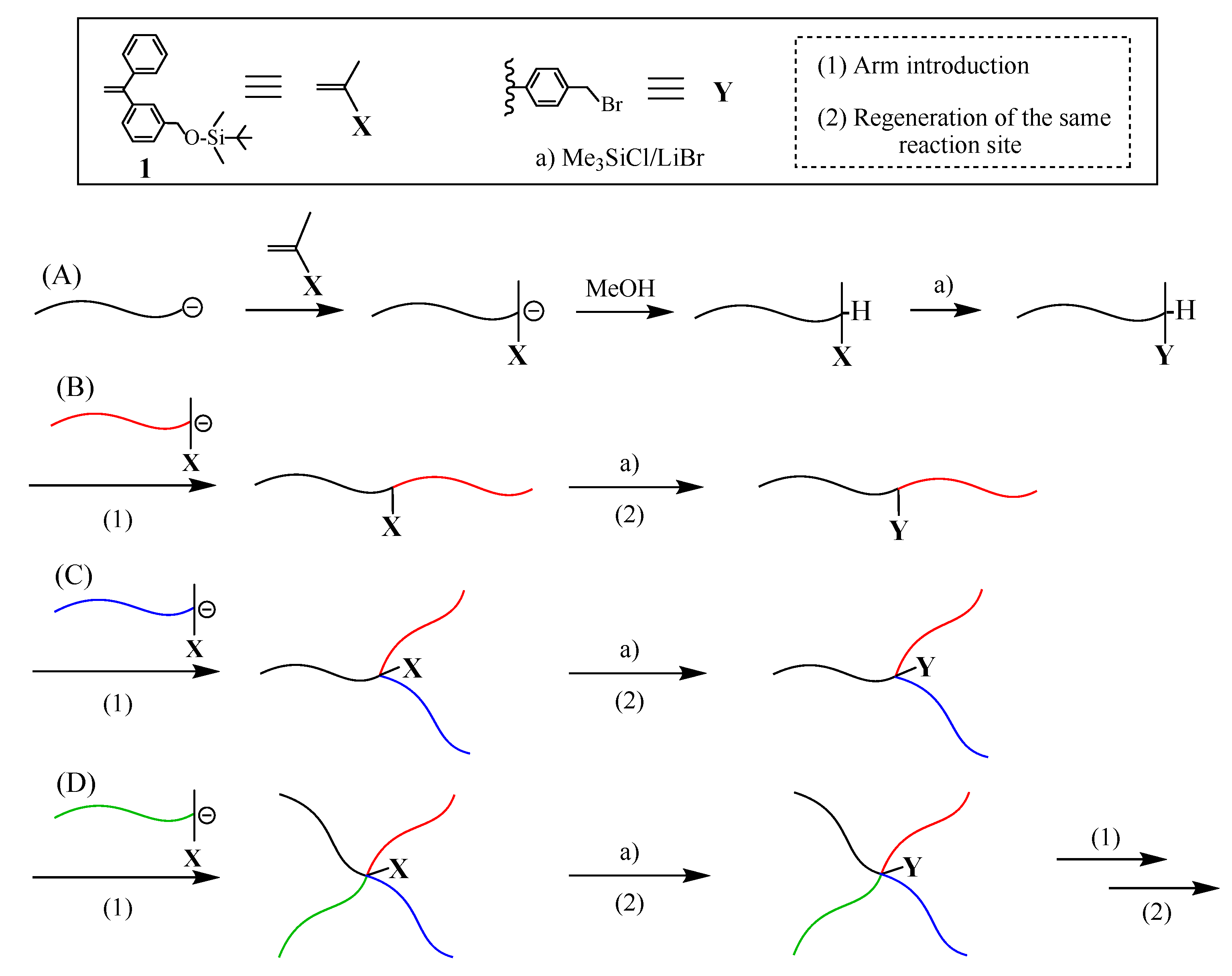
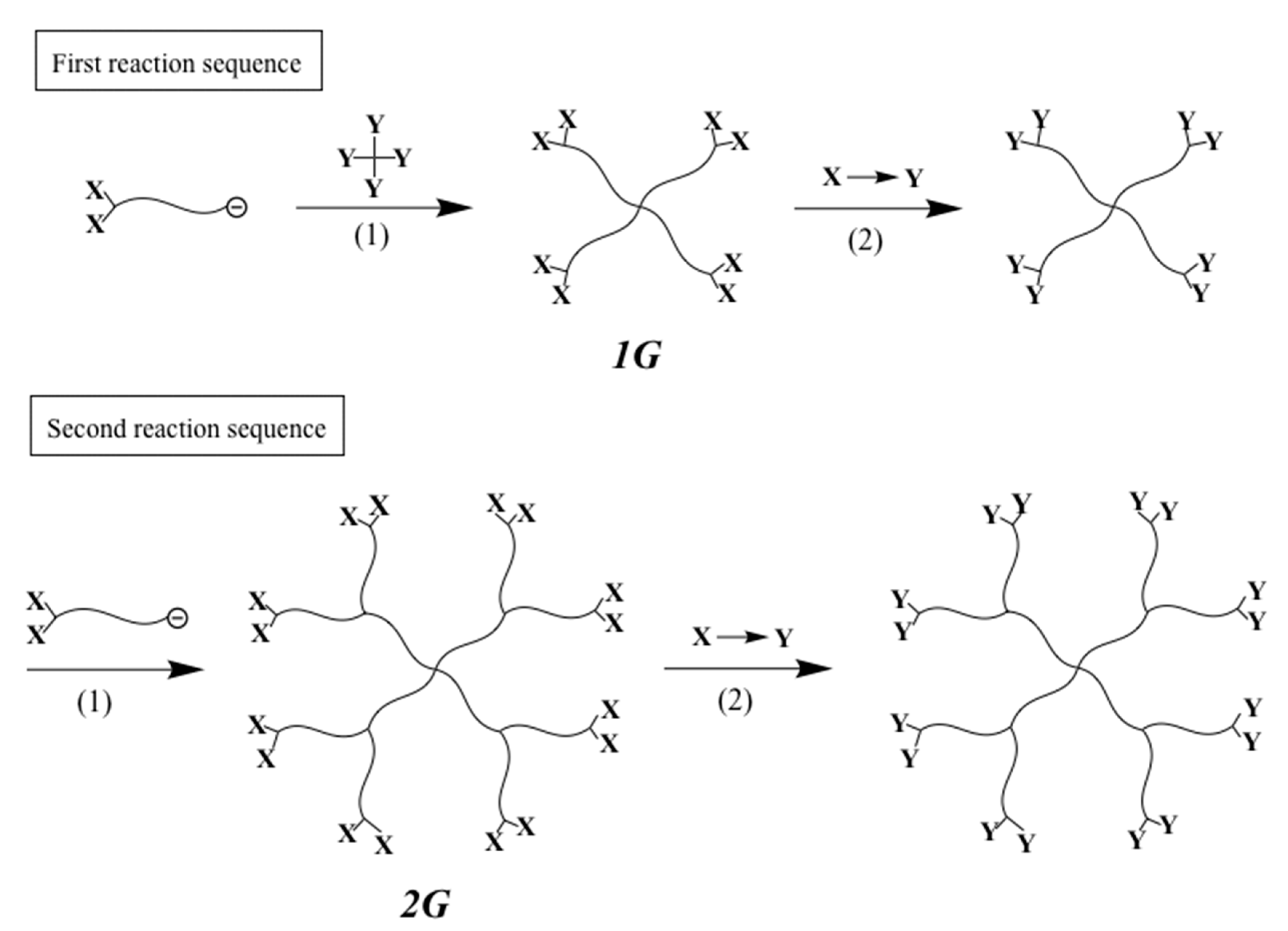
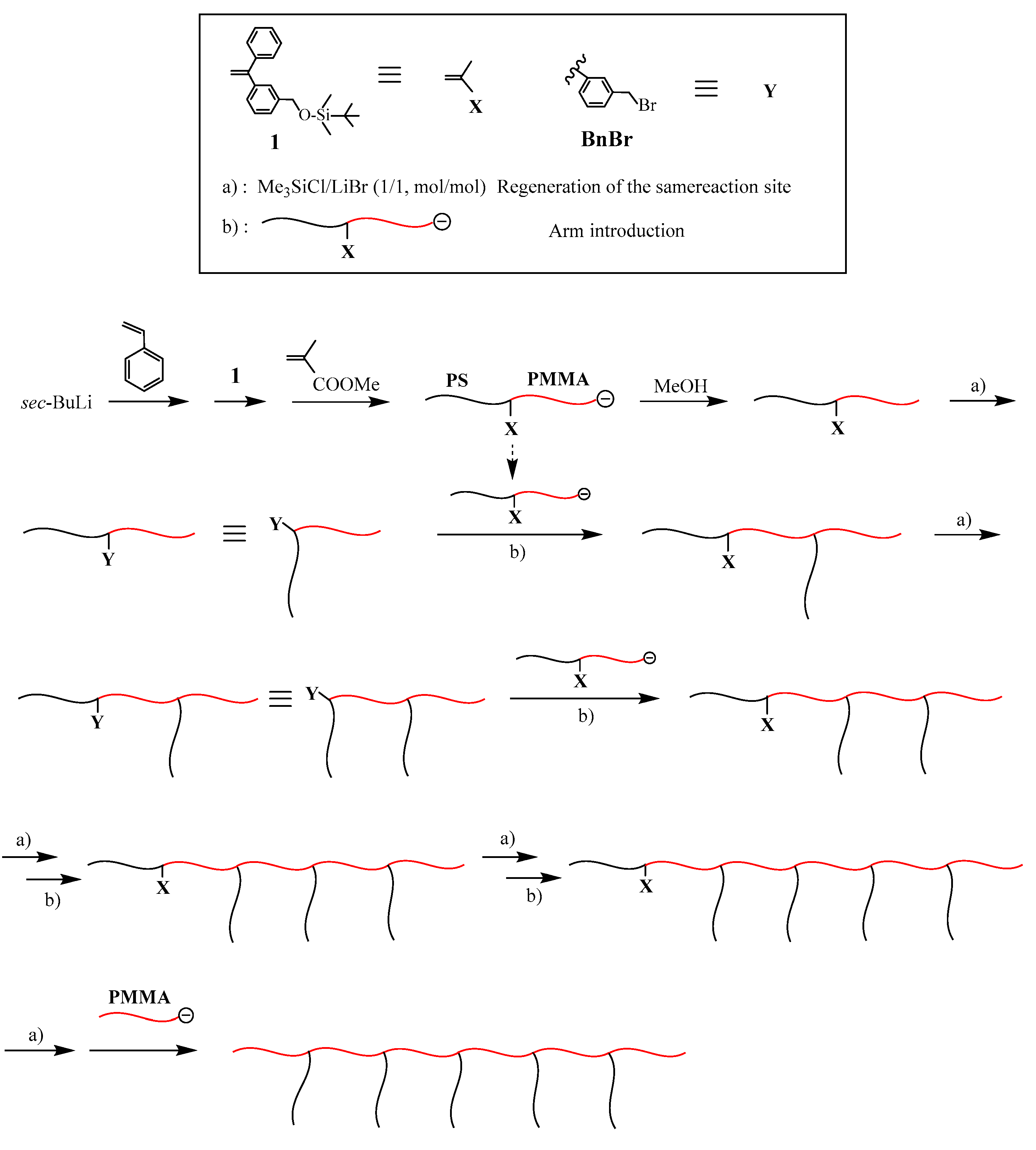
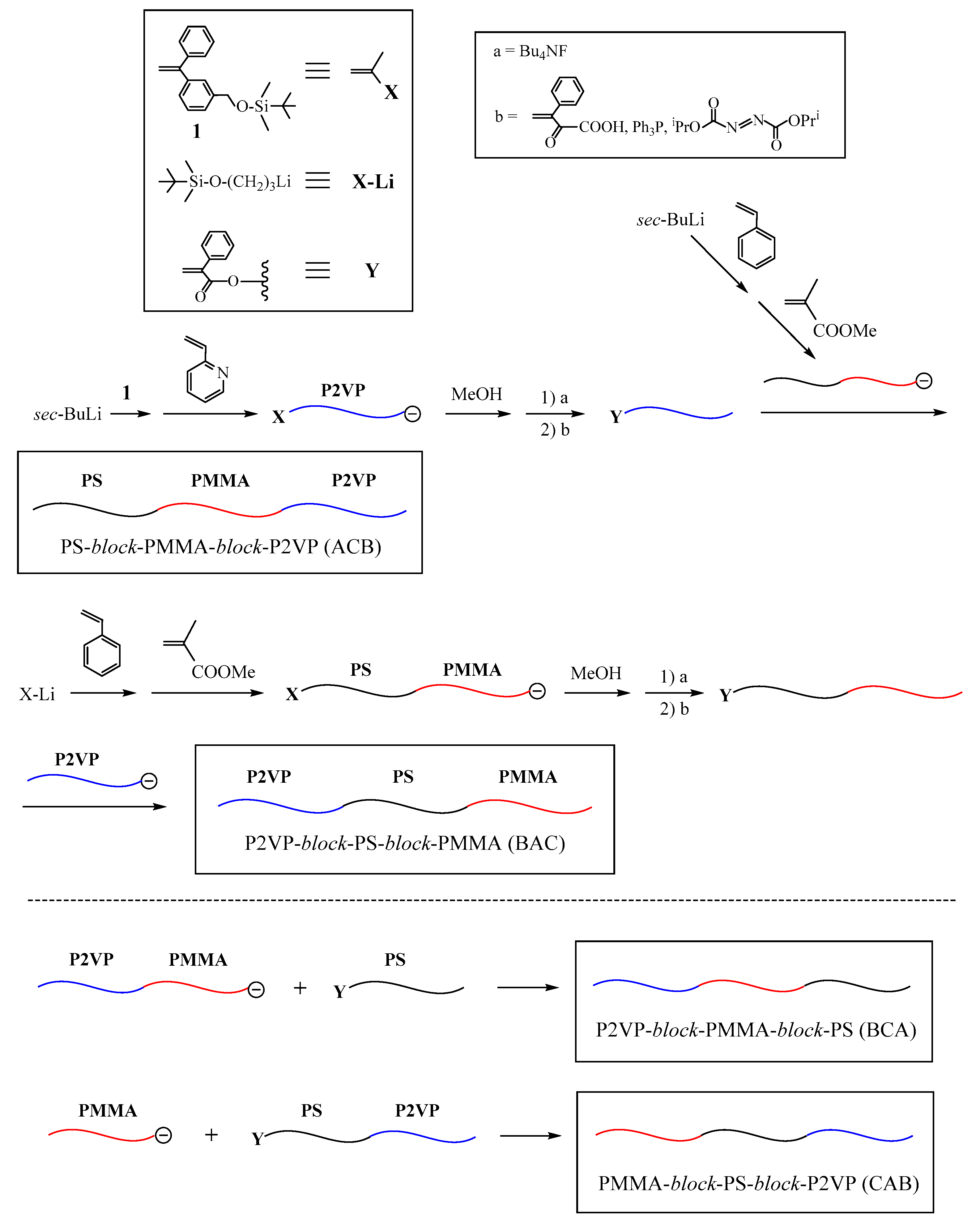
The first successful synthesis of multicomponent (μ-SP) by an iterative methodology is shown in
Scheme 1 [
47,
48]. In the first reaction sequence, a living chain-end-
X-functionalized polymer (A) was prepared by the end-capping of a living polymer (A) with an
X-substituted 1,1-diphenylethylene (DPE). After the termination, the introduced
X terminus was converted to a
Y reaction site, which can react with a living polymer. A living chain-end-
X-functionalized polymer (B) was prepared and in situ reacted with the resulting
Y-functionalized polymer (A), thus forming an in-chain-
X-functionalized diblock copolymer. After converting the
X to
Y reaction site, a newly prepared slightly excess amount of living chain-end-
X-functionalized polymer (C) reacted with the
Y-functionalized diblock copolymer to result in an ABC (μ-SP). The objective coupling product was isolated by fractional precipitation using appropriate solvent system or using preparative size exclusion chromatography (SEC). Similarly, an ABCD (μ-SP) was obtained by iterating the reaction sequence with a living chain-end-
X-functionalized polymer (D).
The living chain-end-
X-functionalized polymer was prepared by end-capping a living anionic polymer with 1-(3-
tert-butyldimethylsilyloxymethylphenyl)-1-phenylethylene (
1). The 3-
tert-butyldimethylsilyloxymethylphenyl ω-terminus was quantitatively converted to the benzyl bromide (BnBr) reaction site with a 1:1 mixture of (CH
3)
3SiCl and LiBr. The results are summarized in
Table 1. All of the polymers possessed the well-controlled
Mn values and compositions and extremely low polydispersity indexes. The successful syntheses of the requisite ABC and ABCD (μ-SP)s are thus obvious.
As can be seen in
Scheme 1, the reaction steps, (1) and (2), in each of all reaction sequences are equal to the “polymer chain (arm in this case) introduction” and the “regeneration of the same reaction site”. The living end-
X-functionalized polymer plays as a key role in order to introduce both the arm and the
X functionality in each sequence. Since the final (μ-SP) still has the
X functionality convertible to the
Y reaction site, this procedure enables further reaction sequence. Thus, the first proposed iterative methodology works very satisfactorily as designed.
The DPE derivative, 1, is capable of not only reacting with a living polymer but also of offering the X function. Thus, the use of 1 is indispensable in the methodology, but may provide a certain restriction. The living polymer, which can react with 1, is essentially limited to highly reactive living polystyrene (PSt), polyisoprene (PIs), poly(1,3-butadiene) (PBd), or living polymers derived from their related monomers. Unfortunately, living poly(2-vinylpyridine) (P2VPy) and poly(methyl methacrylate) (PMMA) with interesting functional groups cannot be used due to their very low or no reactivities for the DPE functionality. The use of living P2VPy and (PMMA) in the linking reaction is possible to introduce P2VPy or (PMMA) segment. In such a case, however, the reaction sequence can no longer be repeated because the X functionality is not introduced in the linking reaction.
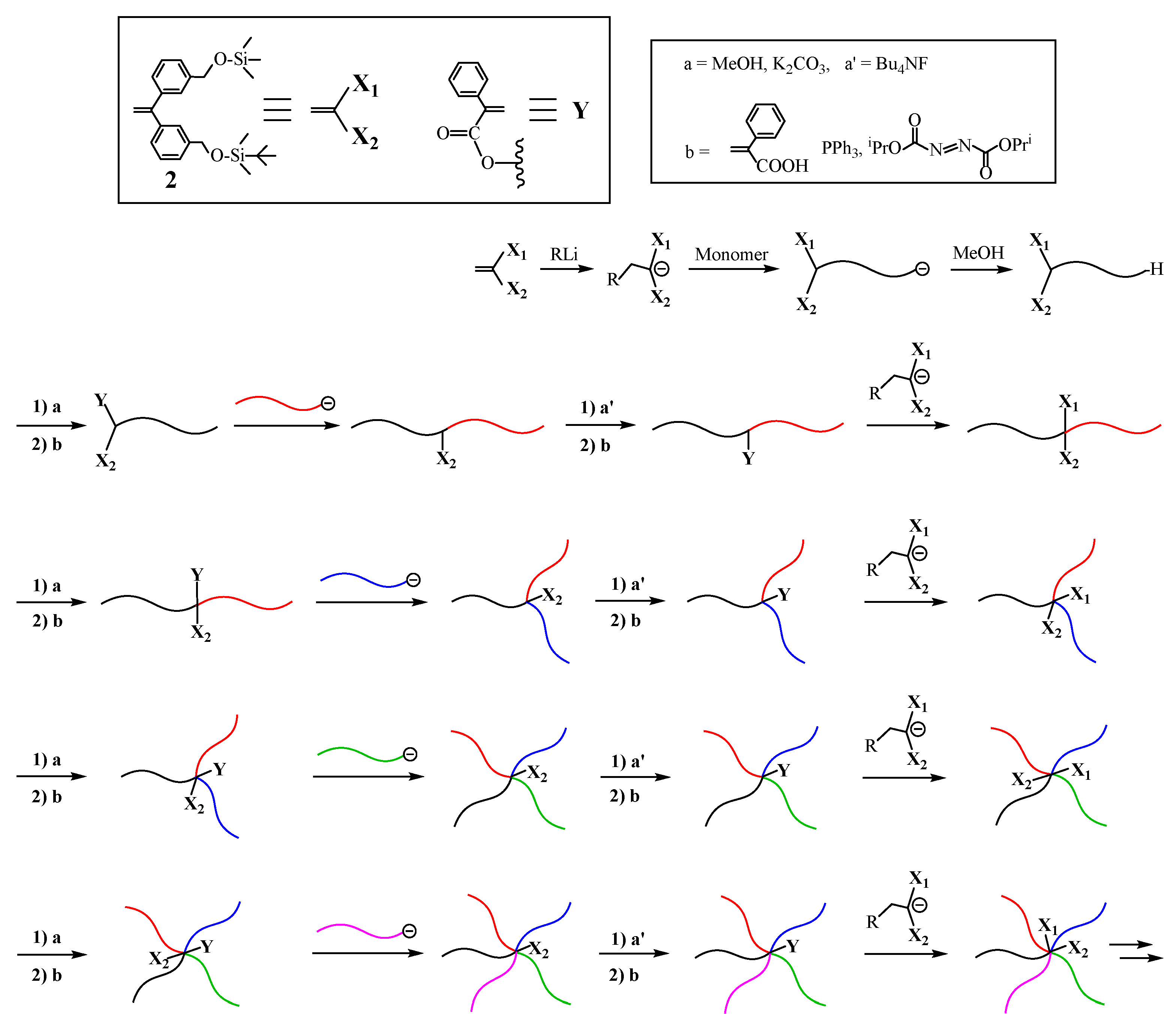
Very recently, a new and improved methodology has been proposed, as illustrated in
Scheme 2 [
49,
50,
51,
52]. The key of this methodology is to utilize two functionalities,
X1 and
X2, which can be deprotected in turn to separately convert to the first and second
Y reaction sites. As the
X1 and
X2 functionalities, trimethylsilyl (TMS) and
tert-butyldimethylsilyl (TBDMS) ethers were selected. As the starting polymer, a living polymer (A) with TMS and TBDMS termini was prepared by the living polymerization with the functional anion obtained from
sec-BuLi and 1-(3-
tert-butyldimethylsilyloxymethylphenyl)-1-(3-trimethylsilyloxymethylphenyl)ethylene (
2). The first reaction sequence involves deprotection of the TMS group and the subsequent conversion of the regenerate OH group to an α-phenylacrylate (PA) reaction site, the reaction of a living polymer (B) with the reaction site, deprotection of the TBDMS group, followed by conversion to the reaction site, and the reaction of the functional anion with the reaction site to reintroduce the TMS and TBDMS ethers.
As was seen in
Scheme 2, the TMS group was deprotected with MeOH containing K
2CO
3. The regenerated OH group was converted to the PA reaction site by the Mitsunobu reaction with α-phenylacrylic acid. The resulting (PA and TBDMS ether)-functionalized polymer was reacted with a living polymer (B) to result in an in-chain-(TBDMS ether)-functionalized AB diblock copolymer. The TBDMS group was then deprotected with (C
4H
9)
4NF, followed by conversion to the PA reaction site. The functional anion, again obtained from
sec-BuLi and
2, reacted with the reaction site to reintroduce the TMS and TBDMS ethers.
An ABC (μ-SP) with TMS and TBDMS ethers at the core was synthesized by iterating the second sequence. The reaction sequence was further continued to yield ABCD and ABCDE (μ-SP)s. Thus, the first and second reaction sites are utilized for the arm introduction and the reintroduction of
X1 and
X2 functionalities convertible to the same reaction sites. The results are summarized in
Table 2. Agreement among the calculated
Mn values, compositions and those observed was satisfactory and low polydispersity indexes were attained in all the resulting (μ-SP)s. Thus, the new methodology efficiently and expectedly operates to successively synthesize ABC, ABCD, and even ABCDE (μ-SP)s. The synthesis of (μ-SP)s with more compositions would be possible, since the two silyl ethers were introduced into the 5-arm (μ-SP).
Unlike the first methodology, the advantage of this new methodology is that various living polymers, ranging from less reactive living (PMMA), P2VPy, to highly reactive living PSt, PIs, and PBd, are usable for the arm introduction because any of these living polymers readily reacts with the PA reaction site with the quantitative conversion of the reactive site. In fact, with this methodology, less reactive living (PMMA), poly(ethyl methacrylate), poly(tert-butyl methacrylate), poly(benzyl methacrylate), and poly(2-methoxyethyl methacrylate) all quantitatively reacted to afford a 5-arm all poly(methacrylate)-based ABCDE (μ-SP) for the first time. In the methodology, the BnBr functionality can also be used as the reaction site. However, as the conversion to the BnBr reaction site is usually carried out under strongly acidic conditions, some poly(alkyl methacrylate)s are not stable and pyridine ring in P2VPy might be formed to pyridinium salt under such conditions.
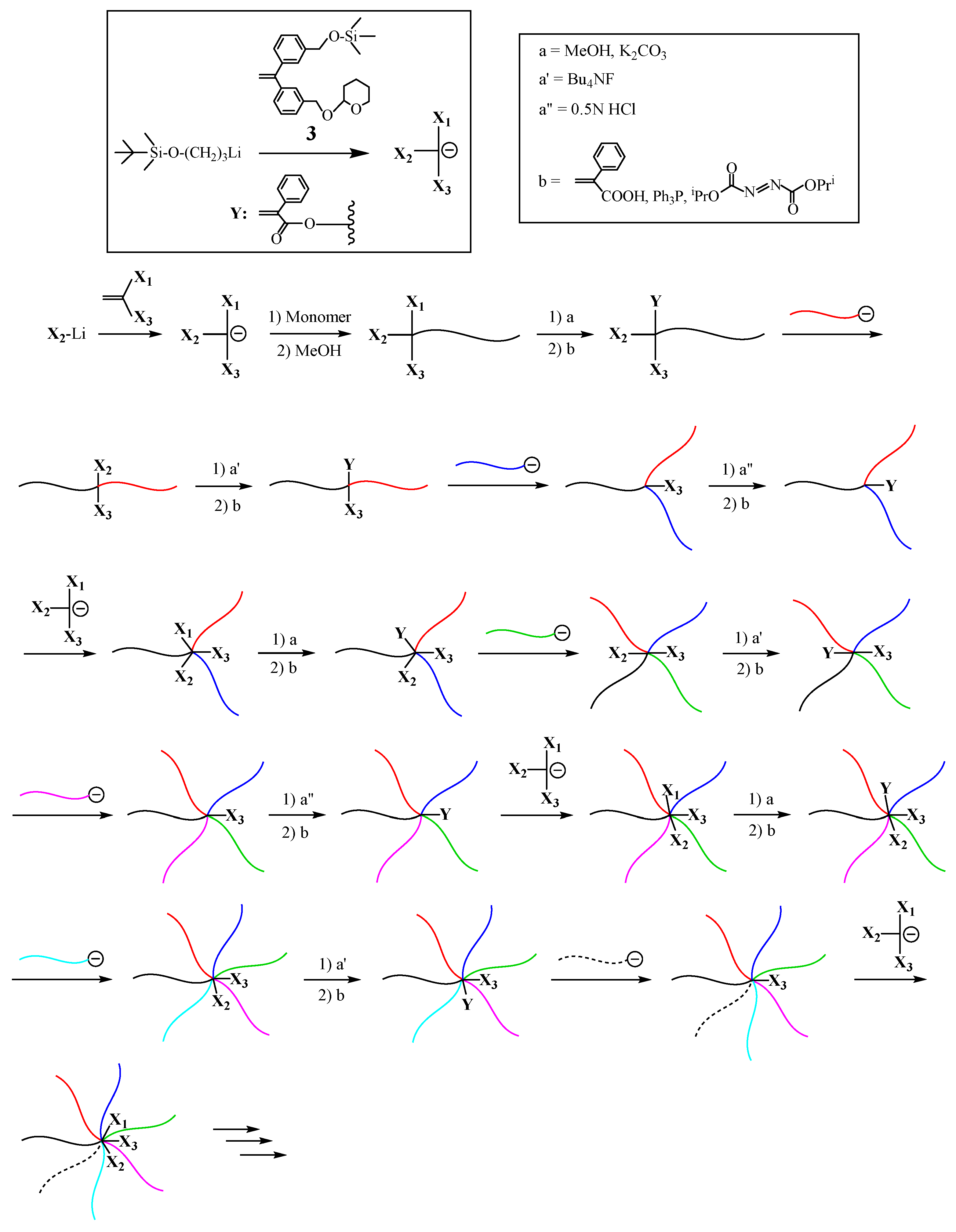
The new iterative methodology was further extended to the methodology with three different protected functionalities,
X1,
X2, and
X3, as illustrated in
Scheme 3 [
53]. The three functionalities are designed to be sequentially converted one order to the first, second, and third
Y reaction sites at different steps. For this design, the TMS, TBDMS, and 2-tetrahydropyranyl (THP) ethers were chosen as the
X1,
X2, and
X3 functionalities. The TMS group was first deprotected with MeOH containing K
2CO
3 and, on the other hand, the TBDMS and THP ethers remained unchanged. The TBDMS group was then deprotected with (C
4H
9)
4NF under the conditions where the THP ether was stable. The THP ether was finally deprotected with 0.5 M HCl. The regenerated OH group in each step was converted to the PA reaction site one by one.
The starting α-terminal (TMS, TBDMS, and THP ethers)-functionalized poly(cyclohexyl methacrylate) (PCHMA) was prepared by the living polymerization of CHMA with the functional anion from 3-tert-butyldimethylsilyloxy-1-propyllithium and 1-(3-(2-tetrahydro-2H-pyranyloxy)methyl)phenyl-1-(3-trimethylsilyloxymethylphenyl)ethylene (3). The reaction sequence involves several steps as follows: the TMS ether deprotection and conversion to the PA reaction site, the linking with a living polymer, the TBDMS ether deprotection and conversion to the reaction site, the second linking reaction with another living polymer, the THP ether deprotection and conversion to the reaction site, and the reaction with the functional anion to reintroduce the TMS, TBDMS, and THP ethers. By iterating such the reaction sequence, the successive synthesis from ABC, ABCD, ABCDE, ABCDEF and to even ABCDEFG (μ-SP)s were successfully achieved.
As summarized in
Table 3, the
Mn values observed by RALLS agreed well with calculated values and low polydispersity indexes were attained in all the (μ-SP)s. Their compositions were close to calculated values. Thus, the successful syntheses of expected (μ-SP)s were evident from the results listed in this table. Since the final ABCDEFG (μ-SP) still has the THP functionality, the reaction sequence is possibly iterated. Thus, the third proposed methodology also works well as designed. The two arms are introduced by the first and second reaction sites and the three ethers are reintroduced by the third reaction site to enable further reaction sequence.
In this section, we proposed three iterative methodologies. In the first methodology, the living end-
X-functionalized polymer was used as the building block in each reaction sequence and the next sequence was continued after converting the
X function to the
Y reaction site. In the last two methodologies shown in
Scheme 2 and
Scheme 3, the functional anions were employed to reintroduce
X1,
X2 and
X1,
X2,
X3 functionalities, with which their reaction sequences could be iterated. It should be mentioned, however, that these methodologies are basically designed in the same concept so that each reaction sequence involves two steps of the arm introduction and regeneration of the same reaction site and can be iterated to synthesize a series of multicomponent (μ-SP)s. As often mentioned, one more important advantage of such iterative methodologies is that, in essence, the use of multistep selective reactions and several reaction sites with different reactivities generally required for the (μ-SP) synthesis are completely avoided because one arm is introduced in each reaction step.
2.2. Dendrimer-Like Hyperbranched Polymers ((DHBH)s)
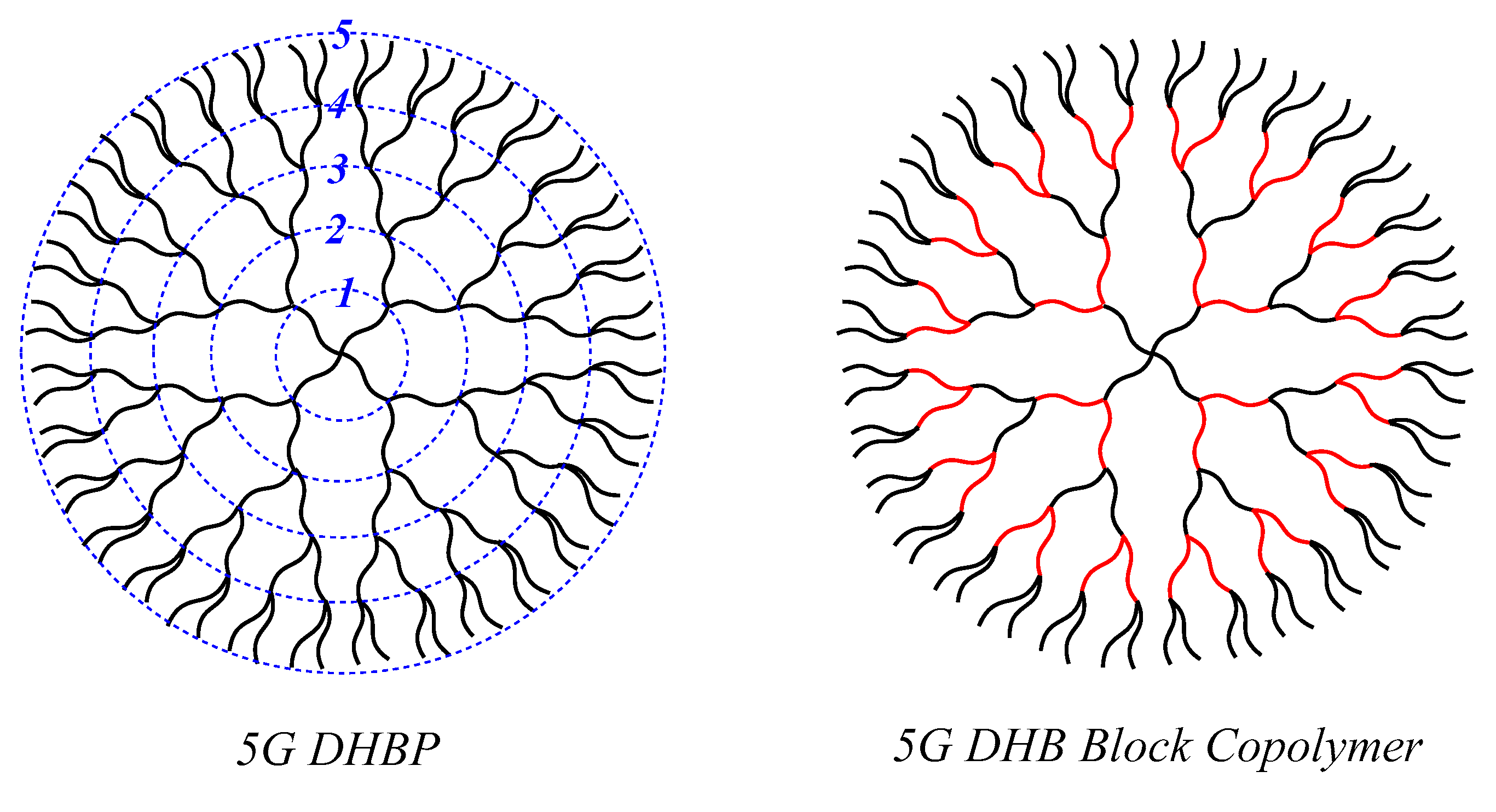
A (DHBP) has emerged as the new class hyperbranched polymer with structural perfection since 1995 [
54]. In order to clearly image the structure of (DHBP), the fifth-generation (5G) (DHBP) and its block copolymer are shown in
Figure 1. These polymers are similar in branched architecture to dendrimers, but comprise several polymers connected between the layers. Accordingly, (DHBP)s are much higher in molecular weight and much larger in molecular size than dendrimers. DHPBs, believed to be globular macromolecules in shape, have many features, such as topologically specific hyperbranched architectures, hierarchic repeated layer structures, so-called “generation”, different branched densities among the core and layers, and many termini [
55,
56,
57,
58,
59].
Although various (DHBP)s have been synthesized to date, most of them were limited to 2G~4G polymers with
Mn values of 10
5 g·mol
−1 order due to several reaction steps for the introduction of additional polymer chains [
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76,
77,
78,
79]. As for high-generation (≥4G) and high-molecular-weight (≥10
6 g·mol
−1) (DHBP)s with well-defined structures, there were reported only two examples synthesized by Gnanou et al. (7G DHB PSt with a
Mn of 1.92 × 10
6 g·mol
−1 and 8G DHB poly(ethylene oxide) with a
Mn of 6.50 × 10
5 g·mol
−1) and several (DHBP)s synthesized by Hirao et al. which will be described in this section [
80,
81,
82,
83,
84,
85,
86,
87].
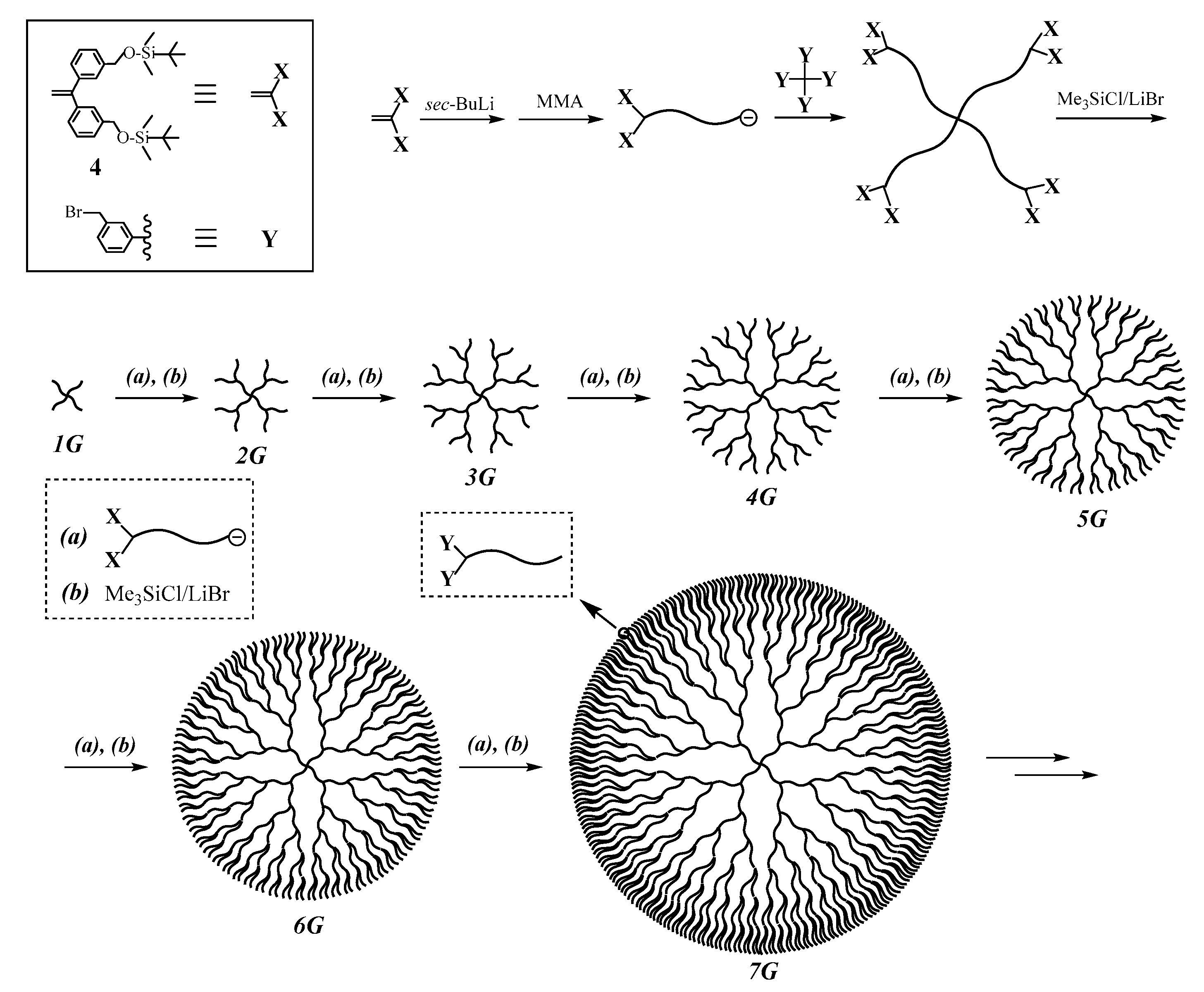
As can be seen in
Figure 1, (DHBP)s comprise several repeating units on the basis of layer structures. It is, therefore, expected that the iterative methodology is applicable to the synthesis of (DHBP)s, as illustrated in
Scheme 4 [
82,
83]. In the first reaction sequence, a living α-chain-end-(
X)
2-functionalized polymer reacted with a core having four
Y reaction sites to result in a 4-arm (SP). The introduced eight
X termini were then converted to the
Y reaction sites. In the second sequence, the resulting (SP) with (
Y)
8 termini was linked with a separately prepared living α-chain-end-(
X)
2-functionalized polymer to yield a 2G (DHBP) with sixteen
X termini. Thus, the living α-chain-end-(
X)
2-functionalized polymer is used as the building block in each reaction sequence. As was seen, the (DHBP) was obtained from the second sequence. The linking of the living polymer with either the core compound or the polymer with (
Y)
8 termini and the conversion to the
Y reaction site are exactly equal to (1) the arm introduction and (2) regeneration of the reaction site, respectively. Since the 2G (DHBP) possesses the sixteen
Y reaction sites, the reaction sequence can be repeated. Indeed, it was repeated to successively synthesize 3G, 4G, 5G, 6G, and even 7G (DHBP)s (see
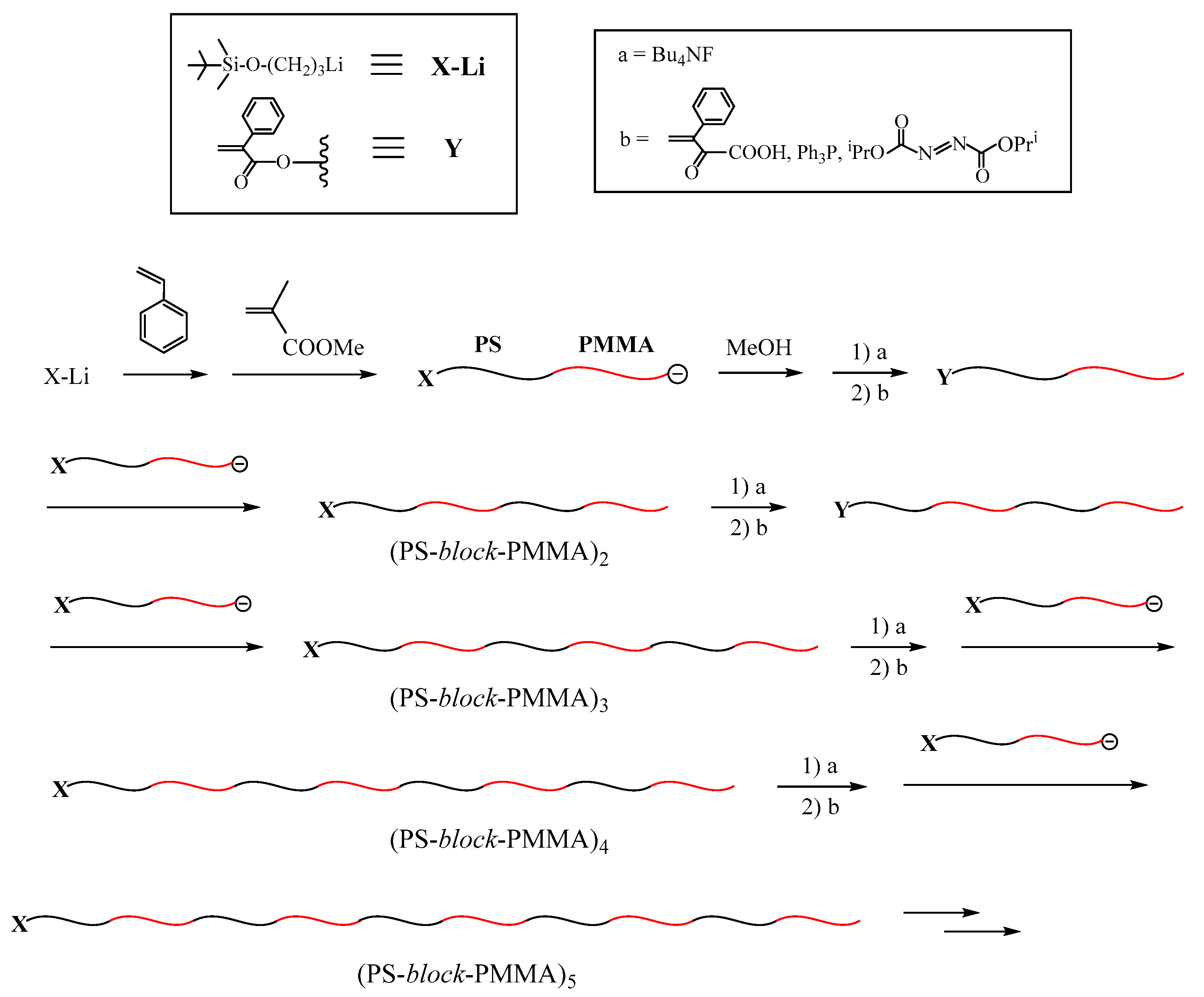
Scheme 5). As can be seen, the polymer obtained in one reaction sequence before becomes the starting polymer in the next sequence [
83].
The building block was prepared by the living polymerization of MMA with the initiator obtained from sec-BuLi and 1,1-bis(3-tert-butyldimethylsilyloxymethylphenyl)ethylene (4). Similar to the previous methodologies, the introduced TBDMS ether (X) is readily and quantitatively converted to the BnBr (Y) reaction site. The linking reactions proceeded with ~100% efficiencies at −40 °C for a few hours with 2 equivalents of living α-chain-end-(TBDMS)2-functinalized (PMMA) to afford 2G, 3G, and 4G polymers. The use of 3–5 equivalents of living (PMMA) and longer reaction times to 48 h were needed to complete the reaction, yielding 5G, 6G, and 7G polymers.
As summarized in
Table 4, the resulting DHB (PMMA)s all had well-controlled
Mn values in agreement with those calculated and low polydispersity indexes, (
Mw/
Mn ≤ 1.03). Agreement between the calculated values and those observed in end-functionality is excellent in each of all samples. The 7G DHB (PMMA) was a huge macromolecule with a
Mw value of 1.97 × 10
6 g·mol
−1 and consisted of 508 (PMMA) segments having 512 BnBr termini, which enable further synthetic sequence of more generation (DHBP)s. Thus, the synthesis of well-defined (DHBP)s was quite successful by employing the iterative methodology using the living α-chain-end-(TBDMS ether)
2-functionalized polymer as the building block. This success also made it possible to synthesize other (DHBP)s made up of poly(
tert-butyl methacrylate) (P
tBMA), PSt, and a mixture of these two polymers by a slightly modified procedure using the PA reaction site. Several amphiphilic generation-based block copolymers with PSt and poly(methacrylic acid) layers could be obtained from the DHB block copolymers consisting of (P
tBMA) and PSt, followed by hydrolysis of the (P
tBMA) segments [
86].
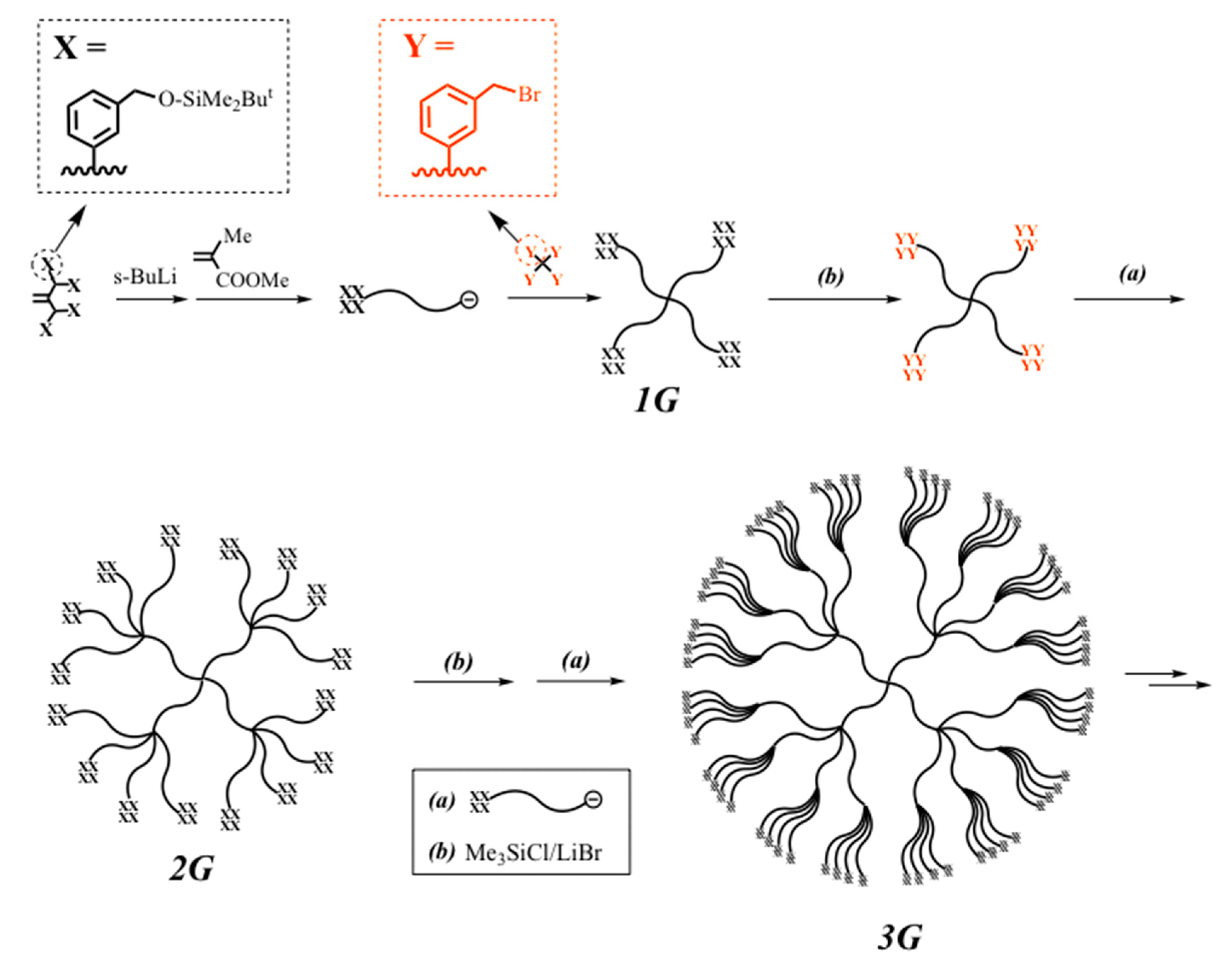
A highly dense 3G DHB (PMMA) with four polymer chains branched at the core as well as in the two layers was synthesized by the iterative methodology with a living α-chain-end-(TBDMS ether)
4-functionalized (PMMA) (see
Scheme 6) [
84]. The resulting polymer had a
Mn value of 9.30 × 10
5 g·mol
−1 and 256 BnBr termini (see
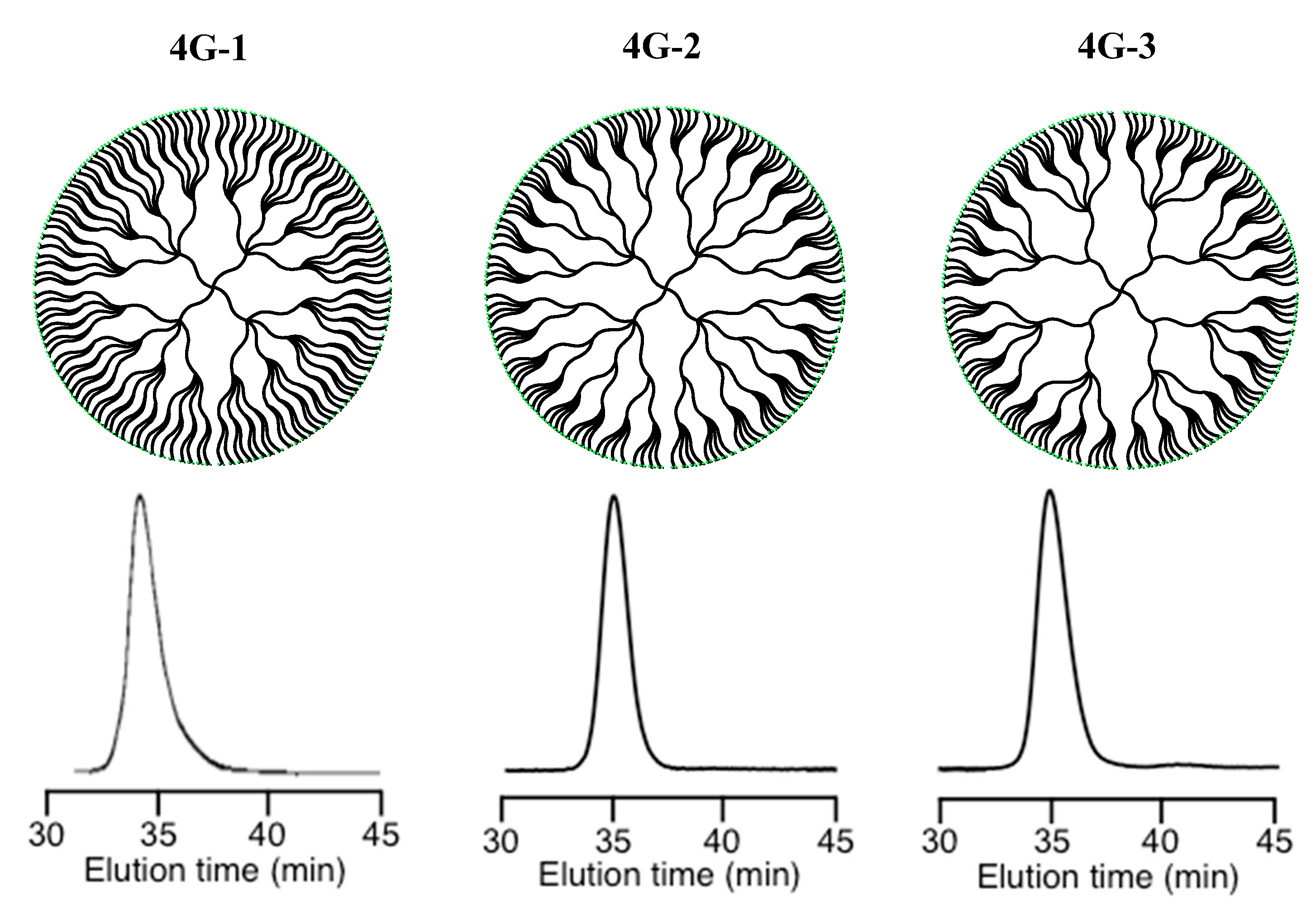
Table 5). Further synthesis was not possible in this case. Instead, three different 4G (DHBP)s shown in
Figure 2 could be synthesized by using the living α-chain-end-(TBDMS)
2-functionalized (PMMA) in either the second, third, or fourth reaction sequence. All of the polymers were around 2 million in
Mn value with 512 BnBr termini even in the 4G polymers (see also
Table 5) [
85].
We demonstrated the effectiveness of the iterative methodology for the synthesis of high-generation and high-molecular-weight (DHBP)s. The methodologies shown in
Scheme 4,
Scheme 5 and
Scheme 6 are basically the same as that shown in
Scheme 1, except for the use of different building blocks and the core compound. Since highly reactive BnBr or PA termini are present in the intermediate and final polymers, various useful functionalities, such as perfluoroalkanes, olefins (double bonds), acetylenes (triple bonds), alkoxy and hydrosilanes, epoxide, thiol, phosphine, ferrocene, and monosaccharide residues, etc., can be introduced at any generation, internal, and external positions [
55]. As a result, well-defined functional hyperbranched and nano-size globular macromolecules with many potential applications are synthesized. Novel and interesting morphological nanostructures and supramolecular assemblies will also be expected from generation-based DHB block polymers [
55,
56,
58,
76,
77,
79].
2.3. Graft Polymers ((GP)s)
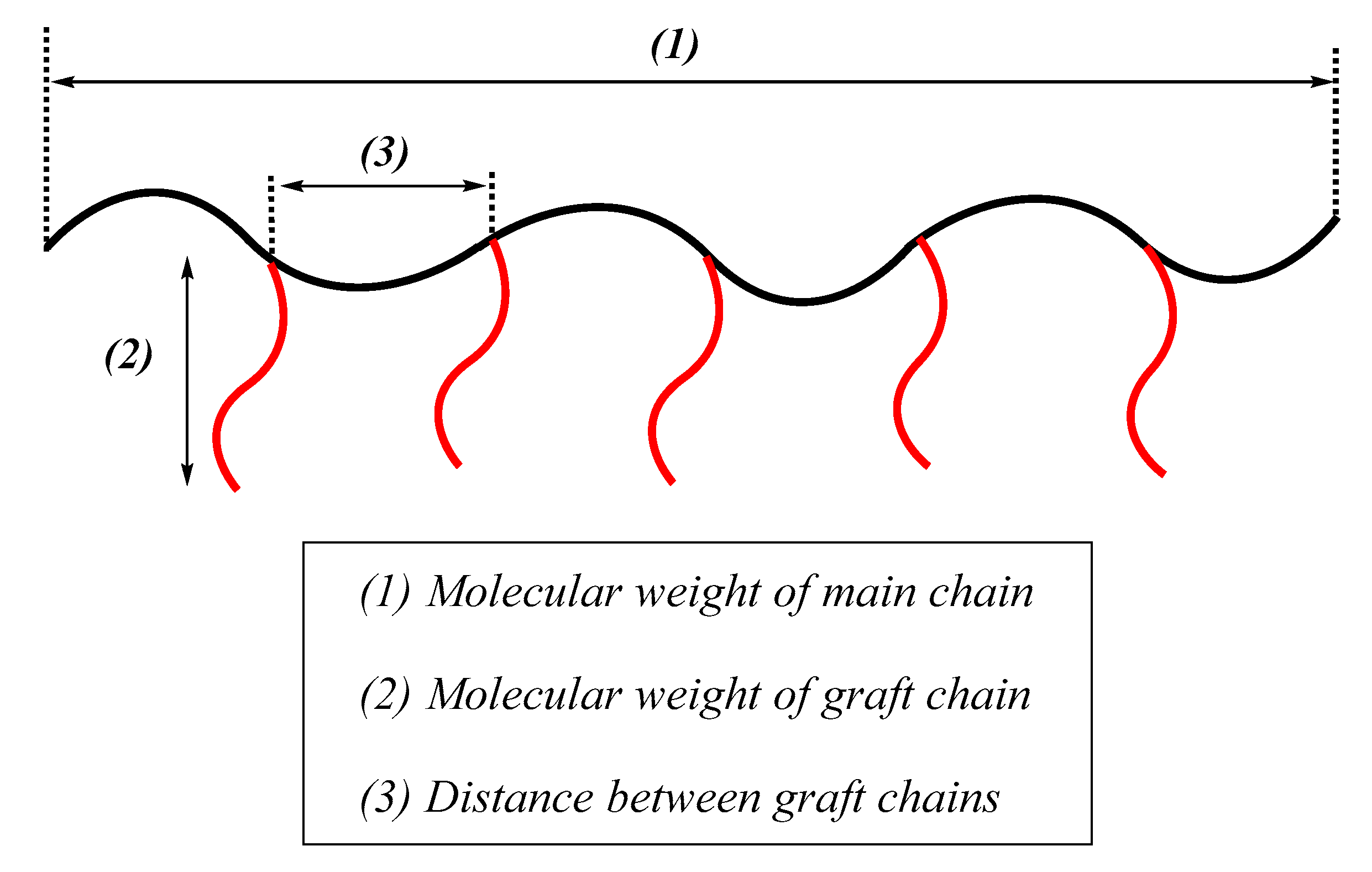
As illustrated in
Figure 3, a (GP) is defined by three structural parameters: (1) molecular weight of the main chain; (2) molecular weight of the graft chain; and (3) distance between the graft chains. An ideal (GP), in which the three parameters are perfectly controlled, is termed an “exactly defined graft polymer (EDGP)” [
88]. Although the physical and mechanical properties as well as morphology of (GP) are significantly influenced by such three structural parameters, their relationships have not been enough elucidated due to the synthetic difficulty of (EDGP) [
1,
2,
89,
90,
91]. In practice, various (GP)s have been synthesized so far, but all of them are not (EDGP)s, except for the PIs–
graft–PSt reported by Hadjichristidis et al. [
88] and several graft polymers synthesized by Hirao et al., which will be introduced in this section [
92,
93,
94,
95,
96,
97].
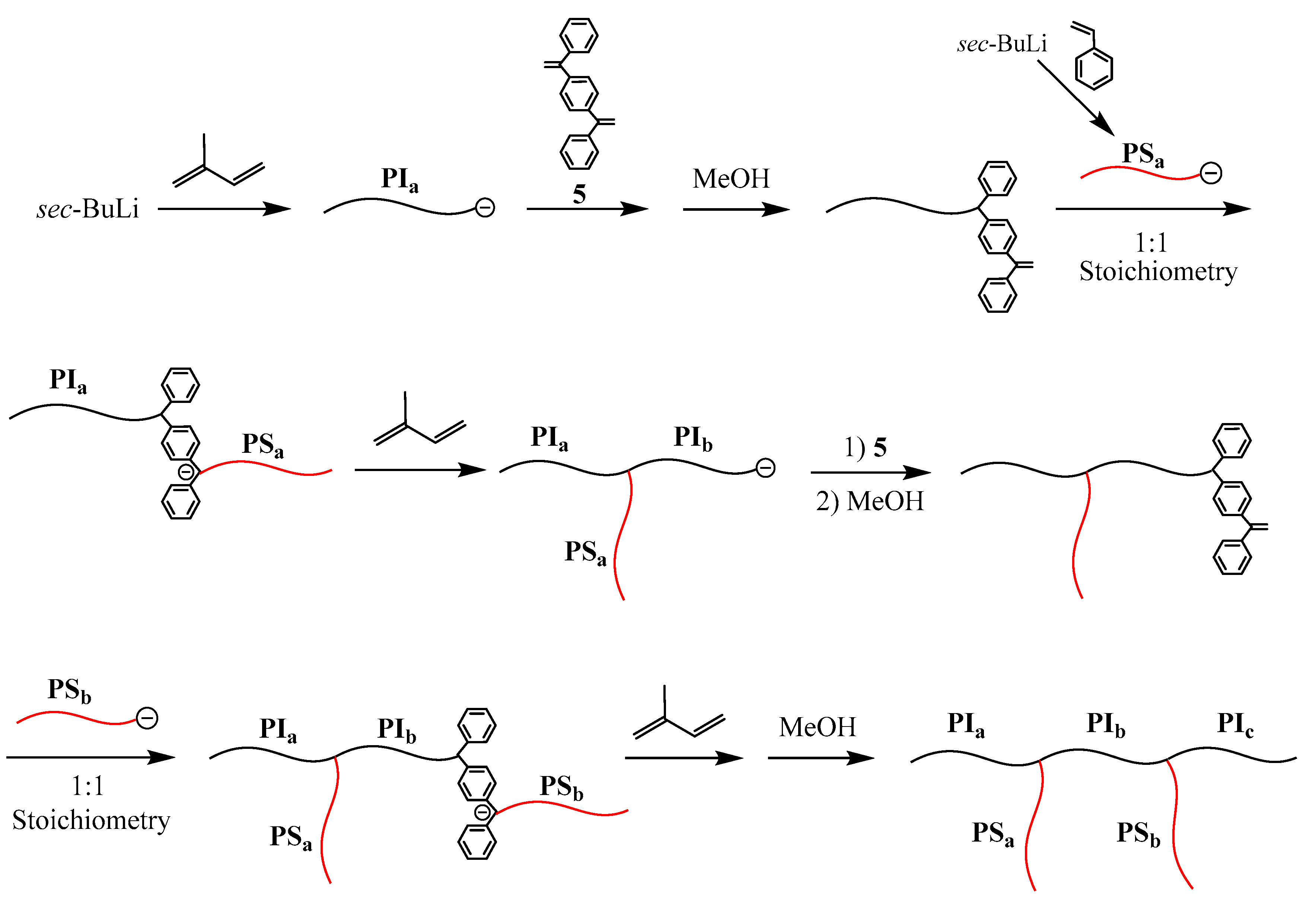
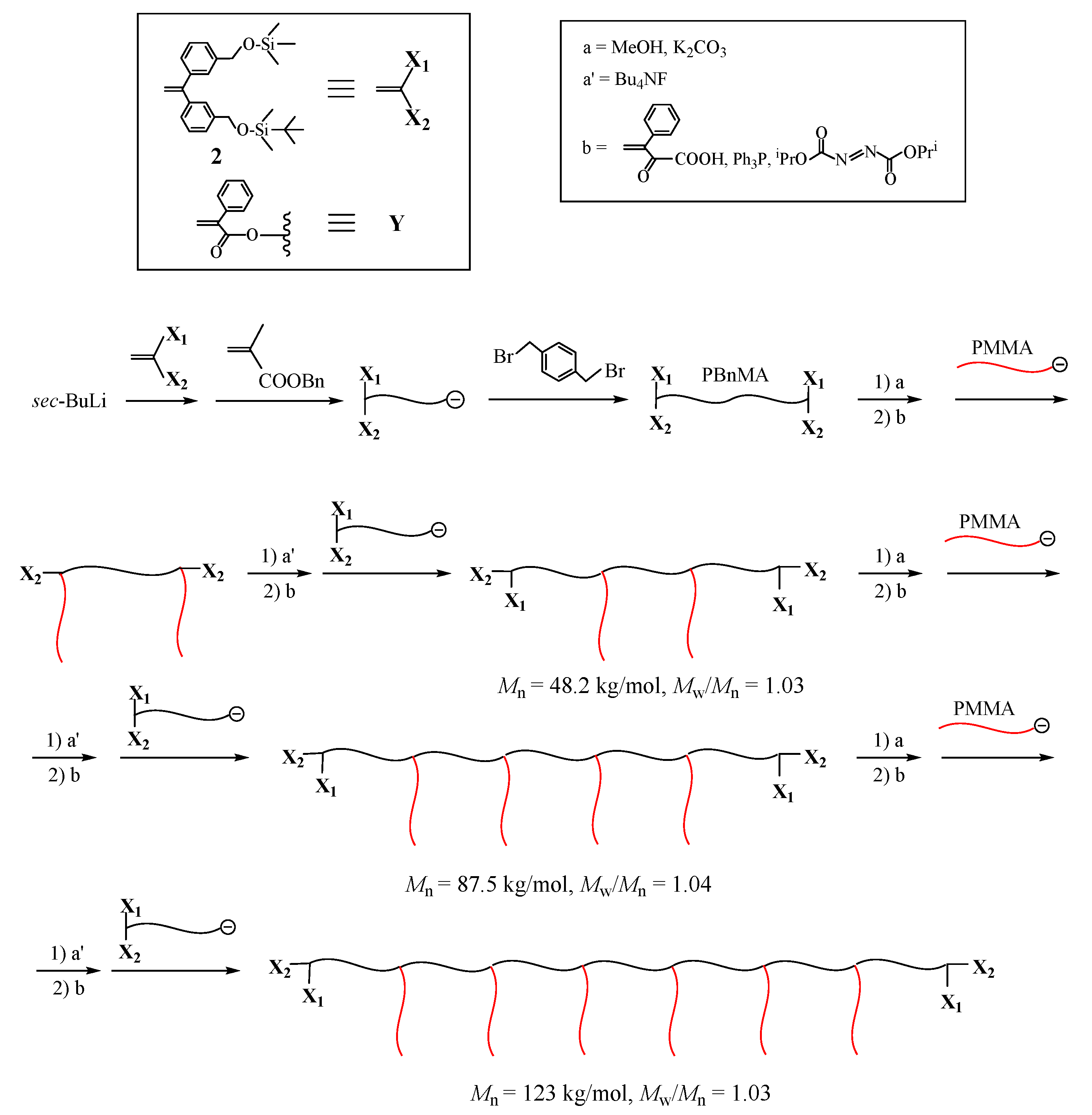
As shown in
Scheme 7, a living PIs reacted with 1,4-bis(phenylethenyl)benzene (
5) to introduce a DPE terminus. After the termination, a living PSt reacted with the DPE terminus to prepare a PIs–
block–PSt in-chain anion, from which isoprene was polymerized. The resulting living 3-arm (SP) reacted with
5 to again introduce the DPE terminus. The same reaction sequence was iterated to synthesize a PIs–
graft–PSt with two PSt graft chains. In the resulting polymer, the three structural parameters are perfectly controlled by the living polymerization of either isoprene or styrene in each step. Although the synthesis of (EDGP)s with more than three graft chains is possible by iterating the reaction sequence, it seems difficult because an exact stoichiometry is required in each reaction between living PSt and the DPE terminus. If the stoichiometry is deviated in the reaction, many side products difficult in separation are by-produced. Later, an (EDGP) having five graft chains was successfully synthesized by an improved methodology based on the termination procedure [
92].
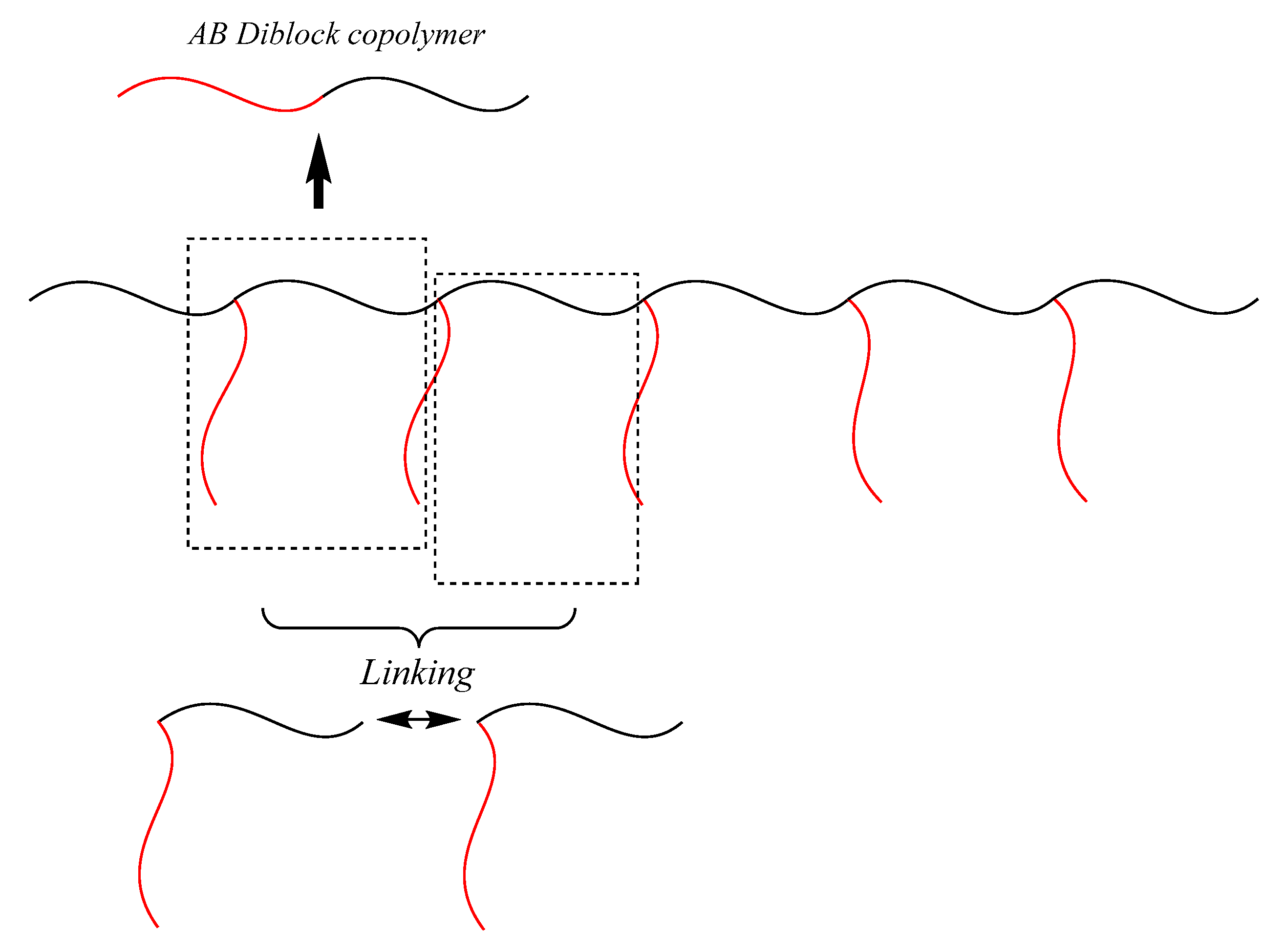
It is considered that the iterative methodologies are applicable because (EDGP)s comprise several repeating units linked each other. As can be seen in
Figure 4, one building unit of graft polymer corresponds to an AB diblock copolymer and the two block copolymers are linked between the chain-end and in-chain of diblock copolymers to make the graft unit. Therefore, if the linking of diblock copolymers can be iterated, a series of exactly defined graft copolymers would be synthesized.
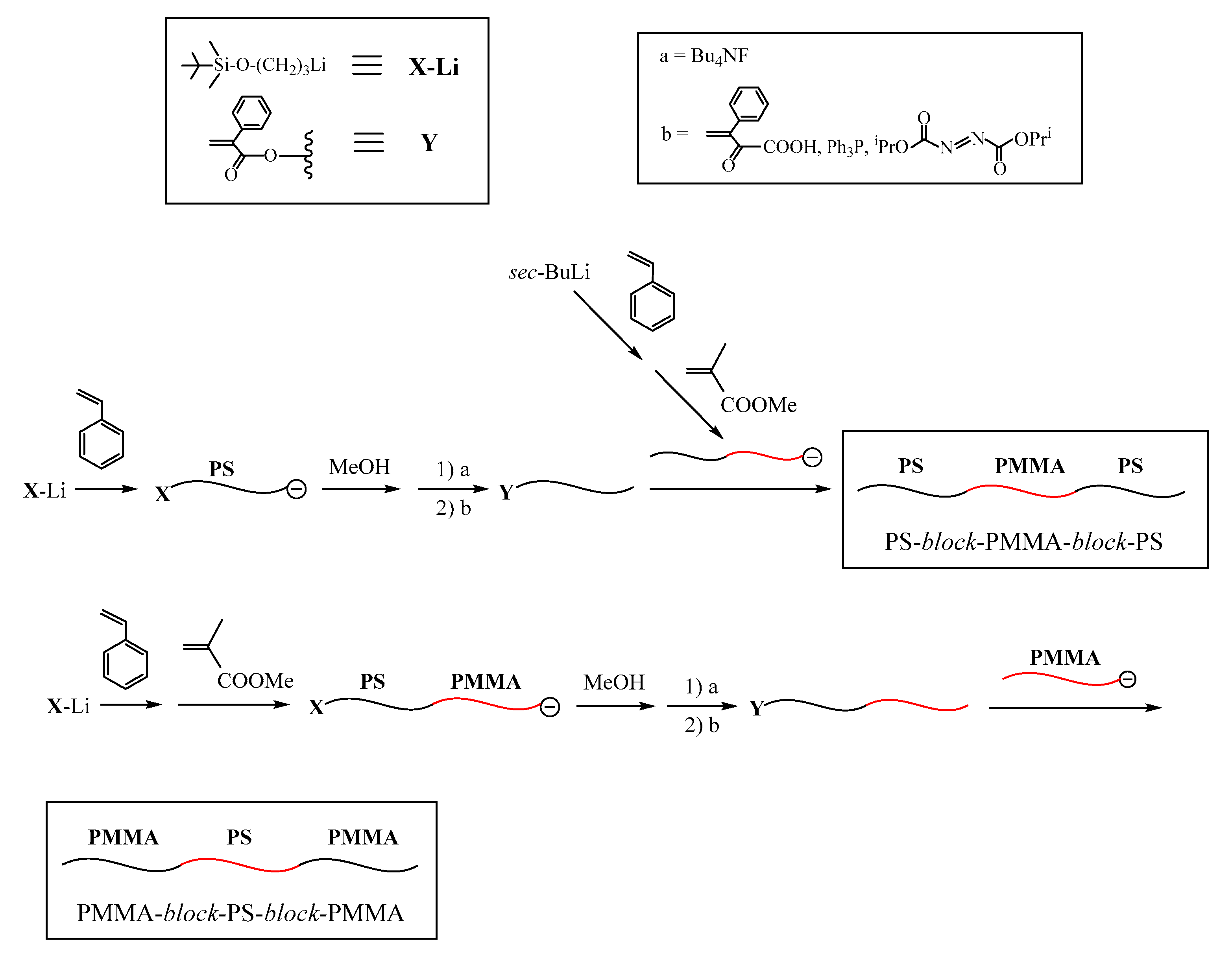
On the basis of the linking manner indicated in
Figure 4, we proposed the iterative methodology as illustrated in
Scheme 8 [
93,
94]. This methodology is basically the same as those shown in
Scheme 1,
Scheme 4,
Scheme 5 and
Scheme 6, except for the use of the living in-chain-
X-functionalized diblock copolymer as the building block. It was prepared by the sequential addition of styrene,
1, and MMA and, after the termination, the TBDMS ether was converted to the BnBr reaction site. Another living in-chain-TBDMS ether-functionalized PSt–
block–PMMA was reacted with the resulting PA-functionalized PSt–
block–PMMA to link the two block copolymers between the chain-end and the in-chain. Since the TBDMS ether was also introduced, repetition of the same reaction sequence is possible. In practice, the reaction sequence was iterated four more times. The final polymer with five graft chains was yielded by linking the intermediate graft copolymer with a living (PMMA). The requisite structures of the synthesized graft copolymers all were clearly evidenced from the results summarized in
Table 6. Thus, the proposed methodology satisfactorily worked as expected.
Each reaction sequence involves the linking reaction between two block copolymers and the conversion to the reaction site, which are equal to the “polymer chain introduction” and “regeneration of the same reaction site” and can be iterated via the regenerated same reaction site. The three parameters of the resulting polymers are controlled and can be intentionally changed by the living first and second blocks. With this in mind, three additional acid-labile segment containing exactly defined PtBMA–graft–PSt, poly(ferrocenylmethyl methacrylate (PFMMA))–graft–PSt, and P2VPy–graft–PSt were also synthesized by the same iterative methodology using a PA reaction site. All of these polymers thus synthesized by the iterative methodology are the first successful (EDGP)s with more than three graft chains.
Recently, we have succeeded in the synthesis of an EDG terpolymer with two different graft chains per one graft point by the iterative methodology using a living in-chain-(
X1 and
X2)-functionalized diblock copolymer (see
Scheme 9) [
97]. Both the
X1 and
X2 functionalities are designed so as to be converted in turn to the first and second
Y reaction sites in different reaction steps. The first graft chain corresponding to the first PSt block was already present. The second graft chain was introduced by linking a living polymer at the first
Y reaction site converted from the
X1 function. Next, a newly prepared living in-chain-(
X1 and
X2)-functionalized block copolymer reacted with the second
Y reaction site from the
X2 function. As the
X1 and
X2 functionalities were thus reintroduced, the same reaction sequence was iterated to continue the synthesis of EDG terpolymers.
As mentioned before, the TMS and TBDMS ethers are used as the X1 and X2 functionalities, respectively. In the first reaction sequence, a living in-chain-(TMS and TBDMS ethers)-functionalized PSt–block–PMMA was prepared by the sequential polymerization of styrene, 2, and MMA. The introduced TMS group in-chain was deprotected with MeOH containing K2CO3 and the regenerated OH group was converted to the PA reaction site. The second graft chain was introduced by linking with a living poly(2-methoxyethyl methacrylate) (P2MEMA). The TBDMS group was then deprotected with (C4H9)4NF, followed by converting to the reaction site. A living in-chain-(TMS and TBDMS ethers)-functionalized PSt–block–PMMA was newly prepared and reacted with the resulting PA-functionalized 3-arm (μ-SP) composed of PSt, (PMMA), and P2MEMA. By this linking reaction, one branched unit was made and the TMS and TBDMS ethers were also reintroduced. Repetition of the reaction sequence is possible via the two silyl ethers. An EDG terpolymer possessing three branched units was yielded after iterating the reaction sequence two more times, followed by linking with a living (PMMA) in the final step. Since all of the repeating reaction sequences proceeded almost quantitatively, the target polymers were always obtained in ca. 100% yields.
The polymers all were obtained with well-controlled
Mn values and compositions (see
Table 7). Accordingly, the proposed methodology also effectively worked to successfully yield the target EDG terpolymers. The three structural parameters of such graft terpolymers are also controlled and intentionally changed by the living polymerization in each step.
With the iterative methodology shown in
Scheme 8, both graft and main chains are advantageously introduced at the same time. We have recently developed an alternative methodology using a living α-chain-end-(
X1 and
X2)-functionalized polymer (see
Scheme 10) [
96]. The same as
Scheme 9, the
X1 and
X2 functionalities are TMS and TBDMS ethers and sequentially deprotected one by one to convert to the first and second PA (
Y) reaction sites. The synthesis was started to prepare a living α-chain-end-(TMS and TBDMS ethers)-functionalized poly(benzyl methacrylate) (PBnMA) by the living polymerization of BnMA with the functional initiator obtained from
sec-BuLi and
2. After the termination and the conversion of the TMS ether to the reaction site, a living (PMMA) reacted with the reaction site to result in an in-chain-TBDMS ether-functionalized (PMMA)–
block–PBnMA. The TBDMS ether was then converted to the reaction site. The in-chain-PA-functionalized (PMMA)–
block–PBnMA thus prepared was linked with a newly prepared living α-chain-end-(TMS and TBDMS ethers)-functionalized PBnMA. By this reaction, one branched unit was made and the TMS and TBDMS ethers were reintroduced. The reaction sequence was iterated twice. In each of the intermediate polymers thus obtained, the TBDMS ether was converted to the reaction site, followed by linking with a living PBnMA, to synthesize the exactly defined PBnMA–
graft–PMMA with two and three (PMMA) graft chains.
The results summarized in
Table 8 clearly show the successful synthesis of the exactly defined (PBnMA–
graft–PMMA)s. Thus, it is possible to synthesize all poly(methacrylate)-based (EDGP)s by this methodology. Needless to say, the synthesis of such (EDGP)s is difficult by the methodology shown in
Scheme 8. Similarly, a series of exactly defined (PBnMA–
graft–P2VPy)s were synthesized by the same methodology, in which living P2VPy was used instead of living (PMMA) in each reaction sequence.
A difunctional PBnMA with the TMS and TBDMS termini was prepared by the coupling reaction of a living α-chain-end-(TMS and TBDMS ethers)-functionalized PBnMA with
p,
p’-xylene dibromide. When this polymer was used as the starting polymer, (EDGP)s with two, four, and six (PMMA) graft chains were readily synthesized with less repetition of the reaction sequence (only three times) (see
Scheme 11).
In summary, we demonstrated the successful syntheses of EDG co- and terpolymers by developing the three iterative methodologies shown in
Scheme 8,
Scheme 9 and
Scheme 10. As often suggested in these methodologies, they are basically the same as the methodologies for the synthesis of (μ-SP)s and (DHBP)s. Three different building blocks, i.e., living in-chain-
X-functionalized diblock copolymer, living in-chain-(
X1 and
X2)-functionalized diblock copolymer, and living α-chain-end-(
X1 and
X2)-functionalized polymer, were employed. It should also be noted that the TBDMS ether (
X), TMS ether (
X1), and TBDMS ether (
X2) functionalities and the BnBr or PA (
Y) reaction site are the same as those used in the previous methodologies.

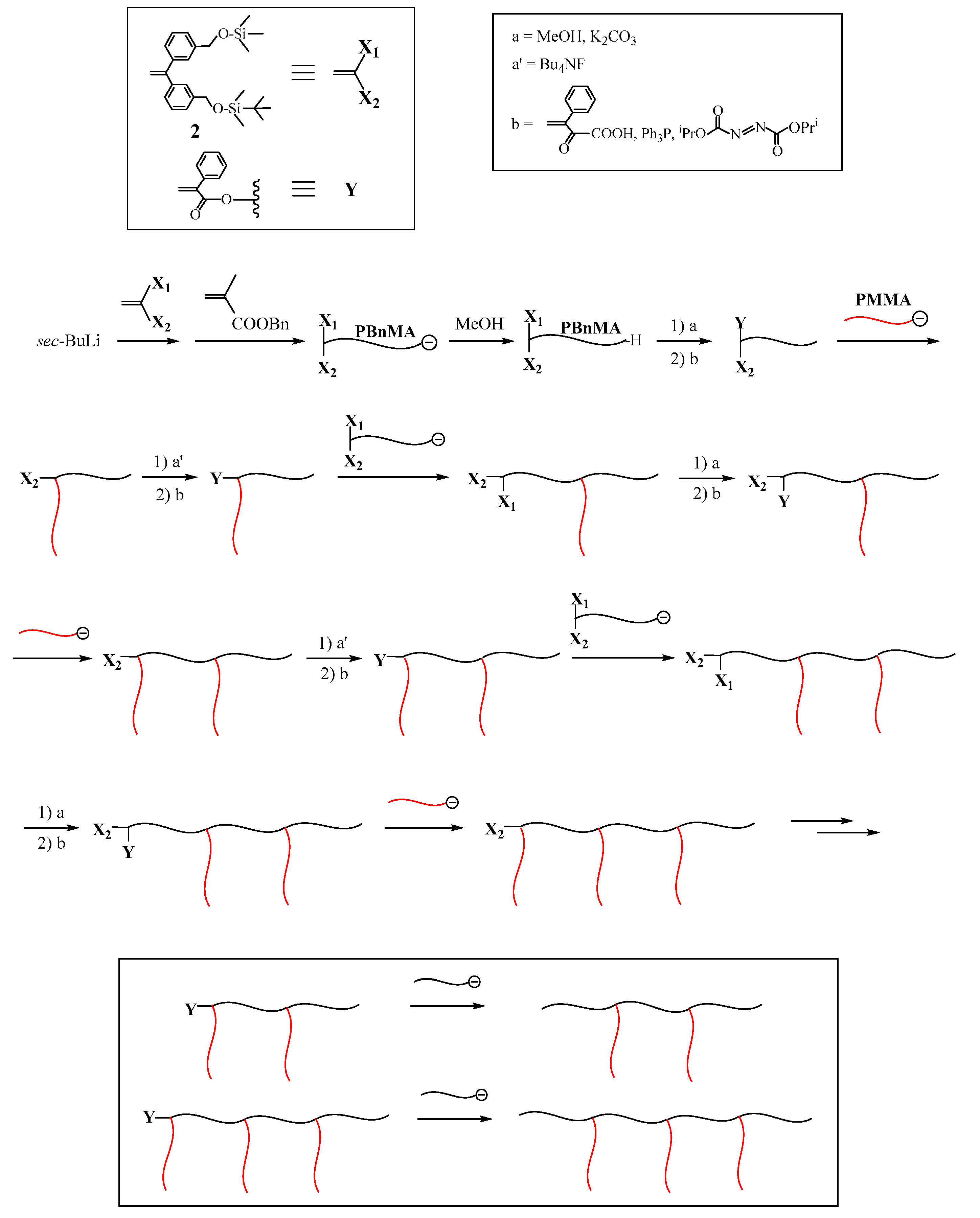















 : Homopolymerization.
: Homopolymerization.  : Living polymerization.
: Living polymerization.  : No polymerization. a) The polymerization proceeds very sluggishly along with unwanted side reacions.
: No polymerization. a) The polymerization proceeds very sluggishly along with unwanted side reacions.