Interfacial Hydrogen Bonds and Their Influence Mechanism on Increasing the Thermal Stability of Nano-SiO2-Modified Meta-Aramid Fibres
Abstract
:1. Introduction
2. Formation, Model Building and Parameter Setting of Hydrogen Bonds
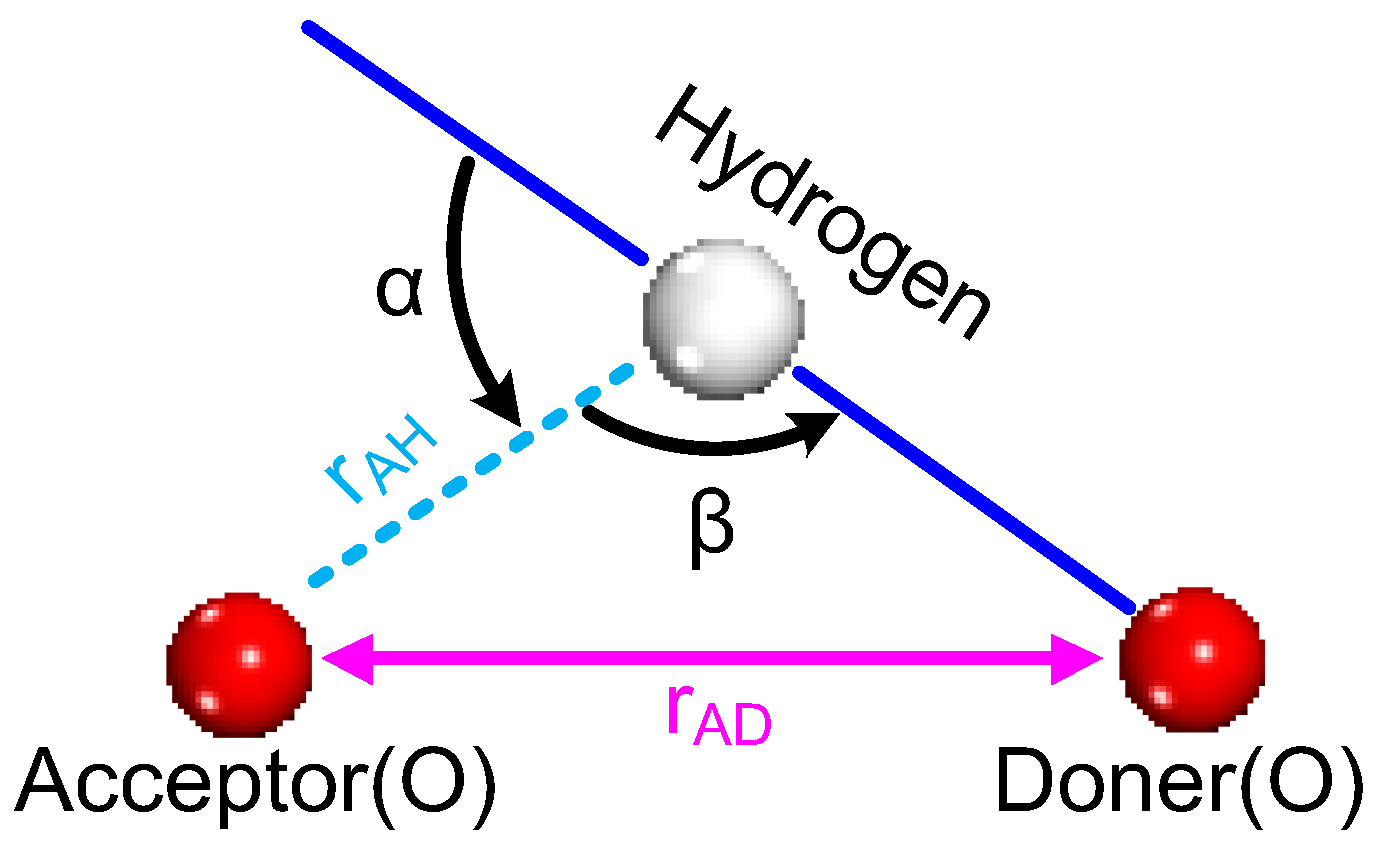
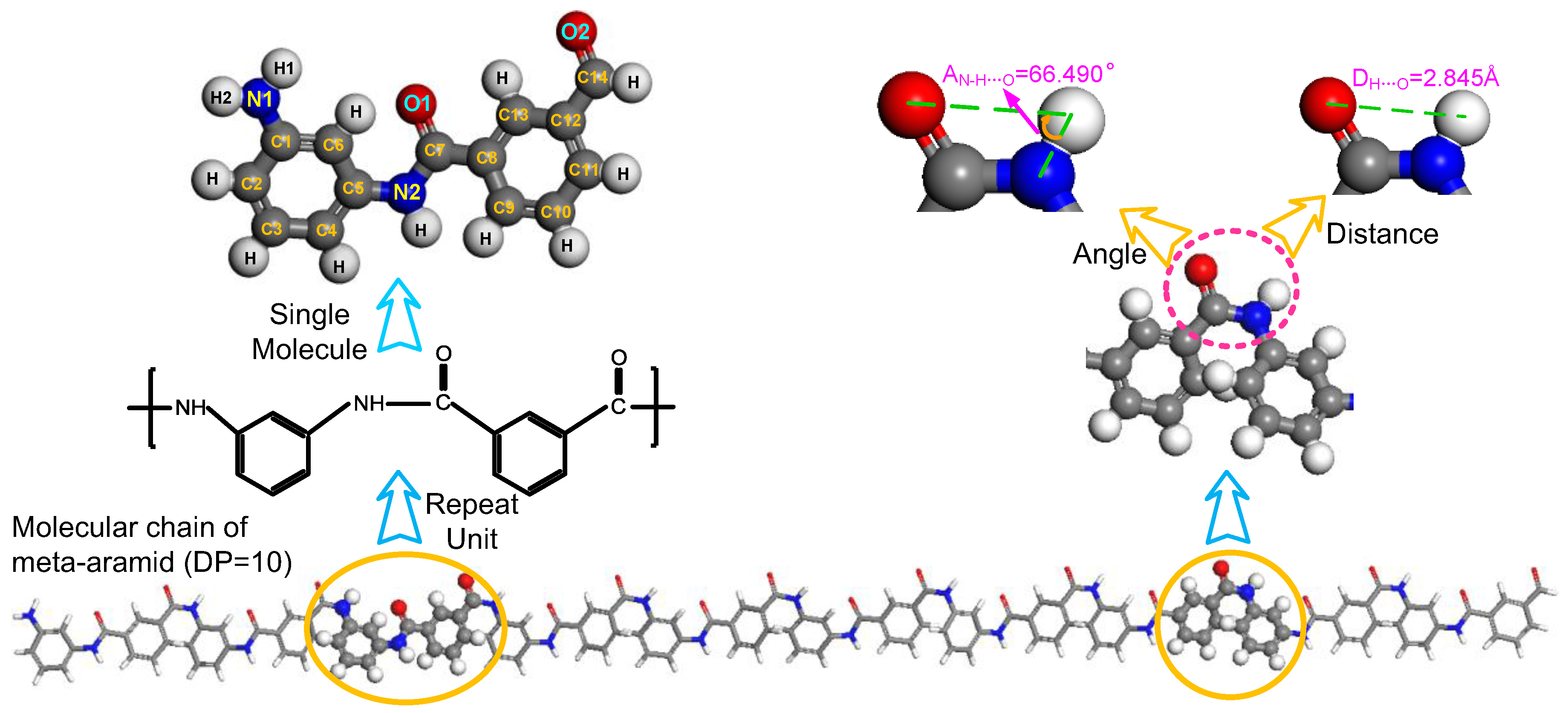
2.1. Formation of Hydrogen Bonds
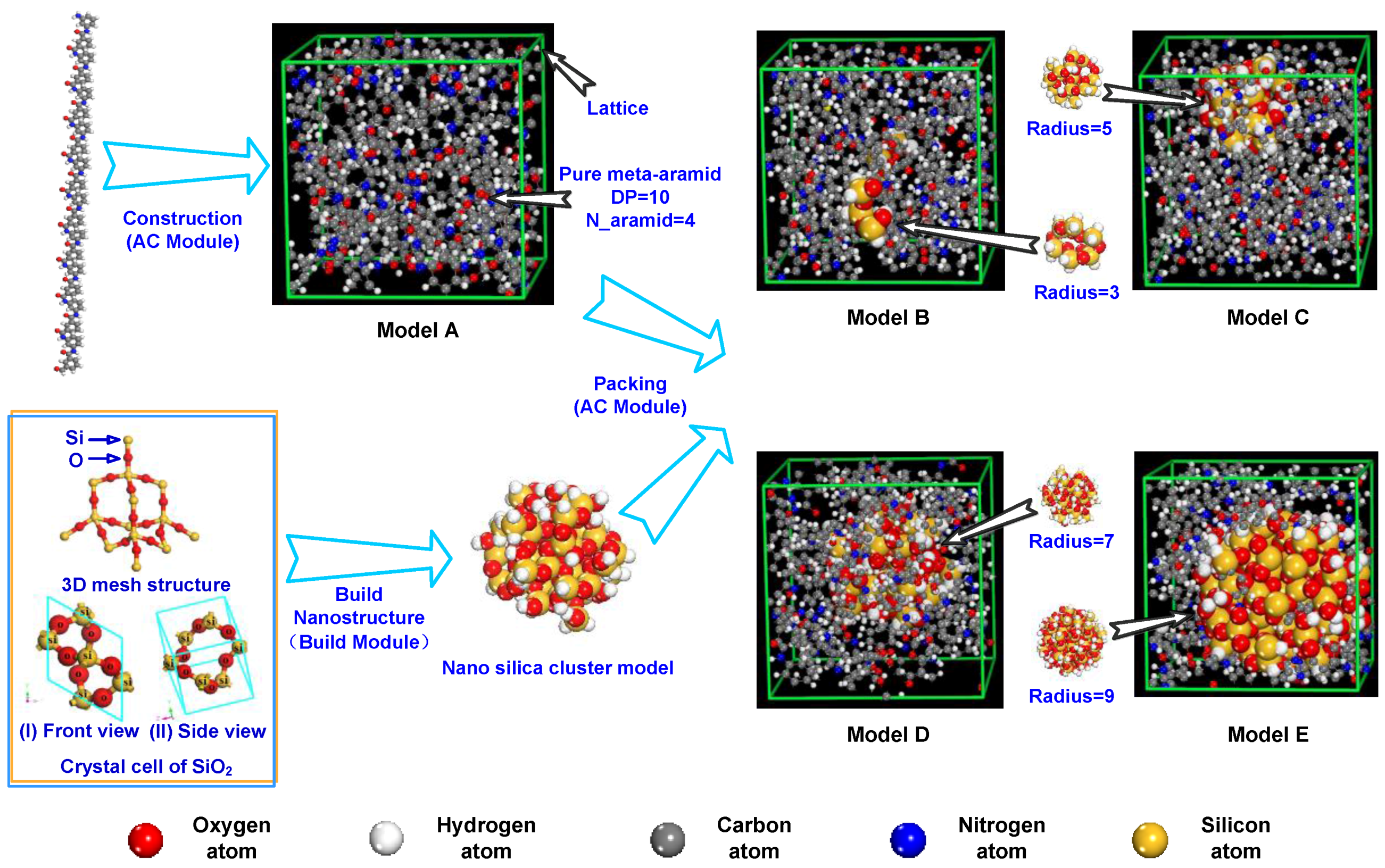
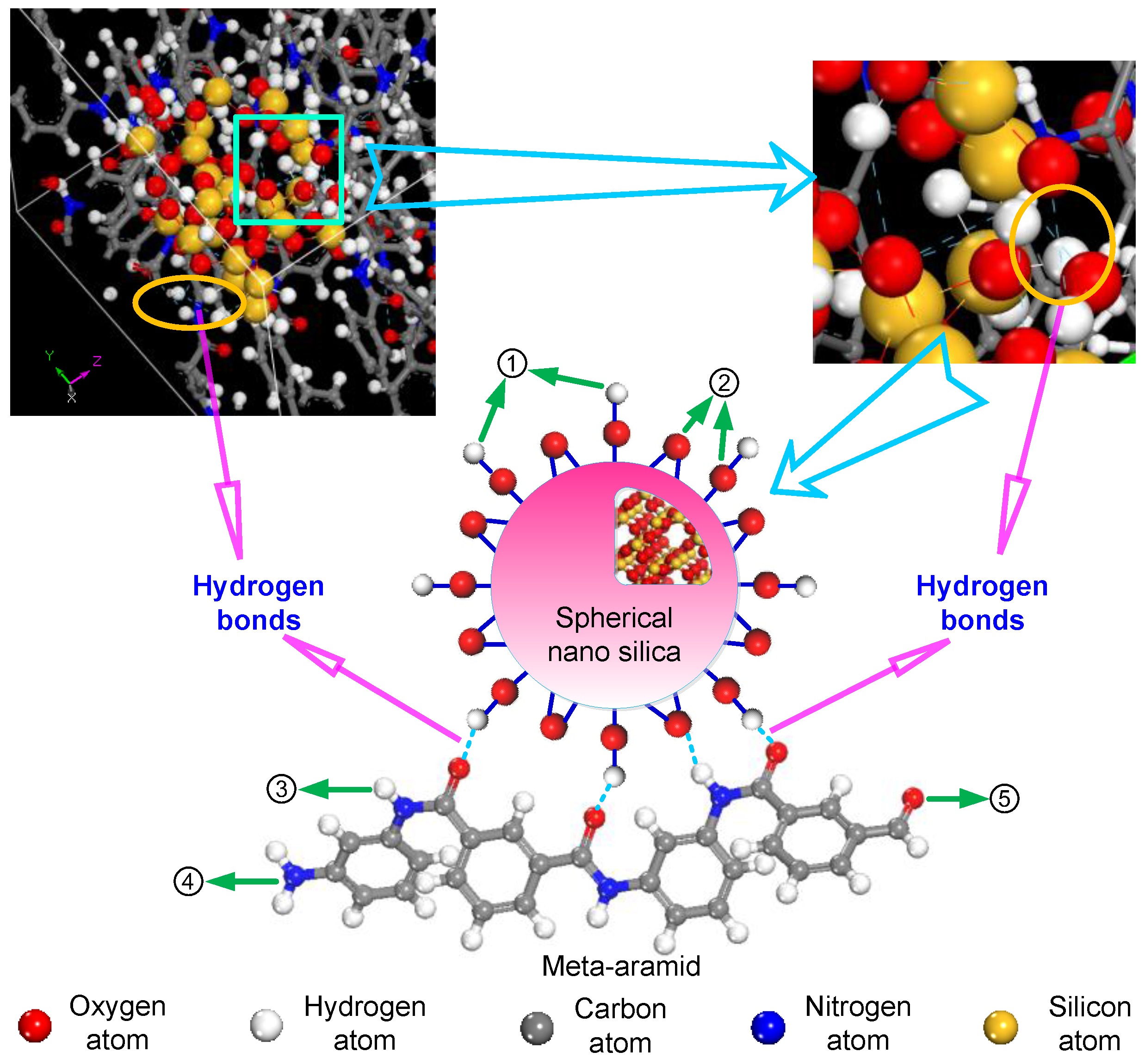
2.2. Model Building
2.3. Simulation Parameter Setting
3. Simulation Results and Discussion
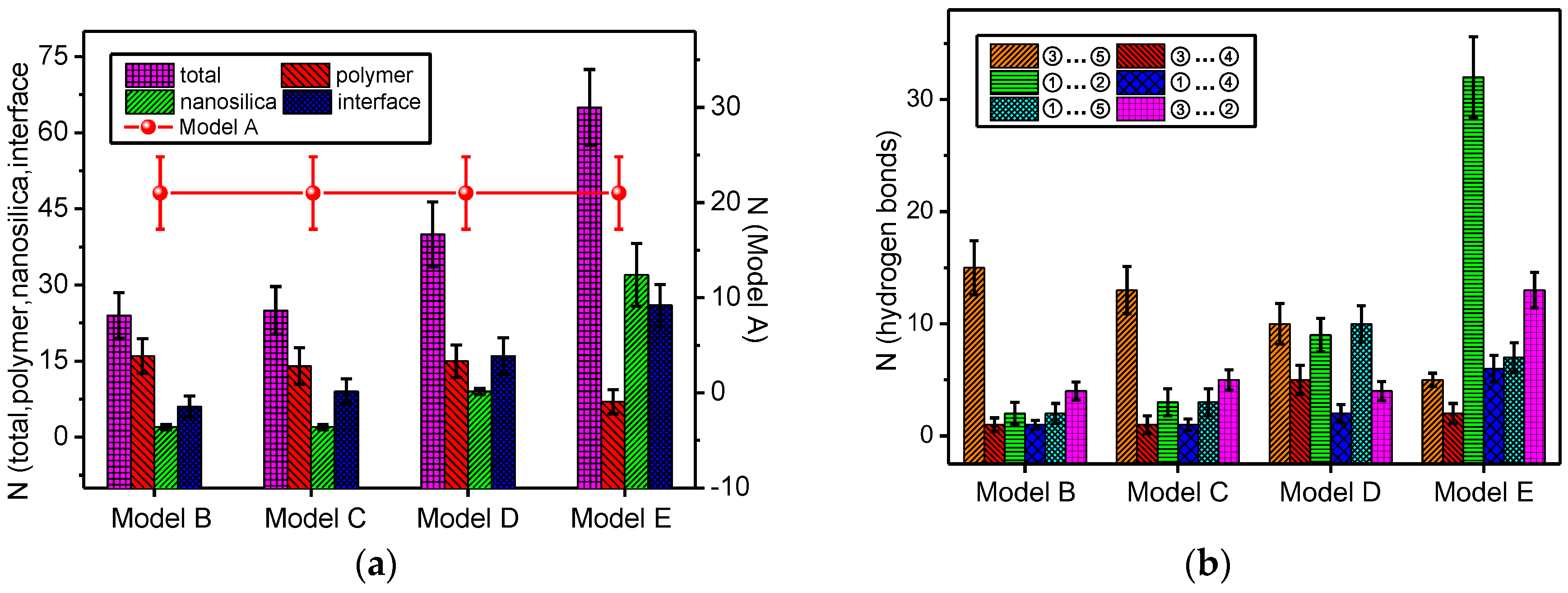
3.1. Change in Number of Hydrogen Bonds in all the Models
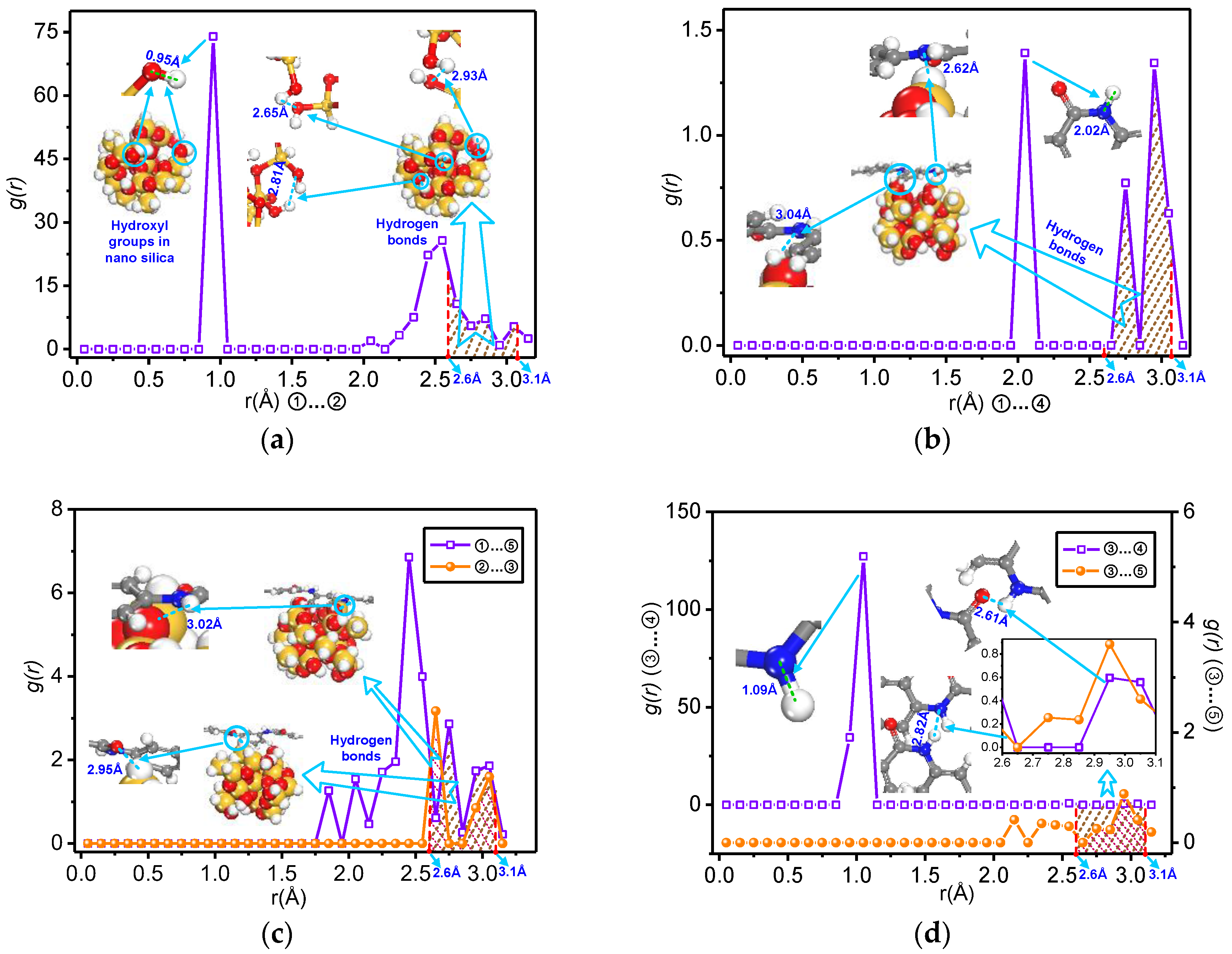
3.2. Analysis of Formation Probability of Interatomic Hydrogen Bonds
3.2.1. Pair Correlation Function
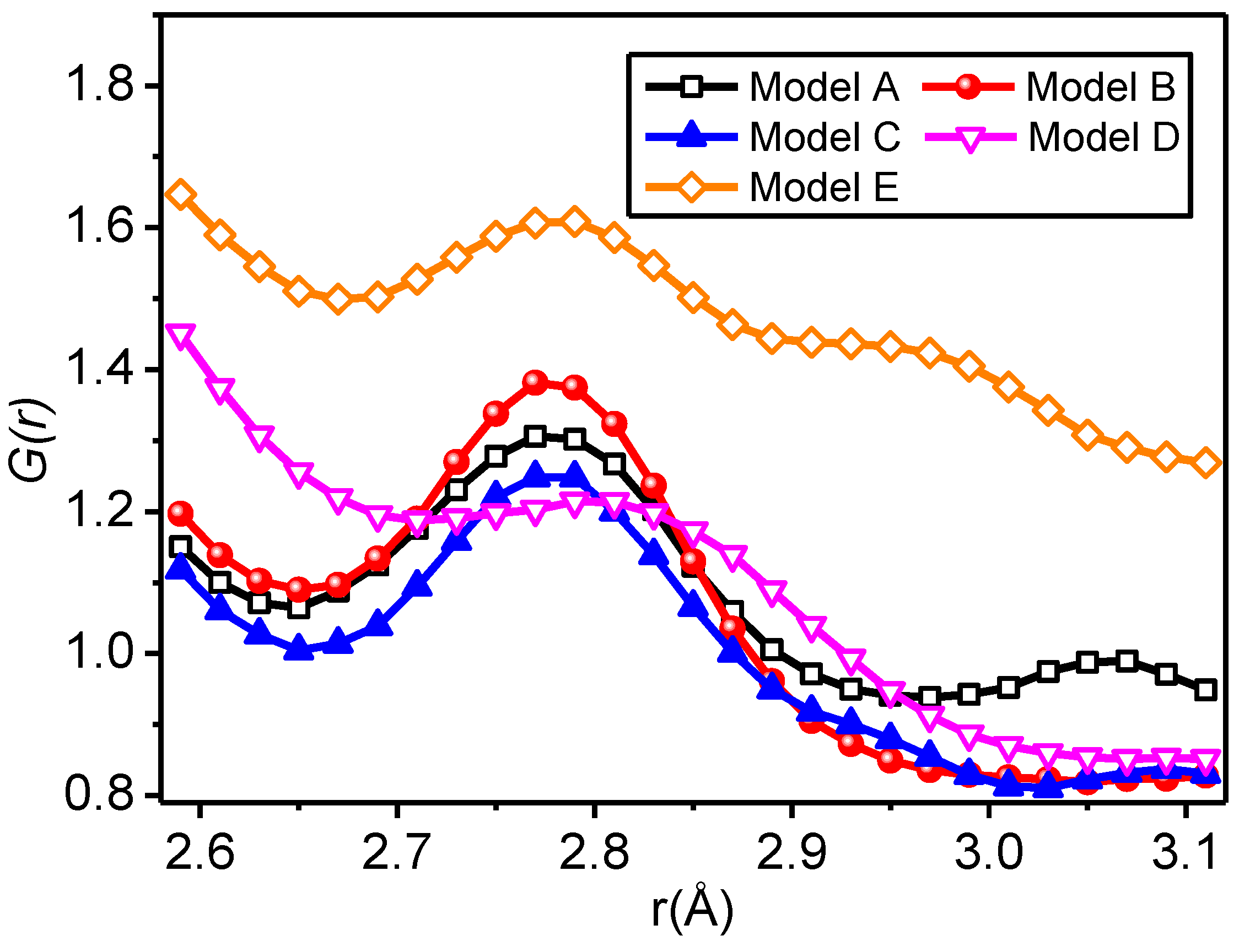
3.2.2. Radial Distribution Function
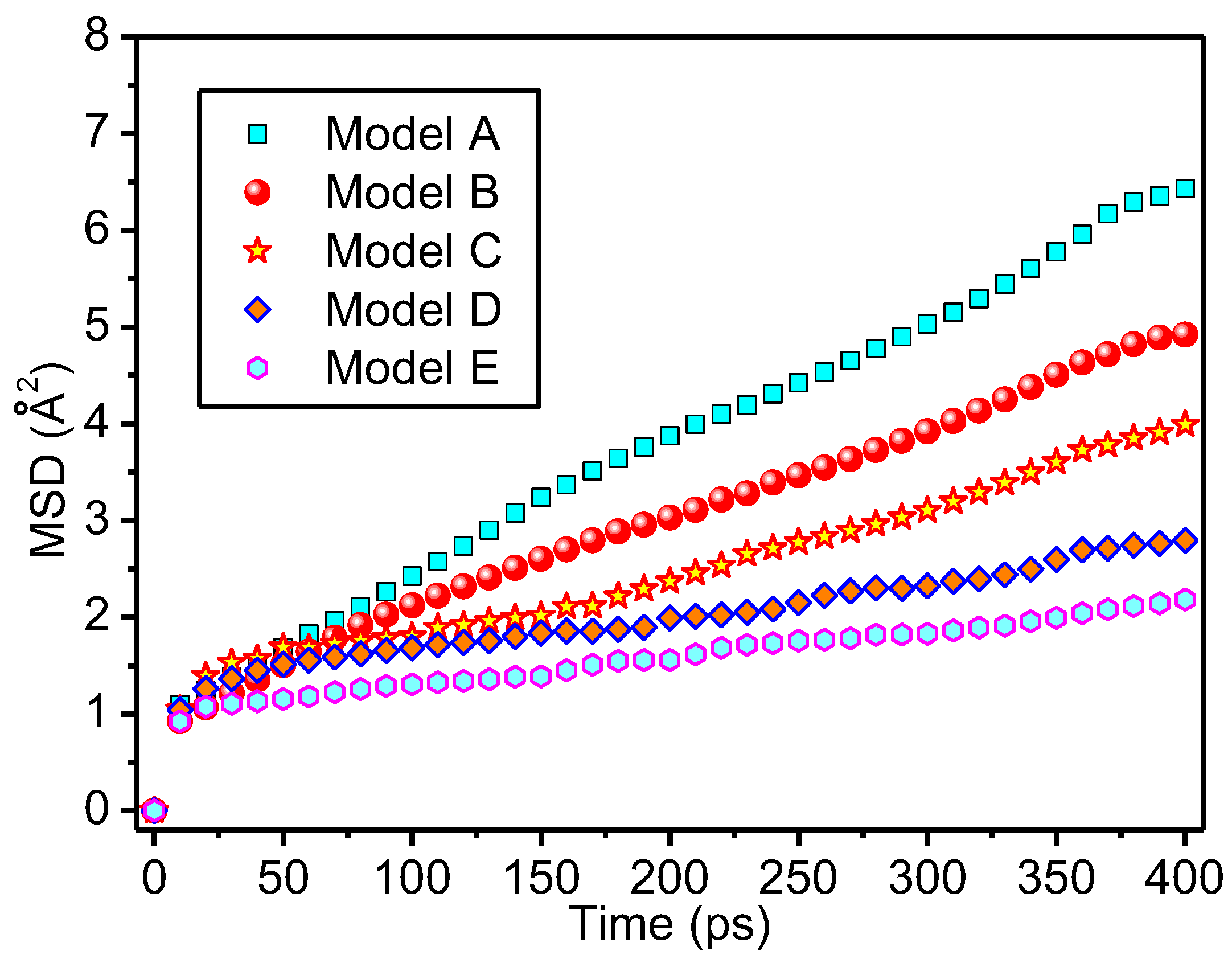
3.3. Thermal Stability of Meta-Aramid Fibres
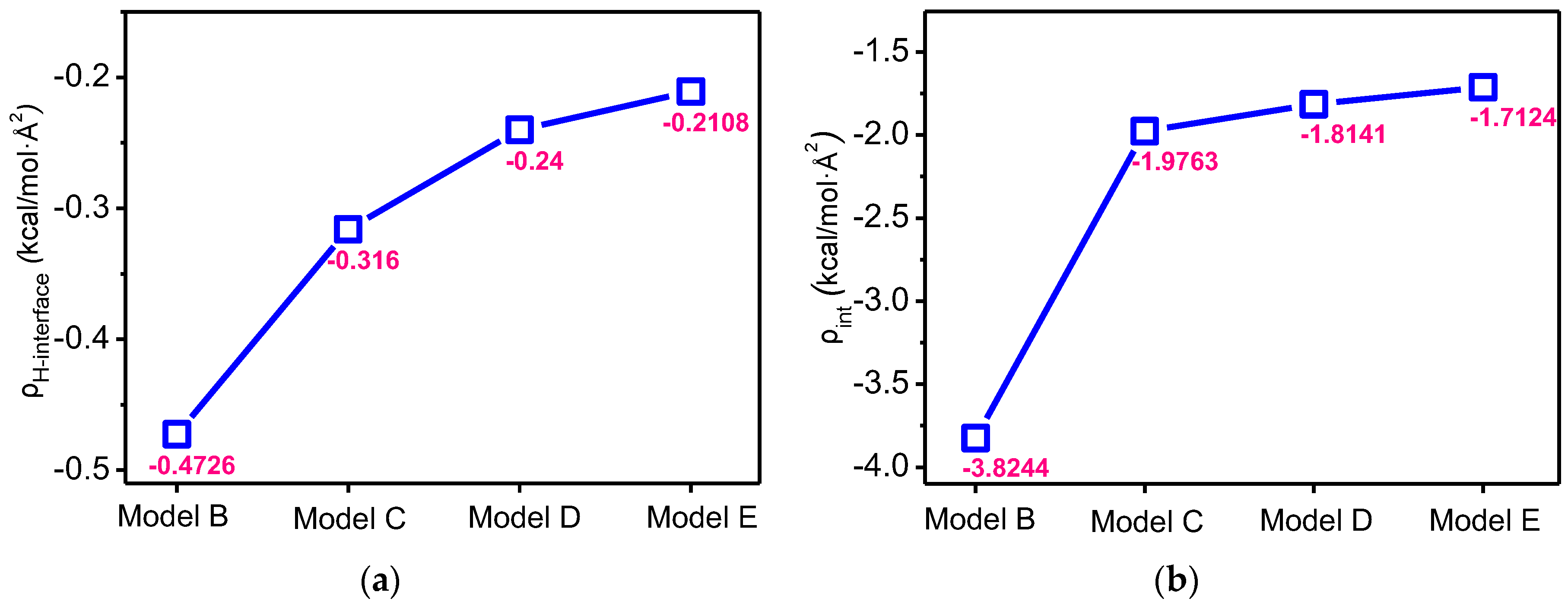
3.4. Effects on Interaction Energy
4. Conclusions
- The total number of the hydrogen bonds of the mixed model increases with doping by nano-silica. With the increase of the nanoparticle radius, the number of hydrogen bonds on the nanoparticle surface and the mixed model interface of nano-SiO2/meta-aramid fibre increases; thus, the increasing trend of the –OH…O hydrogen bonds on the surface of nano-SiO2 is most evident.
- For all of the hydrogen bond types of the SiO2/meta-aramid fibre mixed models, the hydrogen bond formation probability between the oxygen atom and hydrogen atom on the nanoparticle surface is the greatest. One of the effective ways to increase the hydrogen bonds in a SiO2/meta-aramid fibre is to increase the number of hydrogen atoms on the nano-silica surface and oxygen atoms in the meta-aramid fibre. Furthermore, to increase the formation probability of hydrogen bonds in all the models, the atomic distance should be enlarged to 2.7–2.8 Å as far as possible.
- The existence of interfacial hydrogen bonds can restrict the free movement of meta-aramid fibres, lower the chain movement of meta-aramid fibres, and improve the thermal stability of fibres. Additionally, the existence of interfacial hydrogen bonds is one of the important reasons for the formation of the stable interface structure of nanoparticles and meta-aramid fibres. However, at larger time and length scales, some other influence factors (such as Coulombic interactions) which can act on the MSDs should be taken into consideration.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S.H. Dumbbell-like bifunctional Au–Fe3O4 nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Salgueiriño-Maceira, V.; Correa-Duarte, M.A.; Spasova, M.; Liz-Marzán, L.M.; Farle, M. Composite silica spheres with magnetic and luminescent functionalities. Adv. Funct. Mater. 2006, 16, 509–514. [Google Scholar] [CrossRef]
- Zhang, D.H.; Zhang, H.; Zhang, Z.; Chen, Y.F. Industry applications of nanotechnology in high performance insulation composites. Sci. Sin. Chim. 2013, 43, 725–743. [Google Scholar] [CrossRef]
- Bai, G.; Liao, R.J.; Liu, N.; Liu, H.B.; Yang, L.J.; Shakeel, A. Influence of nano-AlN modification on the dielectric properties of meta-aramid paper. High Volt. Eng. 2015, 41, 461–467. [Google Scholar] [CrossRef]
- Chen, L. The development of M-aramid paper and its application. Chain Pulp. Ind. 2016, 37, 28–29. [Google Scholar] [CrossRef]
- Qin, M.; Kong, H.; Zhang, K.; Teng, C.; Yu, M.; Liao, Y. Simple synthesis of hydroxyl and ethylene functionalized aromatic polyamides as sizing agents to improve adhesion properties of aramid fiber/vinyl epoxy composites. Polymers 2017, 9, 143. [Google Scholar] [CrossRef]
- Schuh, C.; Schuh, K.; Lechmann, M.C.; Garnier, L.; Kraft, A. Shape-memory properties of segmented polymers containing aramid hard segments and polycaprolactone soft segments. Polymers 2010, 2, 71–85. [Google Scholar] [CrossRef]
- Seyhan, E.C.; Goksu, C.; Uzunhasanoglu, A.; Ilki, A. Seismic behavior of substandard RC columns retrofitted with embedded aramid fiber reinforced polymer (AFRP) reinforcement. Polymers 2015, 7, 2535–2557. [Google Scholar] [CrossRef]
- Lu, Z.Q.; Wei, N.; Ding, W.; Zhang, M.J. Improving the properties of polyimide fiber-based materials by nanosilica. J. Shaanxi Univ. Sci. Technol. 2017, 35, 6–10. [Google Scholar] [CrossRef]
- Liao, R.J.; Liu, H.B.; Bai, G.; Hao, J.; Li, X.; Lin, Y.D. Space charge characteristics and dielectric properties of nano-SiO2/aramid paper composite. Trans. CES 2016, 31, 40–48. [Google Scholar]
- Li, H.Y.; Wu, Q.L.; Zhou, D.G. Preparation and property analysis of cellulose nano-fibril and nano-silicon dioxide composites. Trans. CSAE 2015, 31, 299–303. [Google Scholar] [CrossRef]
- Ardalan, R.B.; Jamshidi, N.; Arabameri, H.; Joshaghani, A.; Mehrinejad, M.; Sharafi, P. Enhancing the permeability and abrasion resistance of concrete using colloidal nano-SiO2 oxide and spraying nanosilicon practices. Constr. Build. Mater. 2017, 146, 128–135. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, C.; Chen, G.; Zhou, Q.; Lv, C.; Li, X. The influence and mechanism of nano Al2O3 to the thermal stability of cellulose insulation paper. Sci. Sin. Technol. 2015, 45, 1167–1179. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.S.; Li, Y.R. Density-functional study of the adsorption of ZnO on α-Al2O3(0001) surface. Chem. J. Chin. Univ. 2004, 25, 1897–1900. [Google Scholar]
- Lewis, T.J. Interface are the dominaant feature of dielectrics at the nanometric level. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 739–753. [Google Scholar] [CrossRef]
- Cheong, W.C.D.; Zhang, L.C. Molecular dynamics simulation of phase transformations in silicon monocrystals due to nano-indentation. Nanotechnology 2000, 11, 173–180. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.L.; Zu, Y.; Zhao, Y.L. Size and structure effects in the nanotoxic response of nanomaterials. Chin. Sci. Bull. 2011, 56, 108–118. [Google Scholar] [CrossRef]
- Israelachvili, J.; Wennerström, H. Role of hydration and water structure in biological and colloidal interactions. Nature 1996, 379, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xiao, H.; Lackner, K.S.; Chen, X. Capture CO2 from ambient air using nanoconfined ion hydration. Angew. Chem. 2016, 128, 4094–4097. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Chen, X.; Lackner, K.S. The Effect of Moisture on the Hydrolysis of Basic Salts. Chem. Eur. J. 2016, 22, 18326–18330. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tang, Q.; Peng, T.; Zhang, X.; Liu, C.; Shi, X. Molecular characteristics of H2O in hydrate/ice/liquid water mixture. Int. J. Mod. Phys. B 2015, 29, 1550185. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, Y.; Shi, X.; Song, S. Rapid evaporation of water on graphene/graphene-oxide: A molecular dynamics study. Nanomaterials 2017, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, C.; Wang, Q.; Li, X.P.; Hao, J. Molecular simulation research on the micro effect mechanism of interfacial properties of nano SiO2/meta-aramid fiber. Int. J. Heat Technol. 2017, 35, 123–129. [Google Scholar] [CrossRef]
- Heinera, A.P.; Kuuttib, L.; Telemana, O. Comparison of the interface between water and four surfaces of native crystalline cellulose by molecular dynamics simulations. Carbohydr. Res. 1998, 306, 205–220. [Google Scholar] [CrossRef]
- Yin, K.L.; Zou, D.H.; Yang, B.; Zhang, X.H.; Xia, Q.; Xu, D.J. Investigation of H-bonding for the related force fields in materials studio software. Comput. Appl. Chem. 2006, 23, 1335–1340. [Google Scholar] [CrossRef]
- Li, F.; Dong, J.Q.; Shen, Q. The function of hydrogen bond in polymer blends I. Characters and affecting factors of hydrogen bond. Chin. Polym. Bull. 2009, 7, 45–52. [Google Scholar] [CrossRef]
- Li, F.; Dong, J.Q.; Shen, Q. The function of hydrogen bond in polymer blends II. Characters and affecting factors of hydrogen bond. Chin. Polym. Bull. 2009, 8, 54–65. [Google Scholar] [CrossRef]
- Xiao, H.; Shi, X.; Zhang, Y.; Liao, X.; Hao, F.; Lackner, K.S.; Chen, X. The catalytic effect of water in basic hydrolysis of CO32− in hydrated clusters and its implication in the humidity driven CO2 air capture. Phys. Chem. Chem. Phys. 2017. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Müller-Plathe, F. Molecular dynamics simulation of water influence on local structure of nanoconfined polyamide-6,6. J. Phys. Chem. B 2011, 115, 9720–9731. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Rahimi, M.; Müller-Plathe, F. Molecular dynamics simulation of a silica nanoparticle in oligomeric poly(methyl methacrylate): A model system for studying the interphase thickness in a polymer–nanocomposite via different properties. Macromolecules 2013, 46, 8680–8692. [Google Scholar] [CrossRef]
- Eslami, H.; Müller-Plathe, F. Structure and mobility of nanoconfined polyamide-6,6 oligomers: Application of a molecular dynamics technique with constant temperature, Surface Area, and parallel pressure. J. Phys. Chem. B 2009, 113, 5568–5581. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Müller-Plathe, F. How thick is the interphase in an ultrathin polymer film? Coarse-grained molecular dynamics simulations of polyamide-6,6 on graphene. J. Phys. Chem. C 2013, 117, 5249–5257. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhao, Y.; Zhang, C.B. Study of mono-dispersed spherical silica in sol-gel process with orthogonal design. China Powder Sci. Technol. 2003, 9, 4–8. [Google Scholar] [CrossRef]
- Bao, M.R.; Zhu, G.R.; Wang, M.; Gao, C.J. Progress on preparation of mono-dispersed spherical nano-silica. Mater. Rev. 2011, 25, 135–139. [Google Scholar]
- Li, Z.; Okamoto, K.; Ohki, Y.; Tanaka, T. The role of nano and micro particles on partial discharge and breakdown strength in epoxy composites. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 675–681. [Google Scholar] [CrossRef]
- Roman, K.; Thomas, A.; Peter, H.F.M.; Johan, J.S. Anomalous behaviour of the dielectric spectroscopy response of nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 107–117. [Google Scholar] [CrossRef]
- Yang, K.D.; Lee, C.W.; Jang, J.H.; Ha, T.R.; Nam, K.T. Rise of nano effects in electrode during electrocatalytic CO2 conversion. Nanotechnology 2017, 28, 352001. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.W.; Zhu, Y.H.; Li, W.K.; Bai, J.B.; Dang, Z.M. Low dielectric permittivity and high thermal conductivity silicone rubber composites with micro-nano-sized particles. Appl. Phy. Lett. 2012, 101. [Google Scholar] [CrossRef]
- Zha, J.W.; Li, W.K.; Zhu, Y.H.; Dang, Z.M.; Chen, G. Effect of Micro-Si3N4-nano-Al2O3 Cofilled Particles on Thermal Conductivity, Dielectric and Mechanical Properties of Sillicone Rubber Composites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1989–1996. [Google Scholar] [CrossRef]
- Xu, N.Q.; Gu, J.X.; Luo, K.; Zheng, S.B. Preparation of nanon silicon dioxide of large particle size. Chem. Ind. Eng. Prog. (China) 2004, 23, 747–750. [Google Scholar] [CrossRef]
- Qamar, R.; Mohtashim, L.; Elke, D.; Heidemarie, P.; Ludwig, J.; Dieter, G.W.; Dietmar, S. Evidence that ultrafine titanium dioxide induces micronuclei and apoptosis in syrian hamster embryo fibroblasts. Environ. Health Perspect. 2012, 110, 797–800. [Google Scholar]
- Park, M.V.D.Z.; Annema, W.; Salvati, A.; Lesniak, A.; Elsaesser, A.; Barnes, C.; McKerr, G.; Howard, C.V.; Lynch, I.; Dawson, K.A.; et al. In vitro developmental toxicity test detects inhibition of stem cell differentiation by silica nanoparticles. Toxicol. Appl. Pharmacol. 2009, 240, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Li, A.S.; Huang, Y.P.; Liu, J.W.; Lan, M.B.; Gao, F.; Xu, H.L.; Chen, M.C.; Xu, Y.C. Oxidative lesions induced by medical SiO2 nanoparticles on normal human lung cells. Chin. J. Clin. Pharmacol. Ther. 2009, 14, 1115–1120. [Google Scholar]
- Yu, K.O.; Grabinski, C.M.; Schrand, A.M.; Murdock, R.C.; Wang, W.; Gu, B.H.; Schlager, J.J.; Hussain, S.M. Toxicity of amorphous silica nanoparticles in mouse keratinocytes. J. Nanopart. Res. 2009, 11, 15–24. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.J.; Rabolli, V.; Lison, D.; Gonzalez, L.; Kirsch-Volders, M.; Martens, J.A.; Hoet, P.H. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small 2009, 5, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Chelli, R.; Procacci, P.; Cardini, G.; Califano, S. Glycerol condensed phases Part II. A molecular dynamics study of the conformational structure and hydrogen bonding. Phys. Chem. Chem. Phys. 1999, 1, 879–885. [Google Scholar] [CrossRef]
- Chelli, R.; Procacci, P.; Cardini, G.; Valle, R.G.D.; Califano, S. Glycerol condensed phases Part I. A molecular dynamics study. Phys. Chem. Chem. Phys. 1999, 1, 871–877. [Google Scholar] [CrossRef]
- Chen, C.; Li, W.Z. Molecular dynamics simulation of hydrogen bonding characteristics in aqueous glycerol solutions. Acta Phys. Chim. Sin. 2009, 25, 507–512. [Google Scholar]
- Callam, C.S.; Singer, S.J.; Lowary, T.L.; Hadad, C.M. Computational analysis of the potential energy surfaces of glycerol in the gas and aqueous phases: Effects of level of theory, basis set, and solvation on strongly intramolecularly hydrogen-bonded systems. J. Am. Chem. Soc. 2001, 123, 11743–11754. [Google Scholar] [CrossRef] [PubMed]
- Guàrdia, E.; Martí, J.; Padró, J.A.; Saiz, L.; Komolkin, A.V. Dynamics in hydrogen bonded liquids: Water and alcohols. J. Mol. Liq. 2002, 96–97, 3–17. [Google Scholar] [CrossRef]
- Chen, H.T.; Wang, J.F.; Wu, X.L.; Chen, X.H. Technological conditions and properties of nano-SiO2 modified epoxy resin. Insul. Mater. 2008, 41, 40–43. [Google Scholar] [CrossRef]
- Li, C.Y.; Qiu, D.J.; Xie, G.X.; Xiao, X.D.; Ni, X.X. Study on nano-silica toughening modification for epoxy resin. Mater. Prot. 2007, 41, 21–23. [Google Scholar] [CrossRef]
- Liu, D.M. Molecular Dynamics Simulation Study on the Structure and Properties of HMX and PETN and Their Composite Materials. Master’s Thesis, Nanjing University of Science & Technology, Nanjing, China, 2014. [Google Scholar]
- Zhang, Y.; Gu, J.H.; Shang, J.X.; Ma, Y. Fundamentals of Computational Materials, 1st ed.; Beihang University Press: Beijing, China, 2007; pp. 121–122. ISBN 978-7-81077-788-9. [Google Scholar]
- Wernersson, E.; Stenqvist, B.; Lund, M. The mechanism of cellulose solubilization by urea studied by molecular simulaton. Cellulose 2015, 22, 991–1001. [Google Scholar] [CrossRef]
- Liu, X.; Luo, D.F. The concept and discussion of the radial distribution function. J. Liaocheng Teach. Univ. (Nat. Sci.) 2002, 15, 48–49. [Google Scholar]
- Li, B.R.; Li, D. Two definitions of radial distribution function of atomic orbits and their correlations. Chem. Bull. 1982, 4, 62–63. [Google Scholar] [CrossRef]
- Li, B.R. Discrimination of common misconceptions about atomic orbits and electronic cloud patterns. Chem. Bull. 1984, 3, 46–50. [Google Scholar] [CrossRef]
- Hu, J. Molecular Simulation Studies on Aging Mechanism in Crazing Zone of the Insulation Paper in Power Transformers. Master Thesis, Chongqing University, Chongqing, China, 2009. [Google Scholar]
- Hofmann, D.; Fritz, L.; Ulbrich, J.; Paul, D. Molecular simulation of small molecule diffusion and solution in dense amorphous polysiloxanes and polyimides. Comput. Theor. Polym. Sci. 2000, 10, 419–436. [Google Scholar] [CrossRef]
- Afandak, A.; Eslami, H. Ion-Pairing and Electrical Conductivity in the Ionic Liquid 1-n-Butyl-3-methylimidazolium Methylsulfate [Bmim][MeSO4]: Molecular Dynamics Simulation Study. J. Phys. Chem. B 2017, 121, 7699–7708. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.Y.; Shi, F.G.; Zhao, B.; Wang, S.Q.; Brongo, M.; Vasudev, P.K. Interfacial microstructure and reaction at the spin-coated fluorinated polyimide/Al interface: Surface enhanced X-ray diffraction and TEM studies. Appl. Phys. A 1997, 65, 287–290. [Google Scholar] [CrossRef]
- Wang, B. Surface Modification of Inorganic Nanoparticles by Macromolecules. Master’s Thesis, Tsinghua University, Beijing, China, 2005. [Google Scholar]
- Jesionowski, T.; Krysztafkiewicz, A. Influence of silane coupling agents on surface properties of precipitated silicas. Appl. Surf. Sci. 2001, 172, 18–32. [Google Scholar] [CrossRef]

| Model | Eint | ESiO2 | EPolymer | Etotal | EH-interface |
|---|---|---|---|---|---|
| B | −432.3091 | −636.4831 | −1171.2478 | −2240.0400 | −53.4254 |
| C | −620.5648 | −856.8470 | −1476.5172 | −2953.9290 | −99.2254 |
| D | −1116.4885 | −5631.9281 | −1316.9705 | −8065.3871 | −147.7210 |
| E | −1742.1470 | −13,262.3598 | −1506.8031 | −16,511.3099 | −214.4546 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Li, X.; Li, Z.; Hao, J. Interfacial Hydrogen Bonds and Their Influence Mechanism on Increasing the Thermal Stability of Nano-SiO2-Modified Meta-Aramid Fibres. Polymers 2017, 9, 504. https://doi.org/10.3390/polym9100504
Tang C, Li X, Li Z, Hao J. Interfacial Hydrogen Bonds and Their Influence Mechanism on Increasing the Thermal Stability of Nano-SiO2-Modified Meta-Aramid Fibres. Polymers. 2017; 9(10):504. https://doi.org/10.3390/polym9100504
Chicago/Turabian StyleTang, Chao, Xu Li, Zhiwei Li, and Jian Hao. 2017. "Interfacial Hydrogen Bonds and Their Influence Mechanism on Increasing the Thermal Stability of Nano-SiO2-Modified Meta-Aramid Fibres" Polymers 9, no. 10: 504. https://doi.org/10.3390/polym9100504





