Stimuli Responsive Polymer-Based 3D Optical Crystals for Sensing
Abstract
:1. Introduction
2. Composition of Responsive Elements
2.1. Responsive Colloid Particles
2.2. Responsive Polymer Matrixes
3. Sensing Applications
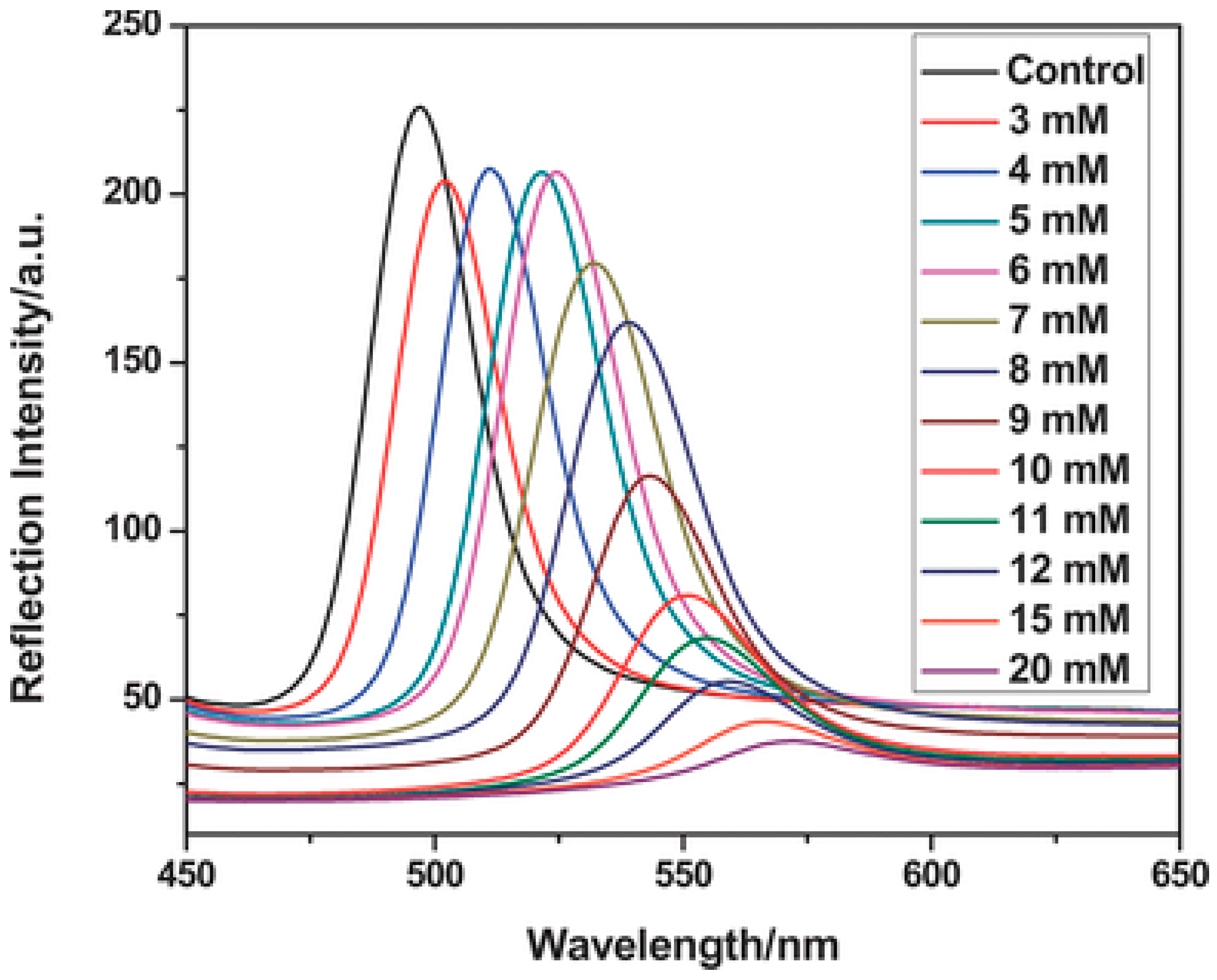
3.1. Chemical Sensors
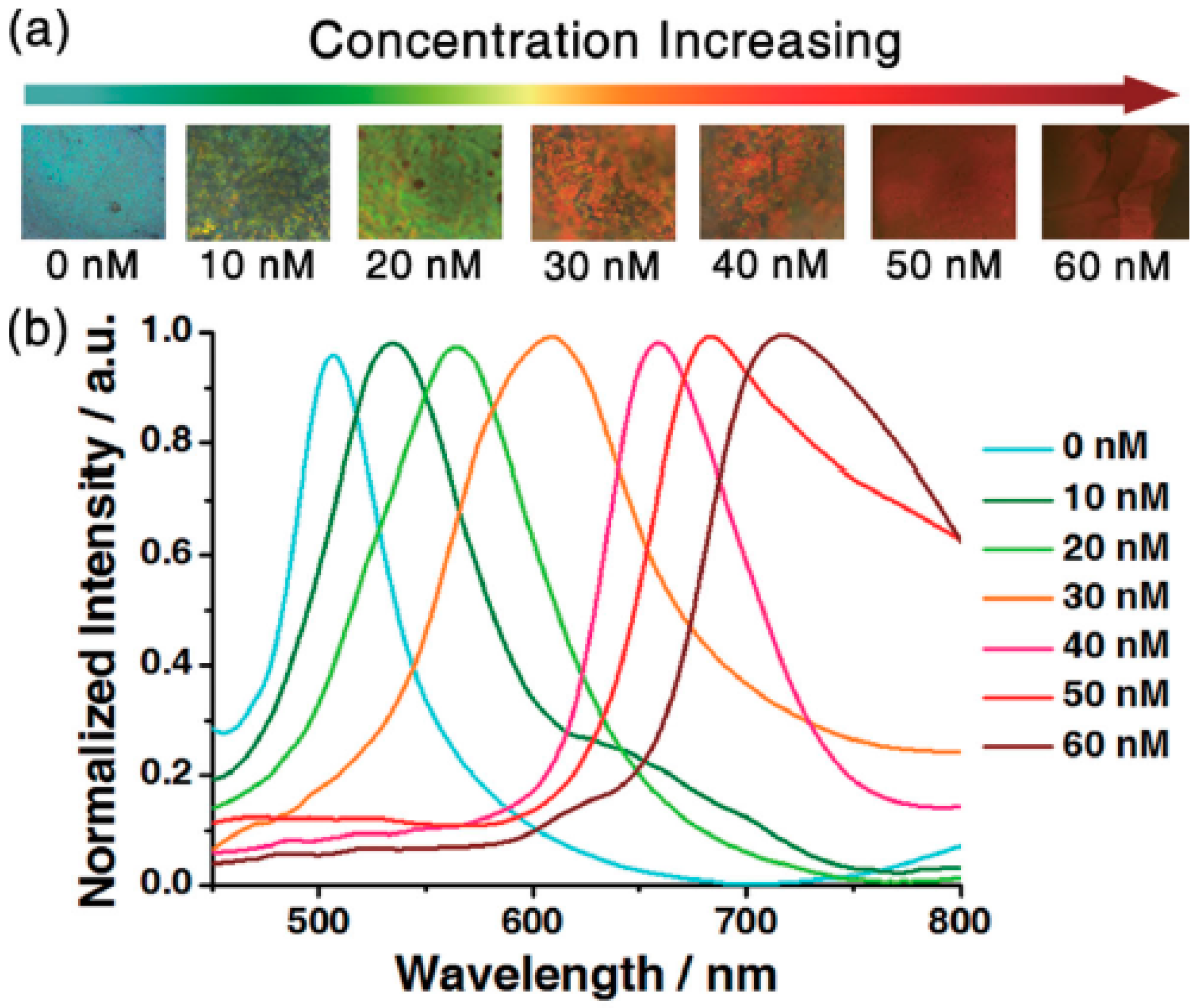
3.2. Biosensors
3.3. Combination of 3DOCs with Other Technologies
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ge, J.; Yin, Y. Responsive photonic crystals. Angew. Chem. Int. Ed. 2011, 50, 1492–1522. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xiu, J.; Tang, B.; Zhao, D.; Zhang, S. Dynamic monitoring of thermally assisted assembly of colloidal crystals. J. Mater. Sci. 2017, 52, 7883–7892. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, L.; Zhu, J.; Ni, H.; Xia, W.; Wang, M. Compact 3D photonic crystals sensing platform with 45 degree angle polished fibers. Opt. Fiber Technol. 2017, 36, 187–194. [Google Scholar] [CrossRef]
- Nakato, T.; Nono, Y.; Mouri, E. Textural diversity of hierarchical macroscopic structures of colloidal liquid crystalline nanosheets organized under electric fields. Colloids Surf. A 2017, 522, 373–381. [Google Scholar] [CrossRef]
- João, C.F.C.; Kullberg, A.T.; Silva, J.C.; Borges, J.P. Chitosan Inverted Colloidal Crystal scaffolds: Influence of molecular weight on structural stability. Mater. Lett. 2017, 193, 50–53. [Google Scholar] [CrossRef]
- Ding, H.; Zhu, C.; Tian, L.; Liu, C.; Fu, G.; Shang, L.; Gu, Z. Structural Color Patterns by Electrohydrodynamic Jet Printed Photonic Crystals. ACS Appl. Mater. Interfaces 2017, 9, 11933–11941. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Mahynski, N.A.; Gang, O.; Panagiotopoulos, A.Z.; Kumar, S.K. Directionally interacting spheres and rods form ordered phases. ACS Nano 2017, 11, 4950–4959. [Google Scholar] [CrossRef] [PubMed]
- Tiu, B.D.B.; Tiu, S.B.; Wen, A.M.; Lam, P.; Steinmetz, N.F.; Advincula, R.C. Free-standing, nanopatterned Janus membranes of conducting polymer–virus nanoparticle arrays. Langmuir 2016, 32, 6185–6193. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Cuevas, K.G.; Wang, L.; Zheng, Z.G.; Bisoyi, H.K.; Li, G.; Tan, L.S.; Vaia, R.A.; Li, Q. Frequency-Driven Self-Organized Helical Superstructures Loaded with Mesogen-Grafted Silica Nanoparticles. Angew. Chem. Int. Ed. 2016, 55, 13090–13094. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, L.; Zhang, G.; Liu, G.; Fan, Q.; Shao, J. Study on the effects of the characteristics of textile substrates on the photonic crystal films and the related structural colors. Surf. Coat. Technol. 2017, 319, 267–276. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, L.; Li, J.; Cleuvenbergen, S.V.; Bartic, C.; Song, K.; Clays, K. Real-Time Fluorescence Detection in Aqueous Systems by Combined and Enhanced Photonic and Surface Effects in Patterned Hollow Sphere Colloidal Photonic Crystals. Langmuir 2017, 33, 4840–4846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.M.; Serpe, M.J. Polymer Mechanochemistry; Boulatov, R., Ed.; Springer: Berlin, Germany, 2015; Volume 369, pp. 377–424. [Google Scholar]
- Zhang, Q.M.; Li, X.; Islam, M.R.; Wei, M.; Serpe, M.J. Light switchable optical materials from azobenzene crosslinked poly(N-isopropylacrylamide)-based microgels. J. Mater. Chem. C 2014, 2, 6961–6965. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. Fabrication methods of biopolymeric microgels and microgel-based hydrogels. Food Hydrocoll. 2017, 62, 262–272. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Wang, W.; Su, Y.-Q.; Hensen, E.J.; Serpe, M.J. Biological Imaging and Sensing with Multiresponsive Microgels. Chem. Mater. 2015, 28, 259–265. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Xu, W.; Serpe, M.J. Optical devices constructed from multiresponsive microgels. Angew. Chem. Int. Ed. 2014, 53, 4827–4831. [Google Scholar] [CrossRef] [PubMed]
- Itabashi, S.; Nishi, H.; Tsuchizawa, T.; Watanabe, T.; Shinojima, H.; Kou, R.; Yamada, K. Integration of Silicon Nano-Photonic Devices for Telecommunications. IEICE Trans. Electron. 2012, E95C, 199–205. [Google Scholar] [CrossRef]
- Chen, A. Introduction to Polymer Photonics for Information Technology; CRC Press-Taylor & Francis Group: Boca Raton, FL, USA, 2008; Volume 133. [Google Scholar]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. TrAC Trends Anal. Chem. 2013, 44, 25–38. [Google Scholar] [CrossRef]
- Guo, C.X.; Guai, G.H.; Li, C.M. Graphene Based Materials: Enhancing Solar Energy Harvesting. Adv. Energy Mater. 2011, 1, 448–452. [Google Scholar] [CrossRef]
- Yu, H.B.; Xiao, Y.; Jin, L.J. A Lysosome-Targetable and Two-Photon Fluorescent Probe for Monitoring Endogenous and Exogenous Nitric Oxide in Living Cells. J. Am. Chem. Soc. 2012, 134, 17486–17489. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.Q.; Lei, T.; Chen, H.; Cai, H.; Luo, X.S.; Poon, A.W. Silicon photonics: from a microresonator perspective. Laser Photonics Rev. 2012, 6, 145–177. [Google Scholar] [CrossRef]
- Schild, H. Poly (N-isopropylacrylamide): experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution property of poly(N-isopropyl acrylamide). J. Macromol. Sci. A 1968, A2, 1441–1455. [Google Scholar] [CrossRef]
- Debord, J.D.; Lyon, L.A. Thermoresponsive photonic crystals. J. Phys. Chem. B 2000, 104, 6327–6331. [Google Scholar] [CrossRef]
- Hong, X.; Peng, Y.; Bai, J.; Ning, B.; Liu, Y.; Zhou, Z.; Gao, Z. A novel opal closest-packing photonic crystal for naked-eye glucose detection. Small 2014, 10, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Lee, D.; Shum, H.C.; Weitz, D.A. Fabrication of Tunable Spherical Colloidal Crystals Immobilized in Soft Hydrogels. Small 2010, 6, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, A.; Qiu, S.; Wong, M.C.; Li, P.C. Dip-in Indicators for Visual Differentiation of Fuel Mixtures Based on Wettability of Fluoroalkylchlorosilane-Coated Inverse Opal Films. ACS Appl. Mater. Interfaces 2015, 7, 28387–28392. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhu, Z.; Xue, M.; Xue, F.; Wang, Q.; Meng, Z.; Lu, W.; Chen, W.; Qi, F.; Yan, Z. Cellulose photonic crystal film sensor for alcohols. Sens. Actuators B 2015, 220, 222–226. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, L.; Guan, Y.; Zhang, Y. Polymerized Microgel Colloidal Crystals: Photonic Hydrogels with Tunable Band Gaps and Fast Response Rates. Angew. Chem. Int. Ed. 2013, 52, 9961–9965. [Google Scholar] [CrossRef] [PubMed]
- Alexeev, V.L.; Das, S.; Finegold, D.N.; Asher, S.A. Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin. Chem. 2004, 50, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Tao, C.A.; Lin, C.X.; Shen, D.Z.; Li, G.T. Label-Free Colorimetric Detection of Trace Atrazine in Aqueous Solution by Using Molecularly Imprinted Photonic Polymers. Chem. Eur. J. 2008, 14, 11358–11368. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, H.; Yang, Q.; Li, M.; Jiang, L.; Song, Y. Hydrophilic–hydrophobic patterned molecularly imprinted photonic crystal sensors for high-sensitive colorimetric detection of tetracycline. Small 2015, 11, 2738–2742. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.; Xu, Y.; Chen, B.; Yang, Z. Responsive photonic encoded breathing microbeads based microfluidic chip for multiplex fluorescent immunoassay. Sens. Actuators B 2017, 242, 1259–1264. [Google Scholar] [CrossRef]
- Lu, W.; Dong, X.; Qiu, L.; Yan, Z.; Meng, Z.; Xue, M.; He, X.; Liu, X. Colorimetric sensor arrays based on pattern recognition for the detection of nitroaromatic molecules. J. Hazard Mater. 2017, 326, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Bearzotti, A.; Venditti, I.; Cametti, C.; Russo, M.V. Role of nanostructured polymers on the improvement of electrical response-based relative humidity sensors. Sens. Actuators B 2016, 225, 96–108. [Google Scholar] [CrossRef]
- Fu, Q.; Zhu, B.; Ge, J. Hierarchically structured photonic crystals for integrated chemical separation and colorimetric detection. Nanoscale 2017, 9, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef]
- Sharma, A.C.; Jana, T.; Kesavamoorthy, R.; Shi, L.; Virji, M.A.; Finegold, D.N.; Asher, S.A. A general photonic crystal sensing motif: creatinine in bodily fluids. J. Am. Chem. Soc. 2004, 126, 2971–2977. [Google Scholar] [CrossRef] [PubMed]
- MacConaghy, K.I.; Geary, C.I.; Kaar, J.L.; Stoykovich, M.P. Photonic crystal kinase biosensor. J. Am. Chem. Soc. 2014, 136, 6896–6899. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, G.; Wu, Y.; Chen, Q.; Wu, Z.; Yu, R. Label-Free Photonic Crystal-Based β-Lactamase Biosensor for β-Lactam Antibiotic and β-Lactamase Inhibitor. Anal. Chem. 2016, 88, 9207–9212. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, X.; Jiang, W.; Fu, D.; Gu, Z. Multiplex bioassays encoded by photonic crystal beads and SERS nanotags. Nanoscale 2016, 8, 17465–17471. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, S.; Tong, J.; Zhu, P.; Diao, G.; Yang, Z. 3D ordered silver nanoshells silica photonic crystal beads for multiplex encoded SERS bioassay. Chem. Commun. 2016, 52, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.D.; Chen, W.; Cunningham, B.T.; Andrade, J.E. Enhanced sandwich immunoassay using antibody-functionalized magnetic iron-oxide nanoparticles for extraction and detection of soluble transferrin receptor on a photonic crystal biosensor. Biosens. Bioelectron. 2015, 74, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.; Li, P.; Lin, B.; Pepper, J. Colorimetric resonant reflection as a direct biochemical assay technique. Sens. Actuators B 2002, 81, 316–328. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, P.; Tang, Q.; Tang, M.; Zang, Z.; Zhao, S. Dually responsive amphiphilic block copolymer with oxidation-responsiveness and tuneable LCST behaviours. Mater. Lett. 2017, 201, 133–136. [Google Scholar] [CrossRef]
- Halligan, S.C.; Dalton, M.B.; Murray, K.A.; Dong, Y.; Wang, W.; Lyons, J.G.; Geever, L.M. Synthesis, characterisation and phase transition behaviour of temperature-responsive physically crosslinked poly(N-vinylcaprolactam) based polymers for biomedical applications. Mater. Sci. Eng. C 2017, 79, 130–139. [Google Scholar] [CrossRef] [PubMed]
- De Baubigny, J.D.; Trégouët, C.; Salez, T.; Pantoustier, N.; Perrin, P.; Reyssat, M.; Monteux, C. One-step fabrication of pH-responsive membranes and microcapsules through interfacial H–bond polymer complexation. Sci. Rep. 2017, 7, 1265. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, T.; Yao, Y.; Zhang, F.; Liu, L.; Liu, Y.; Leng, J. Stimulus methods of Multi-functional Shape Memory Polymer Nanocomposites: A review. Compos. A Appl. Sci. Manuf. 2017, 100, 20–30. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–hydrogel composites: Concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Nikjoo, D.; Aroguz, A.Z. Dual responsive polymeric bionanocomposite gel beads for controlled drug release systems. J. Appl. Polym. Sci. 2017, 134, 45143. [Google Scholar] [CrossRef]
- Chacón, J.V.; Arbeláez, M.I.A.; Jorge, J.H.; Marques, R.F.C.; Jafelicci, M., Jr. pH-responsive poly(aspartic acid) hydrogel-coated magnetite nanoparticles for biomedical applications. Mater. Sci. Eng. C 2017, 77, 366–373. [Google Scholar] [CrossRef] [PubMed]















© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Serpe, M.J.; Mugo, S.M. Stimuli Responsive Polymer-Based 3D Optical Crystals for Sensing. Polymers 2017, 9, 436. https://doi.org/10.3390/polym9110436
Zhang Q, Serpe MJ, Mugo SM. Stimuli Responsive Polymer-Based 3D Optical Crystals for Sensing. Polymers. 2017; 9(11):436. https://doi.org/10.3390/polym9110436
Chicago/Turabian StyleZhang, Qiang, Michael J. Serpe, and Samuel M. Mugo. 2017. "Stimuli Responsive Polymer-Based 3D Optical Crystals for Sensing" Polymers 9, no. 11: 436. https://doi.org/10.3390/polym9110436





