Zwitter-Ionic Polymer Applied as Electron Transportation Layer for Improving the Performance of Polymer Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Devices
2.3. Measurement and Characterization
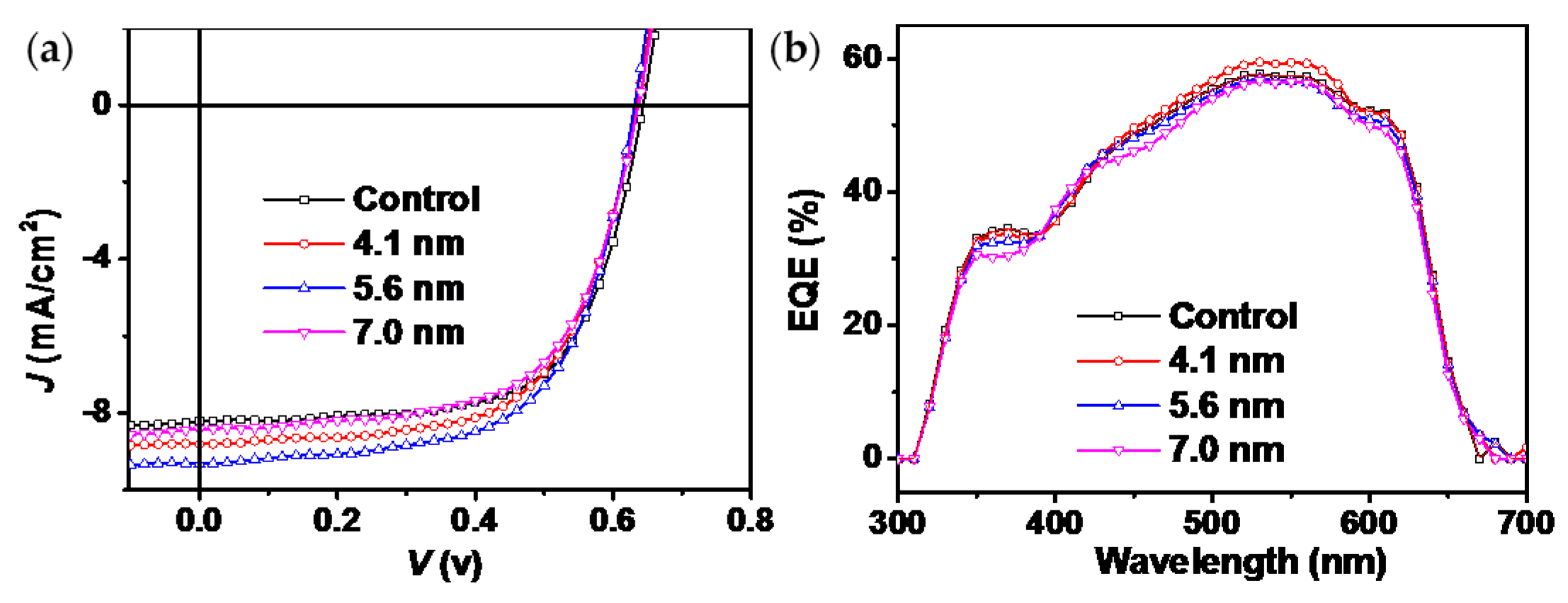
3. Results and Discussion
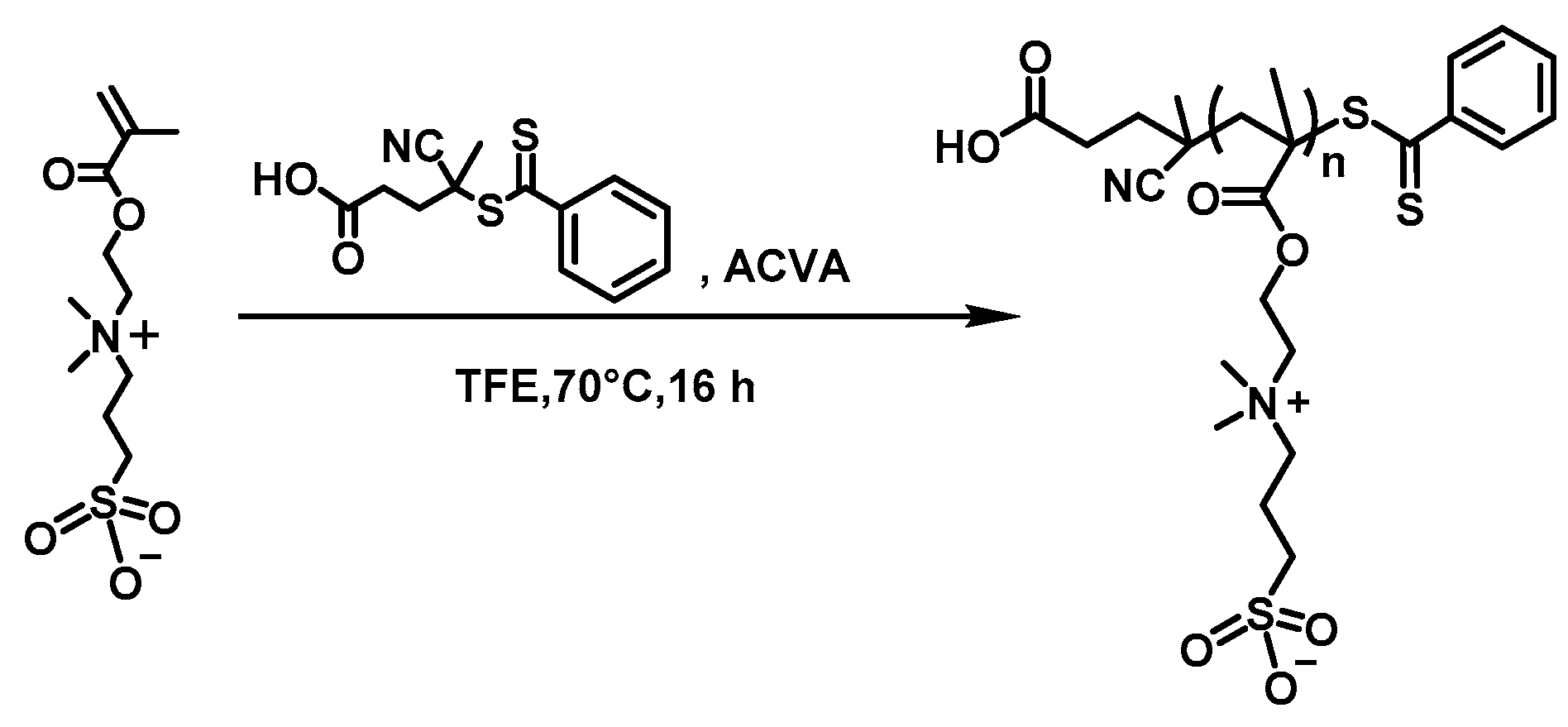
3.1. Synthsis of the PSBMA
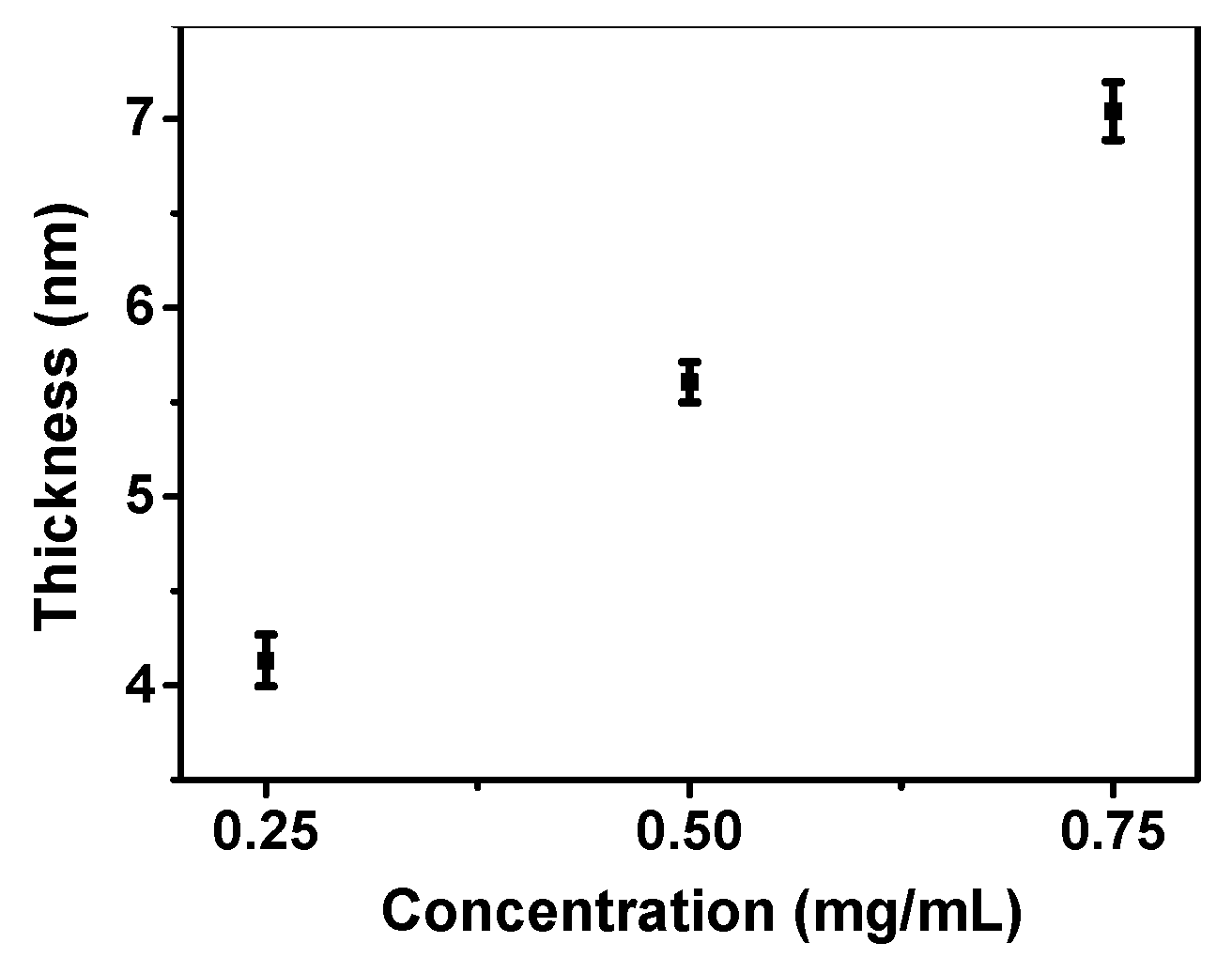
3.2. The Thickness Control of the PSBMA Films
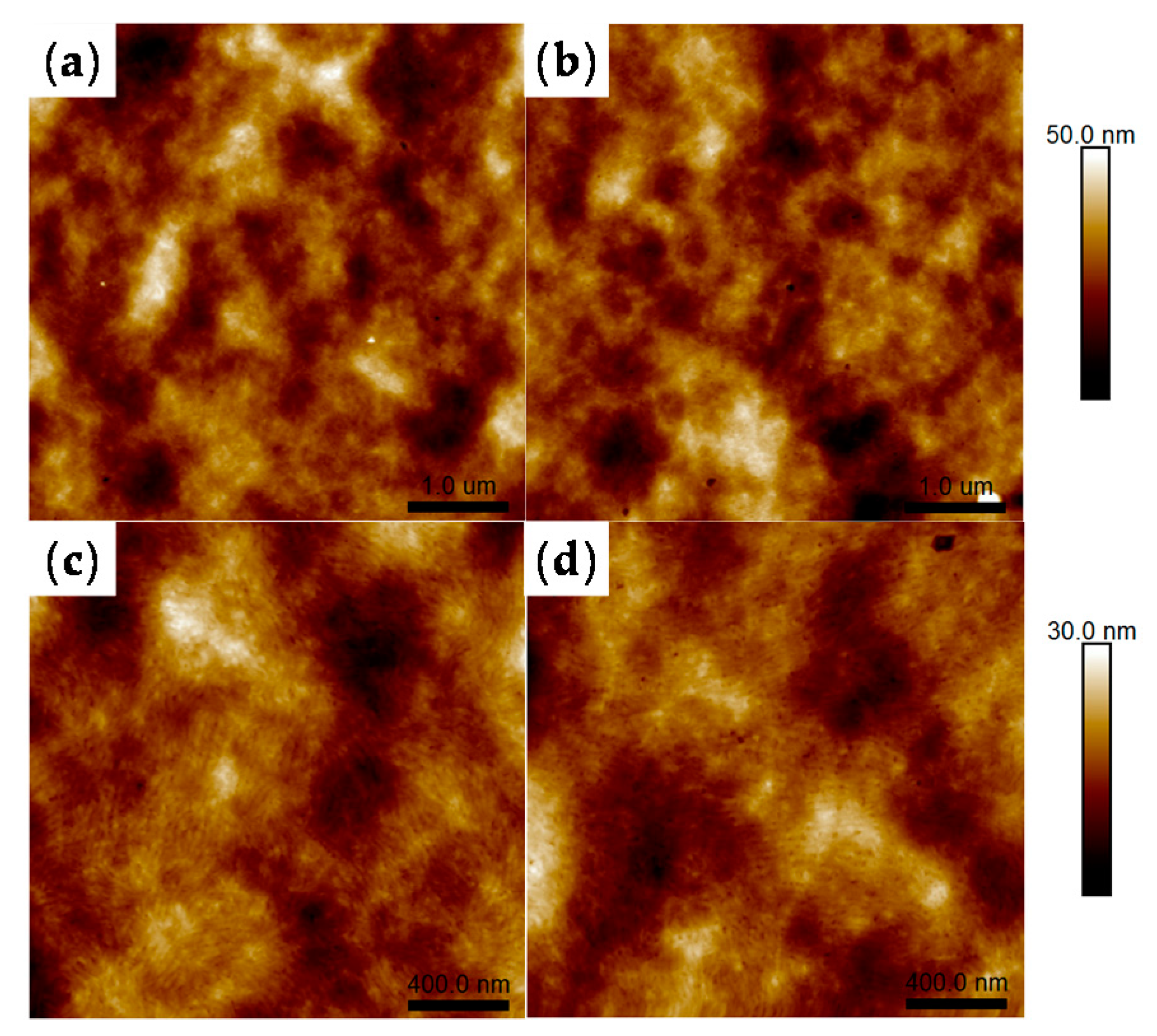
3.3. Morphologies of P3HT:PC61BM and PSBMA@P3HT:PC61BM
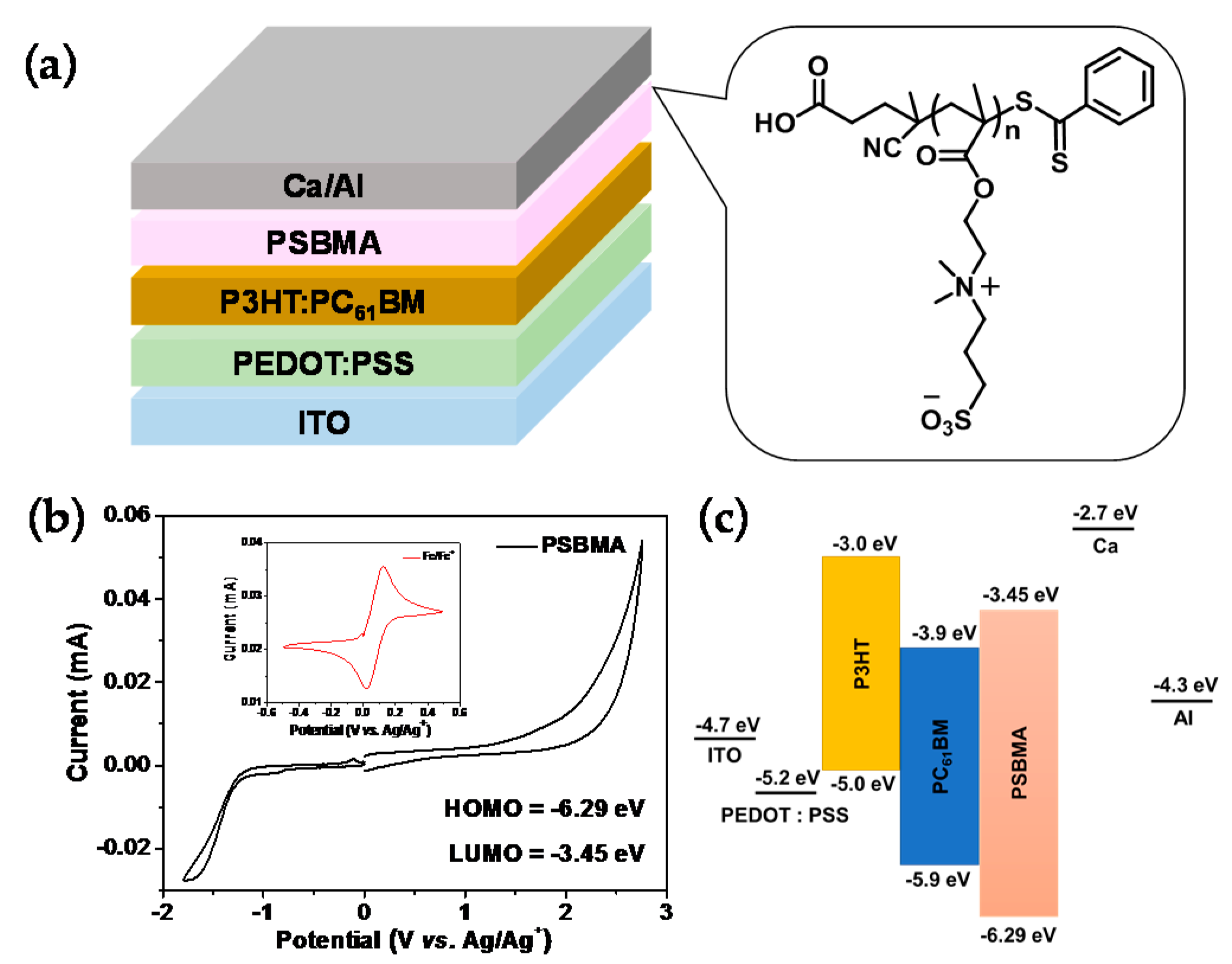
3.4. Electrochemical Properties
3.5. Photovoltaic Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T.P.; Cao, Y. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photonics 2015, 9, 174–179. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Li, S.; Ye, L.; Zhao, W.; Zhang, S.; Mukherjee, S.; Ade, H.; Hou, J. Energy-Level Modulation of Small-Molecule Electron Acceptors to Achieve over 12% Efficiency in Polymer Solar Cells. Adv. Mater. 2016, 28, 9423–9429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Qian, D.; Zhang, S.; Li, S.; Inganas, O.; Gao, F.; Hou, J. Fullerene-Free Polymer Solar Cells with over 11% Efficiency and Excellent Thermal Stability. Adv. Mater. 2016, 28, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Xu, X.; Li, Y.; Li, K.; Peng, Q. Polymer Solar Cells Exceeding 10% Efficiency Enabled via a Facile Star-Shaped Molecular Cathode Interlayer with Variable Counterions. Adv. Funct. Mater. 2016, 26, 4643–4652. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Chen, Y.; Zhang, Z.; Xue, S.; Sun, S.; Zhang, K.; Zhong, C.; Zhang, H.; Pan, Y.; Lv, Y.; et al. Electrochemical route to fabricate film-like conjugated microporous polymers and application for organic electronics. Adv. Mater. 2013, 25, 3443–3448. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhao, Y.; Zhang, J.; Zhang, Y.; Zhu, L.; Lu, K.; Yan, W.; Wei, Z. Oligomeric Donor Material for High-Efficiency Organic Solar Cells: Breaking Down a Polymer. Adv. Mater. 2015, 27, 4229–4233. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.-C.; Chen, P.-H.; Chang, R.; Su, W.-F. Morphological Control Agent in Ternary Blend Bulk Heterojunction Solar Cells. Polymers 2014, 6, 2784–2802. [Google Scholar] [CrossRef]
- Raja, R.; Luo, S.; Hsiow, C.-Y.; Rwei, S.-P.; Wang, L. Novel Two-Dimensional Conjugated Polymer Containing Fluorinated Bithiophene as Donor and Benzoselenodiazole as Acceptor Units with Vinyl-Terthiophene Pendants for Polymer Photovoltaic Cells. Polymers 2017, 9, 272. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, M.; Zhang, Y.; Liu, Z.; Yang, Y.; Zhao, L. Recent Development on Narrow Bandgap Conjugated Polymers for Polymer Solar Cells. Polymers 2017, 9, 39. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, E. Development of Polymer Acceptors for Organic Photovoltaic Cells. Polymers 2014, 6, 382–407. [Google Scholar] [CrossRef]
- Liu, W.; Liang, T.; Chen, Q.; Yu, Z.; Zhang, Y.; Liu, Y.; Fu, W.; Tang, F.; Chen, L.; Chen, H. Solution-Processed 8-Hydroquinolatolithium as Effective Cathode Interlayer for High-Performance Polymer Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 9254–9261. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, W.; Li, X.; Min, C.; Jiu, T.; Zhu, Y.; Dai, N.; Fang, J. Solution-processed hybrid cathode interlayer for inverted organic solar cells. ACS Appl. Mater. Interfaces 2013, 5, 10428–10432. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-M.; Xu, Z.; Hong, Z.; Yang, Y. Interface investigation and engineering—Achieving high performance polymer photovoltaic devices. J. Mater. Chem. 2010, 20, 2575–2598. [Google Scholar] [CrossRef]
- Zeng, H.; Zhu, X.; Liang, Y.; Guo, X. Interfacial Layer Engineering for Performance Enhancement in Polymer Solar Cells. Polymers 2015, 7, 333–372. [Google Scholar] [CrossRef]
- Brabec, C.J.; Shaheen, S.E.; Winder, C.; Sariciftci, N.S.; Denk, P. Effect of LiF/metal electrodes on the performance of plastic solar cells. Appl. Phys. Lett. 2002, 80, 1288–1290. [Google Scholar] [CrossRef]
- Huang, F.; Wu, H.; Wang, D.; Yang, W.; Cao, Y. Novel Electroluminescent Conjugated Polyelectrolytes Based on Polyfluorene. Chem. Mater. 2004, 16, 708–716. [Google Scholar] [CrossRef]
- Na, S.-I.; Oh, S.-H.; Kim, S.-S.; Kim, D.-Y. Efficient organic solar cells with polyfluorene derivatives as a cathode interfacial layer. Org. Electron. 2009, 10, 496–500. [Google Scholar] [CrossRef]
- Yan, L.; Song, Y.; Zhou, Y.; Song, B.; Li, Y. Effect of PEI cathode interlayer on work function and interface resistance of ITO electrode in the inverted polymer solar cells. Org. Electron. 2015, 17, 94–101. [Google Scholar] [CrossRef]
- Udum, Y.; Denk, P.; Adam, G.; Apaydin, D.H.; Nevosad, A.; Teichert, C.; White, M.S.; Sariciftci, N.S.; Scharber, M.C. Inverted bulk-heterojunction solar cell with cross-linked hole-blocking layer. Org. Electron. 2014, 15, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Lin, S.W.; Jiang, J.M.; Su, Y.W.; Wei, K.H. Solution-processed zinc oxide/polyethylenimine nanocomposites as tunable electron transport layers for highly efficient bulk heterojunction polymer solar cells. ACS Appl. Mater. Interfaces 2015, 7, 6273–6281. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chang, J.; Zhang, J.; Jiang, C.; Wu, J.; Zhu, C. A work-function tunable polyelectrolyte complex (PEI:PSS) as a cathode interfacial layer for inverted organic solar cells. J. Mater. Chem. A 2014, 2, 7788–7794. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.W.; Khan, T.M.; Kippelen, B. High performance polymeric charge recombination layer for organic tandem solar cells. Energy Environ. Sci. 2012, 5, 9827–9832. [Google Scholar] [CrossRef]
- Li, P.; Wang, G.; Cai, L.; Ding, B.; Zhou, D.; Hu, Y.; Zhang, Y.; Xiang, J.; Wan, K.; Chen, L.; et al. High-efficiency inverted polymer solar cells controlled by the thickness of polyethylenimine ethoxylated (PEIE) interfacial layers. Phys. Chem. Chem. Phys. 2014, 16, 23792–23799. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Ouyang, X.; Liu, Z.; Peng, R.; Jiang, W.; Li, W.; Zhang, L.; Hong, L.; Lei, T.; Guan, Q.; et al. Highly efficient polymer solar cells using a nonconjugated small-molecule zwitterion with enhancement of electron transfer and collection. J. Mater. Chem. A 2016, 4, 14944–14948. [Google Scholar] [CrossRef]
- Han, J.; Chen, Y.; Chen, W.; Yu, C.; Song, X.; Li, F.; Wang, Y. High Performance Small-Molecule Cathode Interlayer Materials with D-A-D Conjugated Central Skeletons and Side Flexible Alcohol/Water-Soluble Groups for Polymer Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 32823–32832. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, A.K.; Wang, D.H.; Gupta, V.; Zhang, J.; Chand, S.; Bazan, G.C.; Heeger, A.J. Efficient solution-processed small-molecule solar cells with inverted structure. Adv. Mater. 2013, 25, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wu, H.; Cao, Y. Recent advances in polymer solar cells: Realization of high device performance by incorporating water/alcohol-soluble conjugated polymers as electrode buffer layer. Adv. Mater. 2014, 26, 1006–1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, J.; Shao, S.; Deng, Y.; Meng, B.; Xie, Z.; Geng, Y.; Wang, L.; Zhang, F. Printable highly conductive conjugated polymer sensitized ZnO NCs as cathode interfacial layer for efficient polymer solar cells. ACS Appl. Mater. Interfaces 2014, 6, 8237–8245. [Google Scholar] [CrossRef] [PubMed]
- White, M.S.; Olson, D.C.; Shaheen, S.E.; Kopidakis, N.; Ginley, D.S. Inverted bulk-heterojunction organic photovoltaic device using a solution-derived ZnO underlayer. Appl. Phys. Lett. 2006, 89, 143517. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhang, K.C.; Wang, Z.W.; Huang, P.; Zhu, K.; Li, Z.D.; Li, D.H.; Yuan, L.G.; Zhou, Y.; Song, B. Comprehensive Study of Sol-Gel versus Hydrolysis-Condensation Methods To Prepare ZnO Films: Electron Transport Layers in Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 26234–26241. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.-L.; Liang, Z.; Yang, Y.-H.; Ohuchi, F.S.; Jenekhe, S.A.; Cao, G. The effect of SrTiO3:ZnO as cathodic buffer layer for inverted polymer solar cells. Nano Energy 2014, 4, 140–149. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Cao, F.-Y.; Lin, W.-C.; Chen, C.-H.; Hsieh, C.-H. Self-Assembled and Cross-Linked Fullerene Interlayer on Titanium Oxide for Highly Efficient Inverted Polymer Solar Cells. Chem. Mater. 2011, 23, 1512–1518. [Google Scholar] [CrossRef]
- Saehana, S.; Muslimin; Abdullah, M. Electrochemical impedance spectroscopy study of TiO2 based solar cells. J. Renew. Sustain. Energy 2014, 6, 023109. [Google Scholar] [CrossRef]
- Yan, Y.; Cai, F.; Yang, L.; Li, J.; Zhang, Y.; Qin, F.; Xiong, C.; Zhou, Y.; Lidzey, D.G.; Wang, T. Light-Soaking-Free Inverted Polymer Solar Cells with an Efficiency of 10.5% by Compositional and Surface Modifications to a Low-Temperature-Processed TiO2 Electron-Transport Layer. Adv. Mater. 2017, 29, 1604044. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Sun, L.; Shen, W.; Yang, C.; Chen, W.; Yang, R. Facile preparation of TiOX film as an interface material for efficient inverted polymer solar cells. J. Mater. Chem. A 2014, 2, 1732–1737. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, H.; Yang, L.; Shi, M.; Wang, M.; Chen, H. Effect of CsF interlayer on the performance of polymer bulk heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 650–653. [Google Scholar] [CrossRef]
- Chen, F.-C.; Wu, J.-L.; Yang, S.S.; Hsieh, K.-H.; Chen, W.-C. Cesium carbonate as a functional interlayer for polymer photovoltaic devices. J. Appl. Phys. 2008, 103, 103721. [Google Scholar] [CrossRef]
- Gupta, V.; Kyaw, A.K.; Wang, D.H.; Chand, S.; Bazan, G.C.; Heeger, A.J. Barium: An efficient cathode layer for bulk-heterojunction solar cells. Sci. Rep. 2013, 3, 1965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, Z.; Fu, W.; Shi, M.; Chen, H. Phosphate ester side-chain-modified conjugated polymer for hybrid solar cells. J. Appl. Polym. Sci. 2017, 134, 45003. [Google Scholar] [CrossRef]
- Shao, S.; Zheng, K.; Pullerits, T.; Zhang, F. Enhanced performance of inverted polymer solar cells by using poly(ethylene oxide)-modified ZnO as an electron transport layer. ACS Appl. Mater. Interfaces 2013, 5, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ceder, M.; Inganäs, O. Enhancing the Photovoltage of Polymer Solar Cells by Using a Modified Cathode. Adv. Mater. 2007, 19, 1835–1838. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, K.; Huang, F.; Bazan, G.C.; Cao, Y. Amino N-Oxide Functionalized Conjugated Polymers and their Amino-Functionalized Precursors: New Cathode Interlayers for High-Performance Optoelectronic Devices. Adv. Funct. Mater. 2012, 22, 2846–2854. [Google Scholar] [CrossRef]
- Wang, J.; Lin, K.; Zhang, K.; Jiang, X.-F.; Mahmood, K.; Ying, L.; Huang, F.; Cao, Y. Crosslinkable Amino-Functionalized Conjugated Polymer as Cathode Interlayer for Efficient Inverted Polymer Solar Cells. Adv. Energy Mater. 2016, 6, 1502563. [Google Scholar] [CrossRef]
- Zhang, W.; Song, C.; Liu, X.; Fang, J. Realizing Highly Efficient Inverted Photovoltaic Cells by Combination of Nonconjugated Small-Molecule Zwitterions with Polyethylene Glycol. ACS Appl. Mater. Interfaces 2016, 8, 18593–18599. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Page, Z.A.; Duzhko, V.V.; Russell, T.P.; Emrick, T. Conjugated polymeric zwitterions as efficient interlayers in organic solar cells. Adv. Mater. 2013, 25, 6868–6873. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Page, Z.; Ferdous, S.; Liu, F.; Kim, P.; Emrick, T.; Russell, T. Dual Functional Zwitterionic Fullerene Interlayer for Efficient Inverted Polymer Solar Cells. Adv. Energy Mater. 2015, 5, 1500405. [Google Scholar] [CrossRef]
- Duan, C.; Wang, L.; Zhang, K.; Guan, X.; Huang, F. Conjugated Zwitterionic Polyelectrolytes and Their Neutral Precursor as Electron Injection Layer for High-Performance Polymer Light-Emitting Diodes. Adv. Mater. 2011, 23, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wallikewitz, B.H.; Gao, F.; Tu, G.; Müller, C.; Pace, G.; Friend, R.H.; Huck, W.T. Conjugated zwitterionic polyelectrolyte as the charge injection layer for high-performance polymer light-emitting diodes. J. Am. Chem. Soc. 2010, 133, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Page, Z.A.; Duzhko, V.V.; Emrick, T. Conjugated thiophene-containing polymer zwitterions: Direct synthesis and thin film electronic properties. Macromolecules 2012, 46, 344–351. [Google Scholar] [CrossRef]
- Han, D.; Letteri, R.; Chan-Seng, D.; Emrick, T.; Tu, H. Examination of zwitterionic polymers and gels subjected to mechanical constraints. Polymer 2013, 54, 2887–2894. [Google Scholar] [CrossRef]
- Ruderer, M.A.; Müller-Buschbaum, P. Morphology of polymer-based bulk heterojunction films for organic photovoltaics. Soft Matter 2011, 7, 5482–5493. [Google Scholar] [CrossRef]
- Hildner, R.; Köhler, A.; Müller-Buschbaum, P.; Panzer, F.; Thelakkat, M. π-Conjugated Donor Polymers: Structure Formation and Morphology in Solution, Bulk and Photovoltaic Blends. Adv. Energy Mater. 2017, 7, 1700314. [Google Scholar] [CrossRef]
- Ruderer, M.A.; Guo, S.; Meier, R.; Chiang, H.-Y.; Körstgens, V.; Wiedersich, J.; Perlich, J.; Roth, S.V.; Müller-Buschbaum, P. Solvent-Induced Morphology in Polymer-Based Systems for Organic Photovoltaics. Adv. Funct. Mater. 2011, 21, 3382–3391. [Google Scholar] [CrossRef]
- Müller-Buschbaum, P. The active layer morphology of organic solar cells probed with grazing incidence scattering techniques. Adv. Mater. 2014, 26, 7692–7709. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ouyang, X.; Peng, R.; Bai, Y.; Mi, D.; Jiang, W.; Facchetti, A.; Ge, Z. Efficient polymer solar cells based on the synergy effect of a novel non-conjugated small-molecule electrolyte and polar solvent. J. Mater. Chem. A 2016, 4, 2530–2536. [Google Scholar] [CrossRef]
- Ouyang, X.; Peng, R.; Ai, L.; Zhang, X.; Ge, Z. Efficient polymer solar cells employing a non-conjugated small-molecule electrolyte. Nat. Photonics 2015, 9, 520–524. [Google Scholar] [CrossRef]

| ETL | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Ca | 0.64 a (0.63 ± 0.01) b | 8.21 (8.04 ± 0.17) | 65.9 (64.8 ± 0.7) | 3.49 (3.31 ± 0.12) |
| 4.1 nm | 0.64 (0.63 ± 0.01) | 8.81 (8.52 ± 0.20) | 64.7 (63.4 ± 1.0) | 3.51 (3.43 ± 0.05) |
| 5.6 nm | 0.63 (0.63 ± 0.00) | 9.32 (8.99 ± 0.19) | 62.5 (60.7 ± 1.5) | 3.67 (3.44 ± 0.15) |
| 7.0 nm | 0.64 (0.63 ± 0.01) | 8.43 (8.29 ± 0.01) | 64.1 (62.9 ± 0.7) | 3.37 (3.29 ± 0.05) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Li, Z.; Dong, B.; Zhou, Y.; Song, B. Zwitter-Ionic Polymer Applied as Electron Transportation Layer for Improving the Performance of Polymer Solar Cells. Polymers 2017, 9, 566. https://doi.org/10.3390/polym9110566
Chen Q, Li Z, Dong B, Zhou Y, Song B. Zwitter-Ionic Polymer Applied as Electron Transportation Layer for Improving the Performance of Polymer Solar Cells. Polymers. 2017; 9(11):566. https://doi.org/10.3390/polym9110566
Chicago/Turabian StyleChen, Qiaoyun, Zhendong Li, Bin Dong, Yi Zhou, and Bo Song. 2017. "Zwitter-Ionic Polymer Applied as Electron Transportation Layer for Improving the Performance of Polymer Solar Cells" Polymers 9, no. 11: 566. https://doi.org/10.3390/polym9110566





