Extraction and Characterization of Cellulose Nanocrystals from Tea Leaf Waste Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose Fibers
2.3. Extraction of Cellulose Nanocrystals
2.4. Characterizations
2.4.1. Determination of Chemical Composition Analysis
2.4.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.3. X-ray Diffraction (XRD) Analysis
2.4.4. Scanning Electron Microscopy (SEM)
2.4.5. Transmission Electron Microscopy (TEM)
3. Results
3.1. Chemical Composition of Tea Leaf Waste Fiber
3.2. Fourier Transform Infrared (FTIR) Analysis
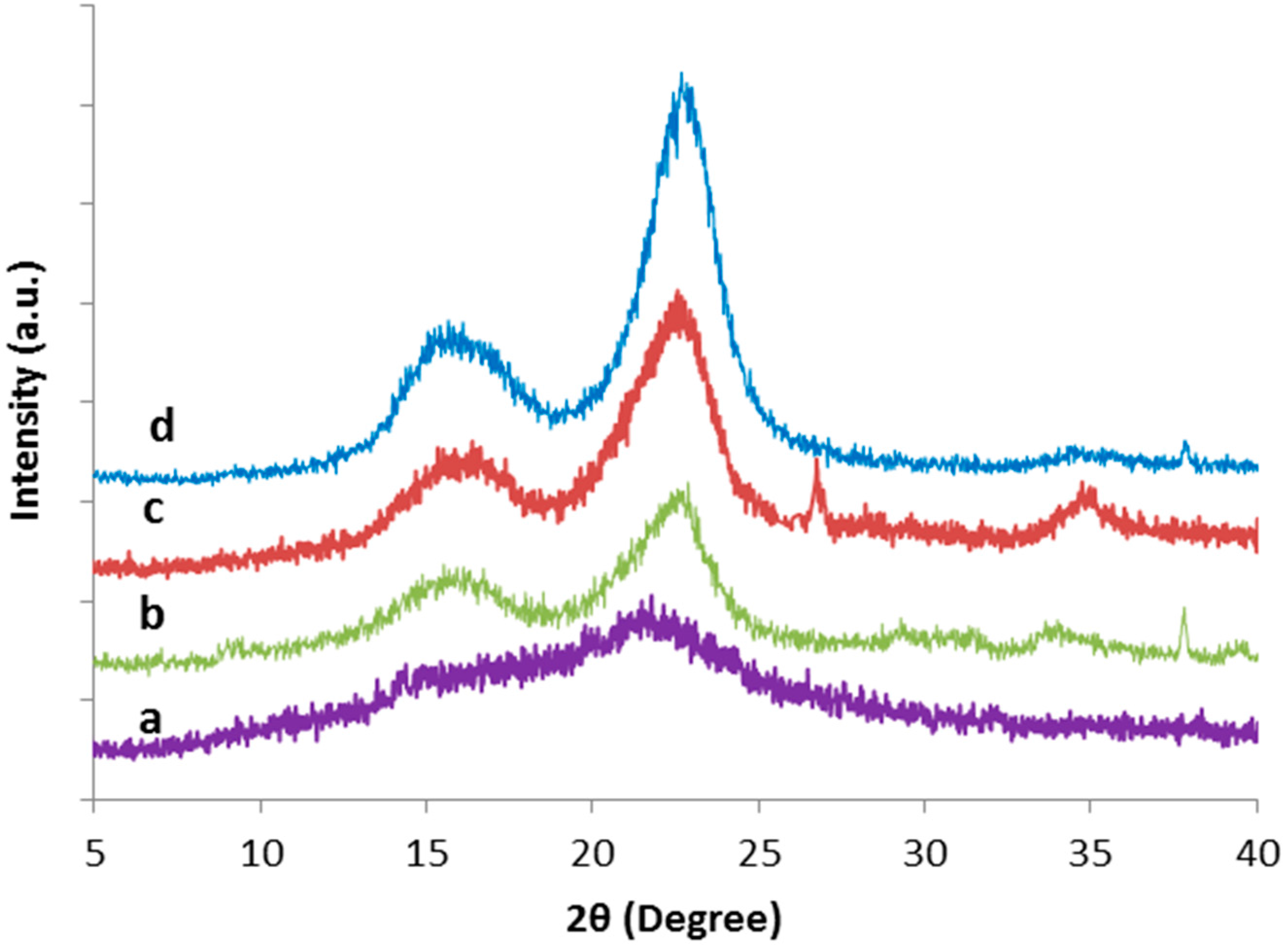
3.3. X-ray Diffraction (XRD) Analysis
3.4. Scanning Electron Microscopy (SEM)
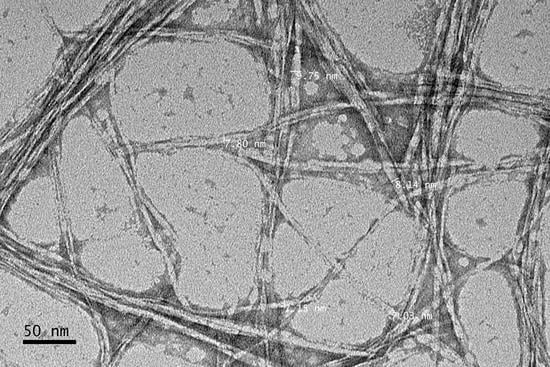
3.5. Transmission Electron Microscopy (TEM)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ku, H.; Wang, H.; Pattarachaiyakoop, N.; Trada, M. A review on the tensile properties of natural fiber reinforced polymer composites. Compos. B. Eng. 2011, 42, 856–873. [Google Scholar] [CrossRef] [Green Version]
- Takagi, H.; Asano, A. Effects of processing conditions on flexural properties of cellulose nanofiber reinforced “green” composites. Compos. A. Appl. Sci. Manuf. 2008, 39, 685–689. [Google Scholar] [CrossRef]
- Takagi, H.; Ichihara, Y. Effect of Fiber Length on Mechanical Properties of “Green” Composites Using a Starch-Based Resin and Short Bamboo Fibers. JSME Int. J. Ser. A 2004, 47, 551–555. [Google Scholar] [CrossRef]
- Then, Y.Y.; Ibrahim, N.A.; Zainuddin, N.; Ariffin, H.; Wan Yunus, W.M.Z.; Chieng, B.W. Surface Modifications of Oil Palm Mesocarp Fiber by Superheated Steam, Alkali, and Superheated Steam-Alkali for Biocomposite Applications. BioResources 2014, 9, 7467–7483. [Google Scholar] [CrossRef]
- Then, Y.Y.; Ibrahim, N.A.; Zainuddin, N.; Ariffin, H.; Wan Yunus, W.M.Z.; Chieng, B.W. The Influence of Green Surface Modification of Oil Palm Mesocarp Fiber by Superheated Steam on the Mechanical Properties and Dimensional Stability of Oil Palm Mesocarp Fiber/Poly(butylene succinate) Biocomposite. Int. J. Mol. Sci. 2014, 15, 15344–15357. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Kaith, B.S.; Kaur, I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—A review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Properties of natural cellulose fibers from hop stems. Carbohydr. Polym. 2009, 77, 898–902. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Gassan, J. Composites reinforced with cellulose based fibres. Prog. Polym. Sci. 1999, 24, 221–274. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of Recent Research into Cellulosic Whiskers, Their Properties and Their Application in Nanocomposite Field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779. [Google Scholar] [CrossRef]
- Wong, S.; Shanks, R. Biocomposites of Natural Fibers and Poly(3-Hydroxybutyrate) and Copolymers: Improved Mechanical Properties through Compatibilization at the Interface. In Biodegradable Polymer Blends and Composites from Renewable Resources; Yu, L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 303–347. [Google Scholar]
- Chang, K. World Tea Production and Trade Current and Future Development; Food and Agricultural Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Liu, Z.; Li, D.; Dai, H.; Huang, H. Preparation and characterization of papain embedded in magnetic cellulose hydrogels prepared from tea residue. J. Mol. Liquids 2017, 232, 449–456. [Google Scholar] [CrossRef]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Rosli, N.A.; Ahmad, I.; Abdullah, I. Isolation and Characterization of Cellulose Nanocrystals from Agave angustifolia Fibre. BioResources 2013, 8, 1893–1908. [Google Scholar] [CrossRef]
- Fortunati, E.; Puglia, D.; Luzi, F.; Santulli, C.; Kenny, J.M.; Torre, L. Binary PVA bio-nanocomposites containing cellulose nanocrystals extracted from different natural sources: Part I. Carbohydr. Polym. 2013, 97, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.L.S.; do Nascimento, D.M.; Souza Filho Mde, M.; Morais, J.P.S.; Vasconcelos, N.F.; Feitosa, J.P.A.; Brígida, A.I.S.; Rosa Mde, F. Improvement of polyvinyl alcohol properties by adding nanocrystalline cellulose isolated from banana pseudostems. Carbohydr. Polym. 2014, 112, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Chieng, B.W.; Lee, S.H.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Isolation and Characterization of Cellulose Nanocrystals from Oil Palm Mesocarp Fiber. Polymers 2017, 9, 355. [Google Scholar] [CrossRef]
- Siqueira, G.; Abdillahi, H.; Bras, J.; Dufresne, A. High reinforcing capability cellulose nanocrystals extracted from Syngonanthus nitens (Capim dourado). Cellulose 2010, 17, 289–298. [Google Scholar] [CrossRef]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining Cellulose Nanofibers with a Uniform Width of 15 nm from Wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Then, Y.Y.; Ibrahim, N.A.; Zainuddin, N.; Chieng, B.W.; Ariffin, H.; Wan Yunus, W.M.Z. Influence of Alkaline-Peroxide Treatment of Fiber on the Mechanical Properties of Oil Palm Mesocarp Fiber/Poly(butylene succinate) Biocomposite. BioResources 2015, 10, 1730–1746. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Physical, chemical and surface properties of wheat husk, rye husk and soft wood and their polypropylene composites. Compos. A. Appl. Sci. Manuf. 2010, 41, 480–488. [Google Scholar] [CrossRef]
- Sain, M.; Panthapulakkal, S. Bioprocess preparation of wheat straw fibers and their characterization. Ind. Crops Prod. 2006, 23, 1–8. [Google Scholar] [CrossRef]
- Le Troedec, M.; Sedan, D.; Peyratout, C.; Bonnet, J.P.; Smith, A.; Guinebretiere, R.; Gloaguen, V.; Krausz, P. Influence of various chemical treatments on the composition and structure of hemp fibres. Compos. A. Appl. Sci. Manuf. 2008, 39, 514–522. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Nicoletti, F.; Vitale, G.; Prestipino, M.; Valenza, A. A new eco-friendly chemical treatment of natural fibres: Effect of sodium bicarbonate on properties of sisal fibre and its epoxy composites. Compos. B. Eng. 2016, 85, 150–160. [Google Scholar] [CrossRef]
- Rayung, M.; Ibrahim, N.A.; Zainuddin, N.; Saad, W.Z.; Abdul Razak, N.I.; Chieng, B.W. The Effect of Fiber Bleaching Treatment on the Properties of Poly(lactic acid)/Oil Palm Empty Fruit Bunch Fiber Composites. Int. J. Mol. Sci. 2014, 15, 14728–14742. [Google Scholar] [CrossRef] [PubMed]
- Razak, N.; Ibrahim, N.; Zainuddin, N.; Rayung, M.; Saad, W. The Influence of Chemical Surface Modification of Kenaf Fiber using Hydrogen Peroxide on the Mechanical Properties of Biodegradable Kenaf Fiber/Poly(Lactic Acid) Composites. Molecules 2014, 19, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, J.X.; Xu, F.; Sun, R.C. Influence of steaming pressure on steam explosion pretreatment of Lespedeza stalks (Lespedeza crytobotrya): Part 1. Characteristics of degraded cellulose. Polym. Degrad. Stab. 2009, 94, 1379–1388. [Google Scholar] [CrossRef]
- Birnin-Yauri, A.U.; Ibrahim, N.A.; Zainuddin, N.; Abdan, K.; Then, Y.Y.; Chieng, B.W. Influence of Kenaf Core Fiber Incorporation on the Mechanical Performance and Dimensional Stability of Oil Palm Fiber Reinforced Poly(lactic acid) Hybrid Biocomposites. BioResources 2016, 11, 3332–3355. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues—Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Joonobi, M.; Harun, J.; Tahir, P.M.; Zaini, L.H.; SaifulAzry, S.; Makinejad, M.D. Characteristic of nanofibers extracted from kenaf core. BioResources 2010, 5, 2556–2566. [Google Scholar]
- Liu, H.; Liu, D.; Yao, F.; Wu, Q. Fabrication and properties of transparent polymethylmethacrylate/cellulose nanocrystals composites. Bioresour. Technol. 2010, 101, 5685–5692. [Google Scholar] [CrossRef] [PubMed]
- Flauzino Neto, W.P.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue—Soy hulls. Ind. Crops Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]




| Material | Cellulose (wt %) | Hemicellulose (wt %) | Lignin (wt %) |
|---|---|---|---|
| Raw TLWF | 16.2 | 68.2 | 18.8 |
| Alkaline-treated TLWF | 58.8 | 22.2 | 5.5 |
| Bleached TLWF | 87.9 | 8.1 | 1.8 |
| Samples | 2θ (Amorphous) (°) | 2θ (002) (°) | CrI (%) | ||
|---|---|---|---|---|---|
| Degree | Intensity (Iam) | Degree | Intensity (I002) | ||
| Raw TLWF | 18.0 | 240 | 21.7 | 410 | 41.5 |
| Alkaline-treated TLWF | 18.9 | 144 | 22.8 | 440 | 67.3 |
| Bleached TLWF | 18.9 | 158 | 22.5 | 616 | 74.4 |
| CNC | 18.7 | 146 | 22.6 | 866 | 83.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Rahman, N.H.; Chieng, B.W.; Ibrahim, N.A.; Abdul Rahman, N. Extraction and Characterization of Cellulose Nanocrystals from Tea Leaf Waste Fibers. Polymers 2017, 9, 588. https://doi.org/10.3390/polym9110588
Abdul Rahman NH, Chieng BW, Ibrahim NA, Abdul Rahman N. Extraction and Characterization of Cellulose Nanocrystals from Tea Leaf Waste Fibers. Polymers. 2017; 9(11):588. https://doi.org/10.3390/polym9110588
Chicago/Turabian StyleAbdul Rahman, Nur Hayati, Buong Woei Chieng, Nor Azowa Ibrahim, and Norizah Abdul Rahman. 2017. "Extraction and Characterization of Cellulose Nanocrystals from Tea Leaf Waste Fibers" Polymers 9, no. 11: 588. https://doi.org/10.3390/polym9110588