Synthesis, Structure, and Dye Adsorption Properties of a Nickel(II) Coordination Layer Built from d-Camphorate and Bispyridyl Ligands
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Synthesis of [Ni4(d-cam)2(d-Hcam)4(bpzpip)4(H2O)2] (1)
2.3. X-ray Data Collection and Structure Refinement
2.4. Dye Adsorption Measurements
3. Results and Discussion
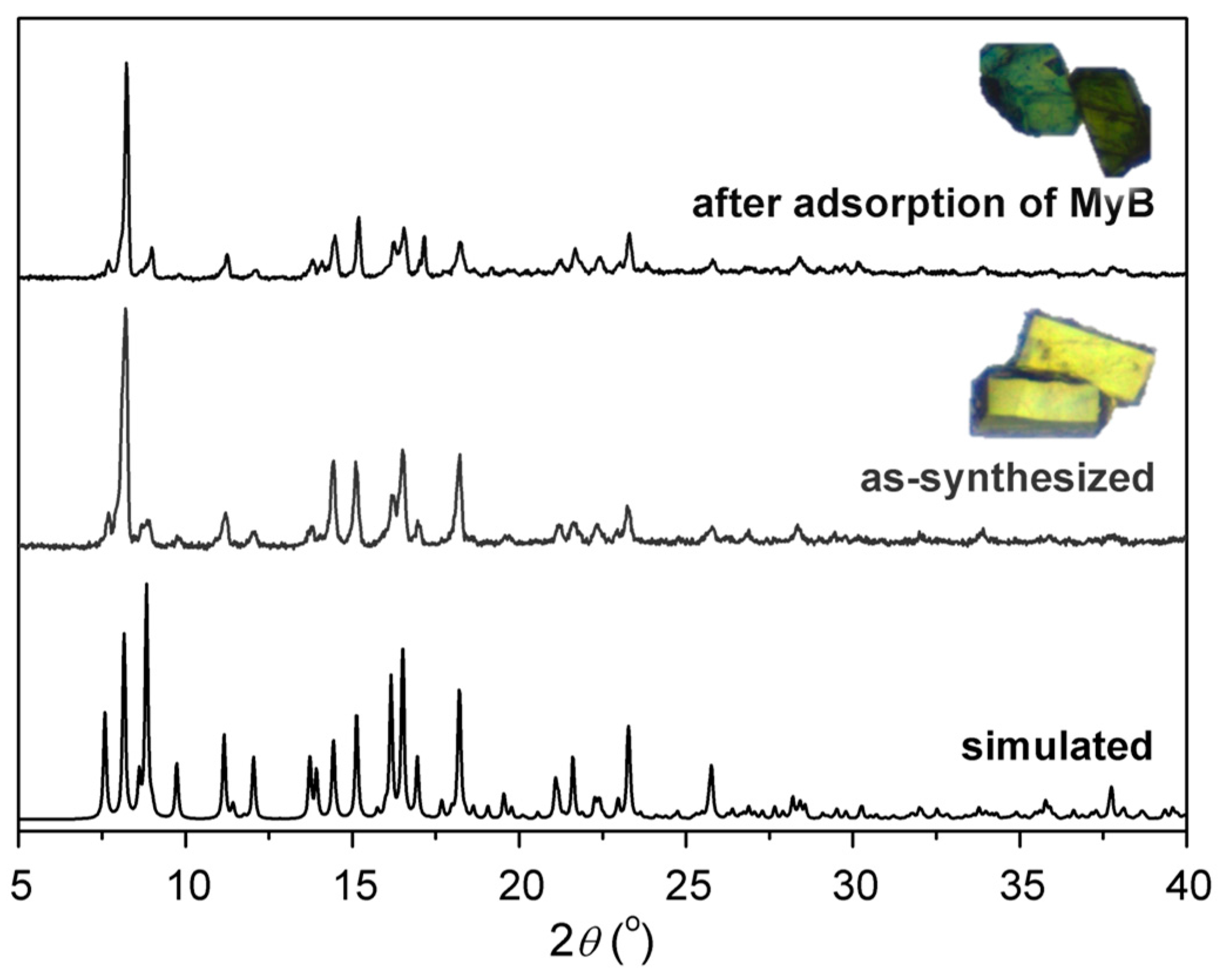
3.1. Syntheses and Characterization
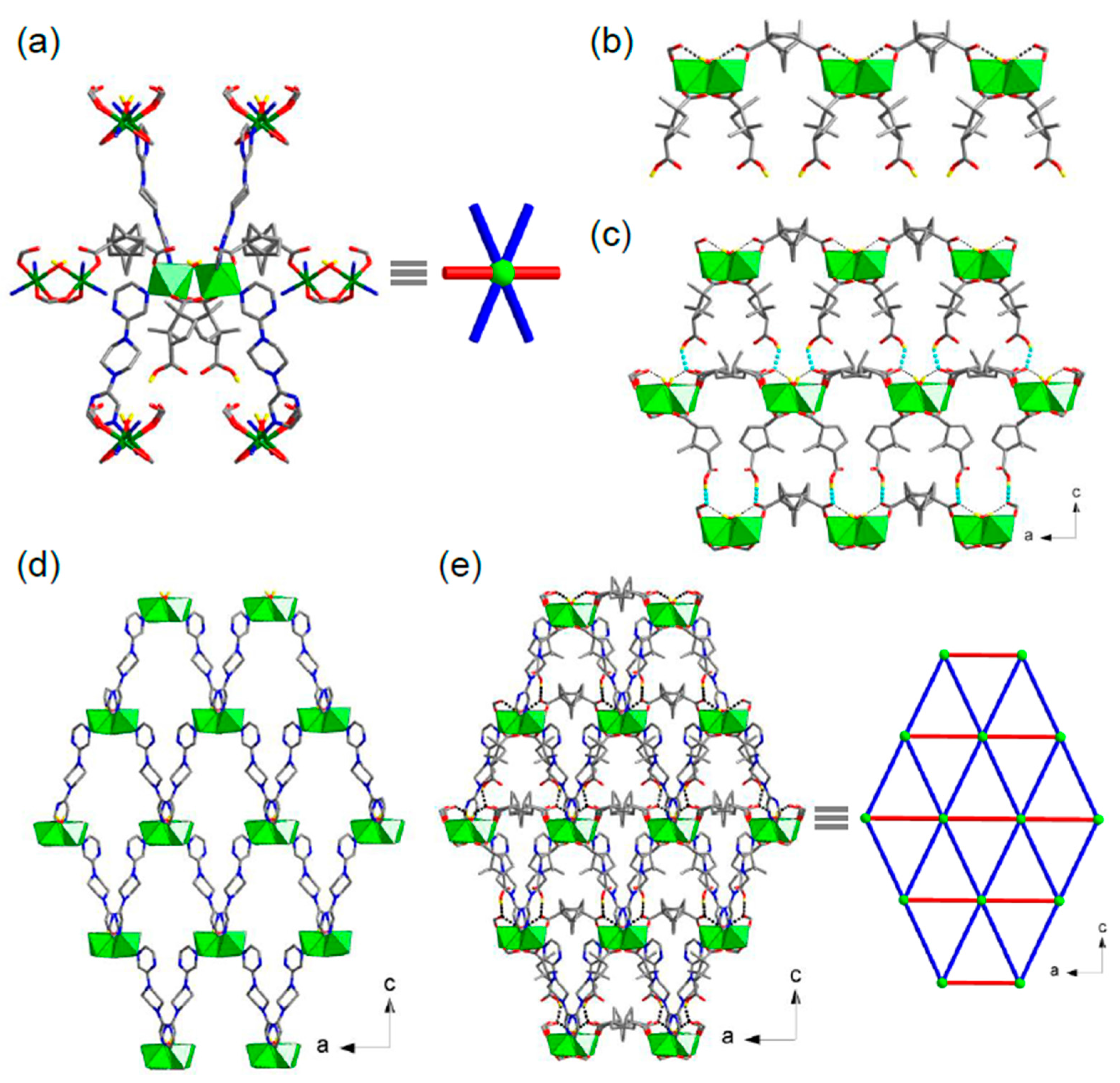
3.2. Crystal Structure of [Ni4(d-cam)2(d-Hcam)4(bpzpip)4(H2O)2] (1)
3.3. Thermogravimetric and N2 Adsorption Properties
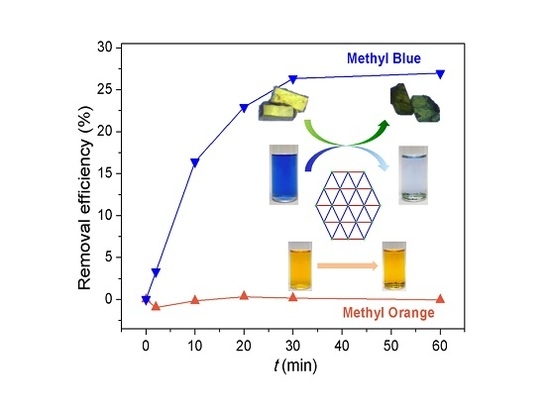
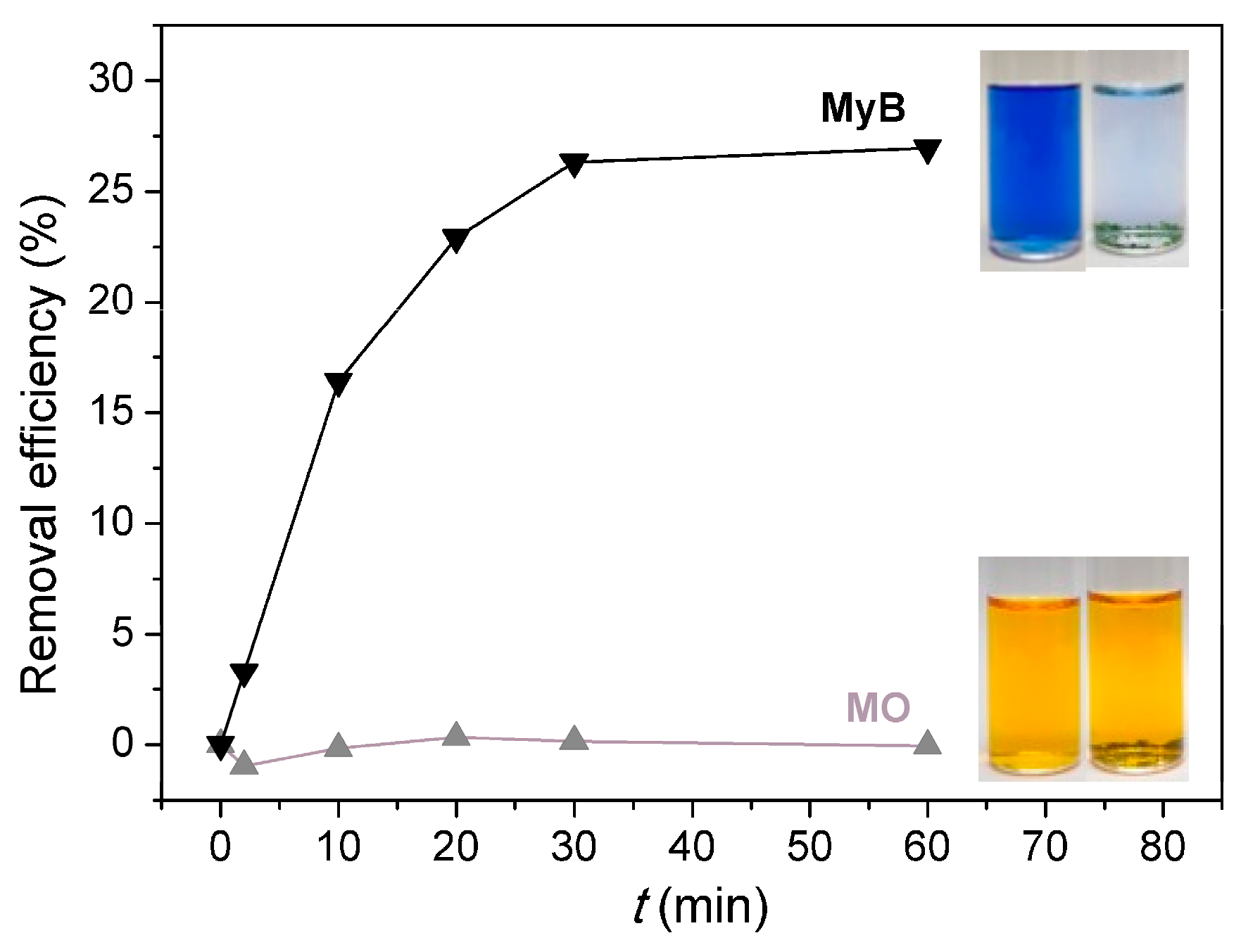
3.4. Dye Adsorption Behaviors
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, J.; Furukawa, H.; Zhang, Y.-B.; Yaghi, O.M. High methane storage working capacity in metal−organic frameworks with acrylate links. J. Am. Chem. Soc. 2016, 138, 10244–10251. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- De Voorde, B.V.; Bueken, B.; Denayer, J.; Vos, D.D. Adsorptive separation on metal–organic frameworks in the liquid phase. Chem. Soc. Rev. 2014, 43, 5766–5788. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.-Y.; Dai, M.; Young, D.J.; Ren, Z.-G.; Lang, J.-P. Luminescent Zn(II) coordination polymers for highly selective sensing of Cr(III) and Cr(VI) in water. Inorg. Chem. 2017, 56, 4668–4678. [Google Scholar]
- Zhao, Y.; Xu, X.; Qiu, L.; Kang, X.; Wen, L.; Zhang, B. Metal−organic frameworks constructed from a new thiophene-functionalized dicarboxylate: Luminescence sensing and pesticide removal. ACS Appl. Mater. Interfaces 2017, 9, 15164–15175. [Google Scholar] [CrossRef] [PubMed]
- Varadwaj, G.B.B.; Oyetade, O.A.; Rana, S.; Martincigh, B.S.; Jonnalagadda, S.B.; Nyamori, V.O. Facile synthesis of three-dimensional Mg−Al layered double hydroxide/partially reduced graphene oxide nanocomposites for the effective removal of Pb2+ from aqueous solution. ACS Appl. Mater. Interfaces 2017, 9, 17290–17305. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215–216, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Song, Z.; Che, G.; Ren, A.; Li, P.; Liu, C.; Zhang, J. Adsorption behavior of metal–organic frameworks for methylene blue from aqueous solution. Microporous Mesoporous Mater. 2014, 193, 27–34. [Google Scholar] [CrossRef]
- Dias, E.M.; Petit, C. Towards the use of metal–organic frameworks for water reuse: A review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J. Mater. Chem. A 2015, 3, 22484–22506. [Google Scholar] [CrossRef]
- Wang, C.-C.; Li, J.-R.; Lv, X.-L.; Zhang, Y.-Q.; Guo, G. Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Pan, B.; Zhang, S. Photodegradation of acid orange 7 in a UV/acetylacetone process. Chemosphere 2013, 93, 2877–2882. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, W.; Zhao, J. Semiconductor-mediated photodegradation of pollutants under visible-light irradiation. Chem. Soc. Rev. 2010, 39, 4206–4219. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-L.; Zheng, T.-R.; Li, M.; Qian, L.-L.; Li, B.-L.; Li, H.-Y. A series of five-coordinated copper coordination polymers for efficient degradation of organic dyes under visible light irradiation. RSC Adv. 2017, 7, 23432–23443. [Google Scholar] [CrossRef]
- Du, P.-Y.; Li, H.; Fu, X.; Gu, W.; Liu, X. A 1D anionic lanthanide coordination polymer as an adsorbent material for the selective uptake of cationic dyes from aqueous solutions. Dalton Trans. 2015, 44, 13752–13759. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.-M.; Zhao, S.-Y.; Liu, P.; Wei, C.; Wu, Y.-L.; Xia, C.-K.; Xie, J.-M. Three N−H functionalized metal−organic frameworks with selective CO2 uptake, dye capture, and catalysis. Inorg. Chem. 2014, 53, 7692–7699. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Bai, Y.-L.; Zhang, L.; Sun, D.; Fang, J.; Zhu, S. Two nanocage anionic metal–organic frameworks with rht topology and {[M(H2O)6]6}12+ charge aggregation for rapid and selective adsorption of cationic dyes. Chem. Commun. 2014, 50, 14674–14677. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-J.; Wu, J.-Y. Synthesis, characterization, and dye capture of a 3D Cd(II)–carboxylate pcu network. Polyhedron 2017, 122, 124–130. [Google Scholar] [CrossRef]
- Wen, L.; Xu, X.; Lv, K.; Huang, Y.; Zheng, X.; Zhou, L.; Sun, R.; Li, D. Metal−organic frameworks constructed from d-camphor acid: Bifunctional properties related to luminescence sensing and liquid-phase separation. ACS Appl. Mater. Interfaces 2015, 7, 4449–4455. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, Z.; Xu, J.; Xu, W.; Sang, P.; Zhao, L.; Zhu, H.; Sun, D.; Guo, W. A NbO-type copper metal–organic framework decorated with carboxylate groups exhibiting highly selective CO2 adsorption and separation of organic dyes. J. Mater. Chem. A 2016, 4, 13844–13851. [Google Scholar] [CrossRef]
- Huo, M.; Yang, W.; Zhang, H.; Zhang, L.; Liao, J.; Lin, L.; Lu, C. A new POM–MOF hybrid microporous material with ultrahigh thermal stability and selective adsorption of organic dyes. RSC Adv. 2016, 6, 111549–111555. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Wu, J.-Y.; Lee, C.-F.; Lee, C.-C.; Lai, L.-L.; Lu, K.-L. Flexible “piperazine-pyrazine” building blocks: Conformational isomerism of “equatorial-axial” sites toward the constructions of silver(I) coordination chains. CrystEngComm 2010, 12, 3388–3390. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Ashiq, M.N. Adsorption of dyes from aqueous solutions on activated charcoal. J. Hazard. Mater. 2007, 139, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhuo, S.P.; Xing, W.; Cui, H.Y.; Dai, X.D.; Liu, X.M.; Yan, Z.F. Aqueous dye adsorption on ordered mesoporous carbons. J. Colloid Interface Sci. 2007, 310, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Liu, H.; Zhang, G.; Zou, H.; Zhang, Y.; Zhou, M.; Gu, Y. Magnetic field assisted adsorption of methyl blue onto organo-bentonite. Appl. Clay Sci. 2012, 55, 177–180. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Adsorption of dyes using different types of clay: A review. Appl. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef]
- Tsai, W.T.; Hsien, K.J.; Hsu, H.C. Adsorption of organic compounds from aqueous solution onto the synthesized zeolite. J. Hazard. Mater. 2009, 166, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Z.H. Characterisation and environmental application of an Australian natural zeolite for basic dye removal from aqueous solution. J. Hazard. Mater. 2006, 136, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Cai, X.; Tan, S.; Li, H.; Liu, J.; Yang, W.D. Adsorption characteristics of acrylonitrile, p-toluenesulfonic acid, 1-naphthalenesulfonic acid and methyl blue on graphene in aqueous solutions. Chem. Eng. J. 2011, 173, 144–149. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, Q.; Lu, C.; Li, Z.; Wang, W. Layered double hydroxide−carbon dot composite: High-Performance adsorbent for removal of anionic organic dye. ACS Appl. Mater. Interfaces 2014, 6, 20225–20233. [Google Scholar] [CrossRef] [PubMed]
- Fronczak, M.; Krajewska, M.; Demby, K.; Bystrzejewski, M. Extraordinary adsorption of methyl blue onto sodium-doped graphitic carbon nitride. J. Phys. Chem. C 2017, 121, 15756–15766. [Google Scholar] [CrossRef]
- Lu, Z.; Chu, W.; Tan, R.; Tang, S.; Xu, F.; Song, W.; Zhao, J. Facile synthesis of β-SrHPO4 with wide applications in the effective removal of Pb2+ and methyl blue. J. Chem. Eng. Data 2017, 62, 3501–3511. [Google Scholar] [CrossRef]
- Long, Y.; Xiao, L.; Cao, Q. Co-polymerization of catechol and polyethylenimine on magnetic nanoparticles for efficient selective removal of anionic dyes from water. Powder Technol. 2017, 310, 24–34. [Google Scholar] [CrossRef]
- Tan, P.; Hu, Y. Improved synthesis of graphene/β-cyclodextrin composite for highly efficient dye adsorption and removal. J. Mol. Liq. 2017, 242, 181–189. [Google Scholar] [CrossRef]
- Cramer, A.J.; Cole, J.M. Removal or storage of environmental pollutants and alternative fuel sources with inorganic adsorbents via host–guest encapsulation. J. Mater. Chem. A 2017, 5, 10746–10771. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, C.; Xu, W. Magnetic dendritic materials for highly efficient adsorption of dyes and drugs. ACS Appl. Mater. Interfaces 2010, 2, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Choi, E.-J.; Jhung, S.H. Adsorption of naproxen and clofibric acid over a metal–organic framework MIL-101 functionalized with acidic and basic groups. Chem. Eng. J. 2013, 219, 537–544. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Tong, M.; Liu, D.; Yang, Q.; Devautour-Vinot, S.; Maurin, G.; Zhong, C. Influence of framework metal ions on the dye capture behavior of MIL-100 (Fe, Cr) MOF type solids. J. Mater. Chem. A 2013, 1, 8534–8537. [Google Scholar] [CrossRef]
- Huo, S.-H.; Yan, X.-P. Metal–organic framework MIL-100(Fe) for the adsorption of malachite green from aqueous solution. J. Mater. Chem. 2012, 22, 7449–7455. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1402. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in Solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]

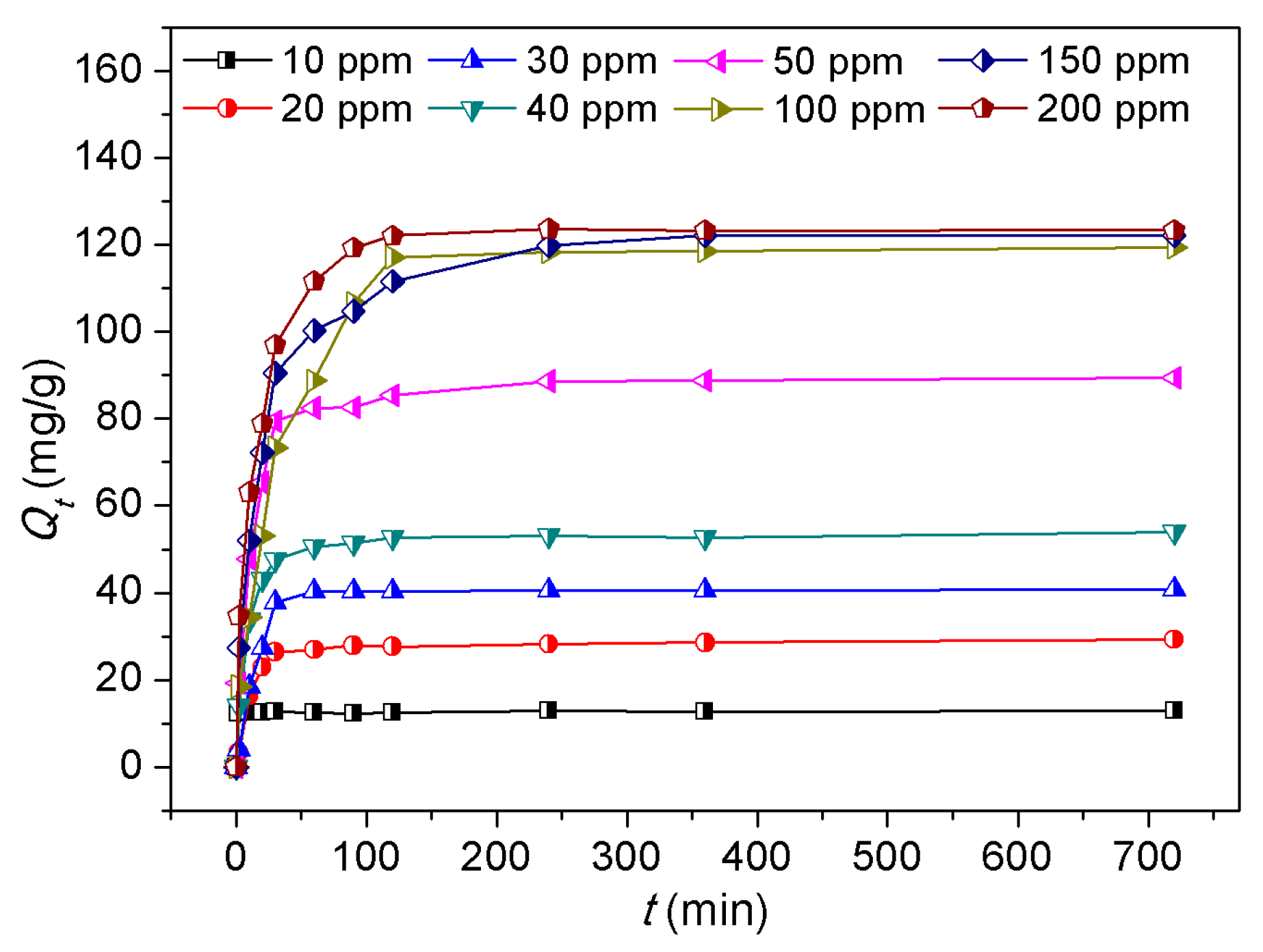
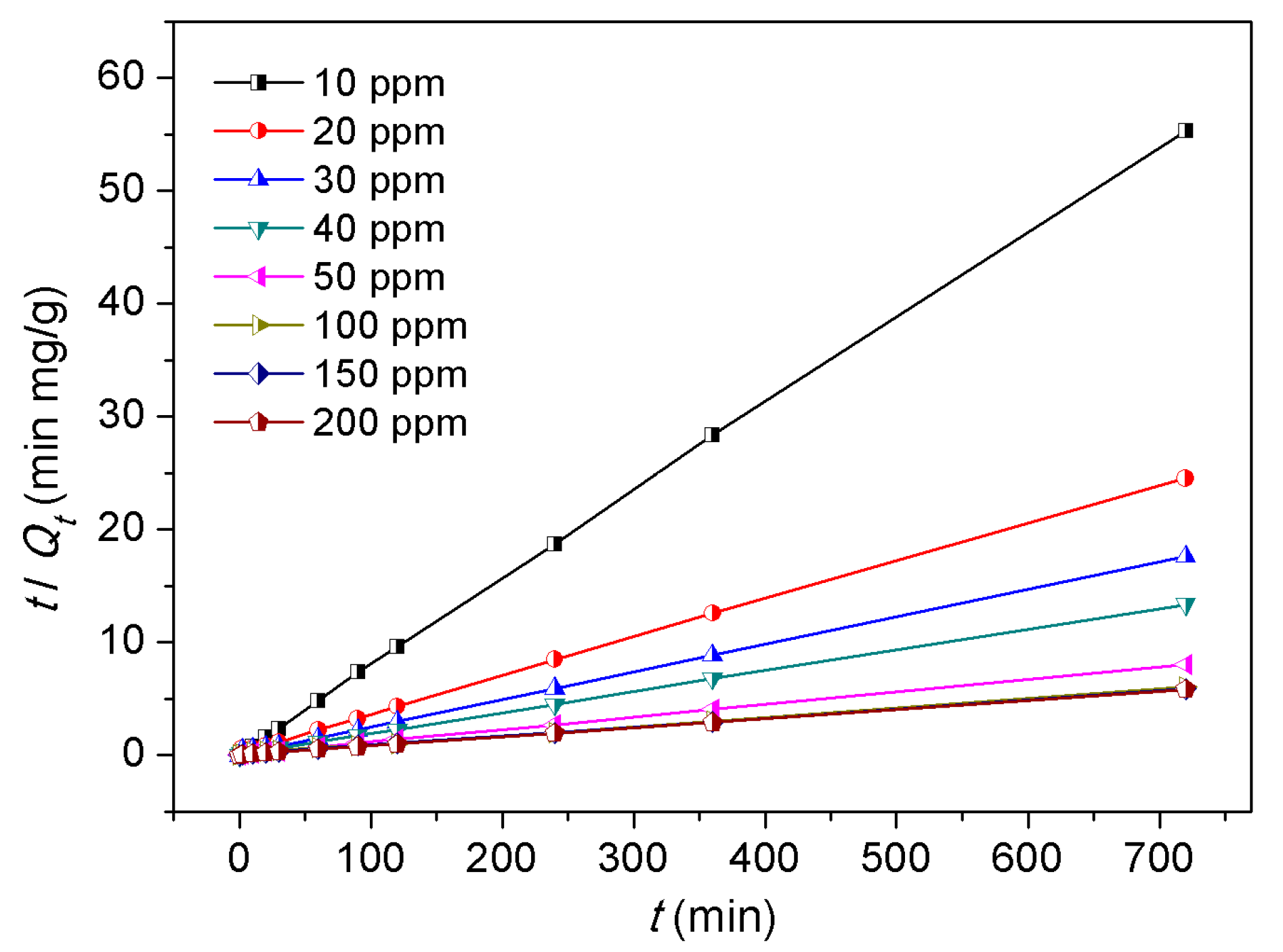

| Langmuir parameters | Freundlich parameters | ||||
|---|---|---|---|---|---|
| Qm/mg·g−1 | KL/L·mg−1 | R2 | n | KF/mg·g−1 | R2 |
| 185.5 | 0.014 | 0.8701 | 1.412 | 4.429 | 0.8573 |
| Adsorbents | Adsorption capacity/mg·g−1 | Ref. |
|---|---|---|
| Activated charcoal | 25.25 | [28] |
| Graphene | 50 | [25] |
| Organo-bentonite without magnetic exposure | 92.96 | [30] |
| Organo-bentonite with magnetic exposure | 98.15 | [30] |
| Magnetic chitosan-graphene oxide (MGGO) | 98.52 | [10] |
| Layered double hydroxide (LDH)-carbon dot composite | 185 | [35] |
| 1 | 185.5 | This work |
| Sodium-doped graphitic carbon nitride | 200–360 | [36] |
| β-Type strontium hydrogen phosphate (β-SrHPO4) nanosheet | 335.68 | [37] |
| Poly(catechol-polyethylenimine)-coated Fe3O4 nanoparticle (Fe3O4 ≅ catechol/PEI) | 344.8 | [38] |
| Graphene/β-cyclodextrin (GNS/β-CD) composite | 580.4 | [39] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-J.; Wu, J.-Y. Synthesis, Structure, and Dye Adsorption Properties of a Nickel(II) Coordination Layer Built from d-Camphorate and Bispyridyl Ligands. Polymers 2017, 9, 661. https://doi.org/10.3390/polym9120661
Tsai M-J, Wu J-Y. Synthesis, Structure, and Dye Adsorption Properties of a Nickel(II) Coordination Layer Built from d-Camphorate and Bispyridyl Ligands. Polymers. 2017; 9(12):661. https://doi.org/10.3390/polym9120661
Chicago/Turabian StyleTsai, Meng-Jung, and Jing-Yun Wu. 2017. "Synthesis, Structure, and Dye Adsorption Properties of a Nickel(II) Coordination Layer Built from d-Camphorate and Bispyridyl Ligands" Polymers 9, no. 12: 661. https://doi.org/10.3390/polym9120661