Thermal Transport in Soft PAAm Hydrogels
Abstract
1. Introduction
2. Materials and Methods
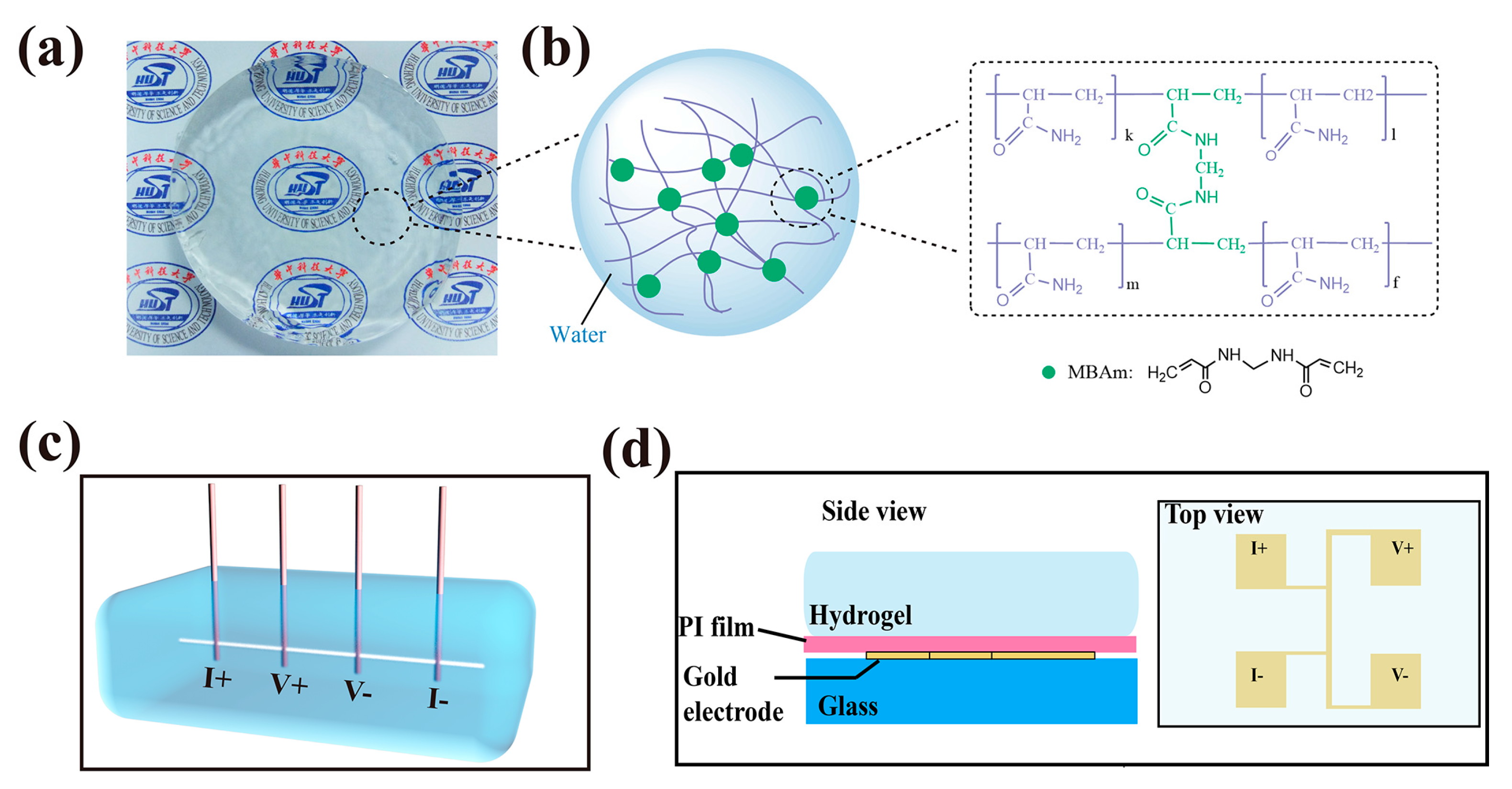
2.1. Materials Synthesis
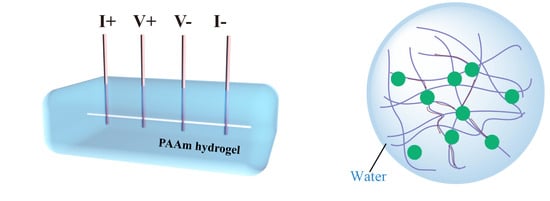
2.2. 3ω Method
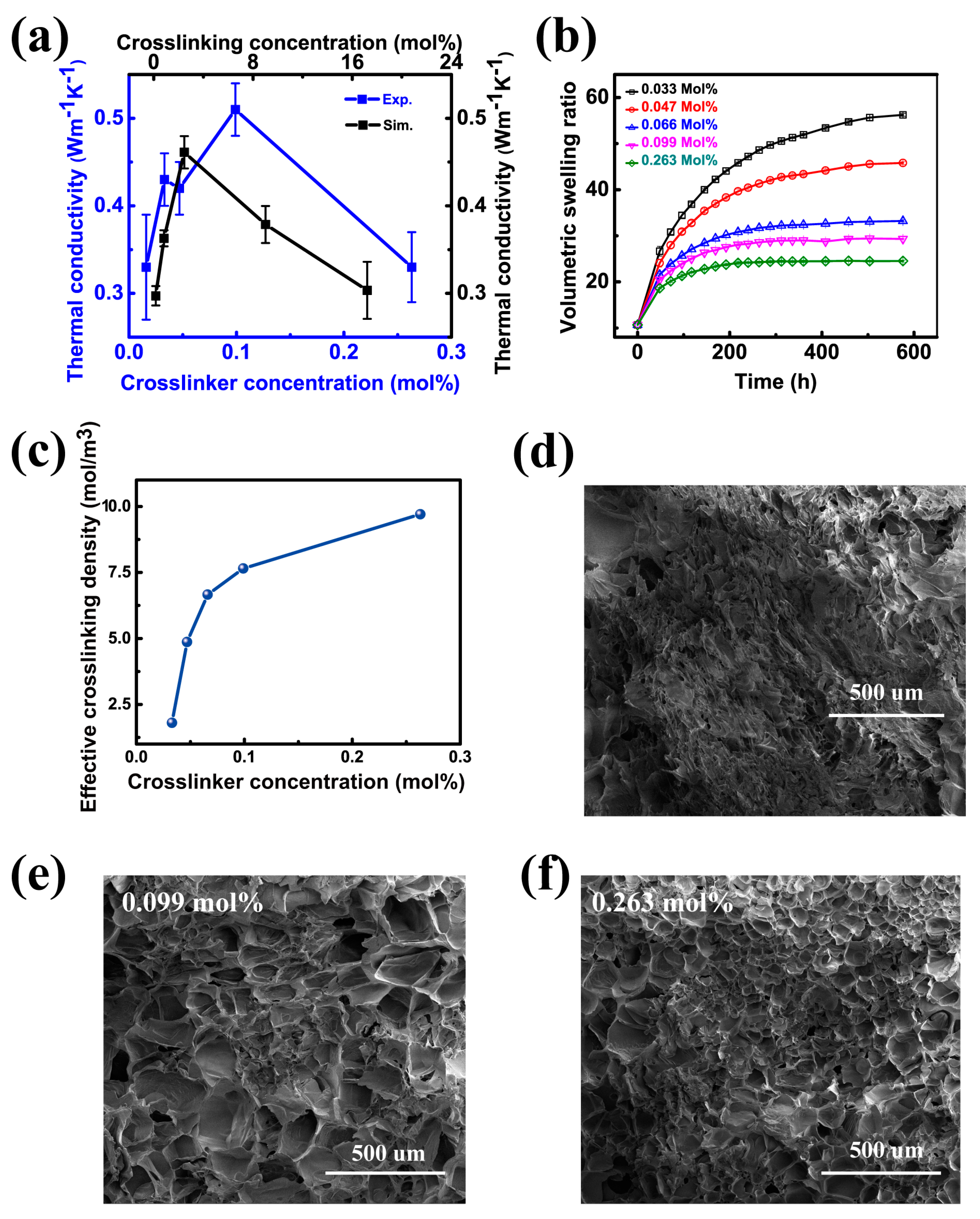
2.3. Equilibrium Swelling Ratio Measurement
2.4. EMD Simulation
2.5. Scanning Electron Microscope (SEM)
3. Results and Discussion
3.1. The Crosslinking Effect
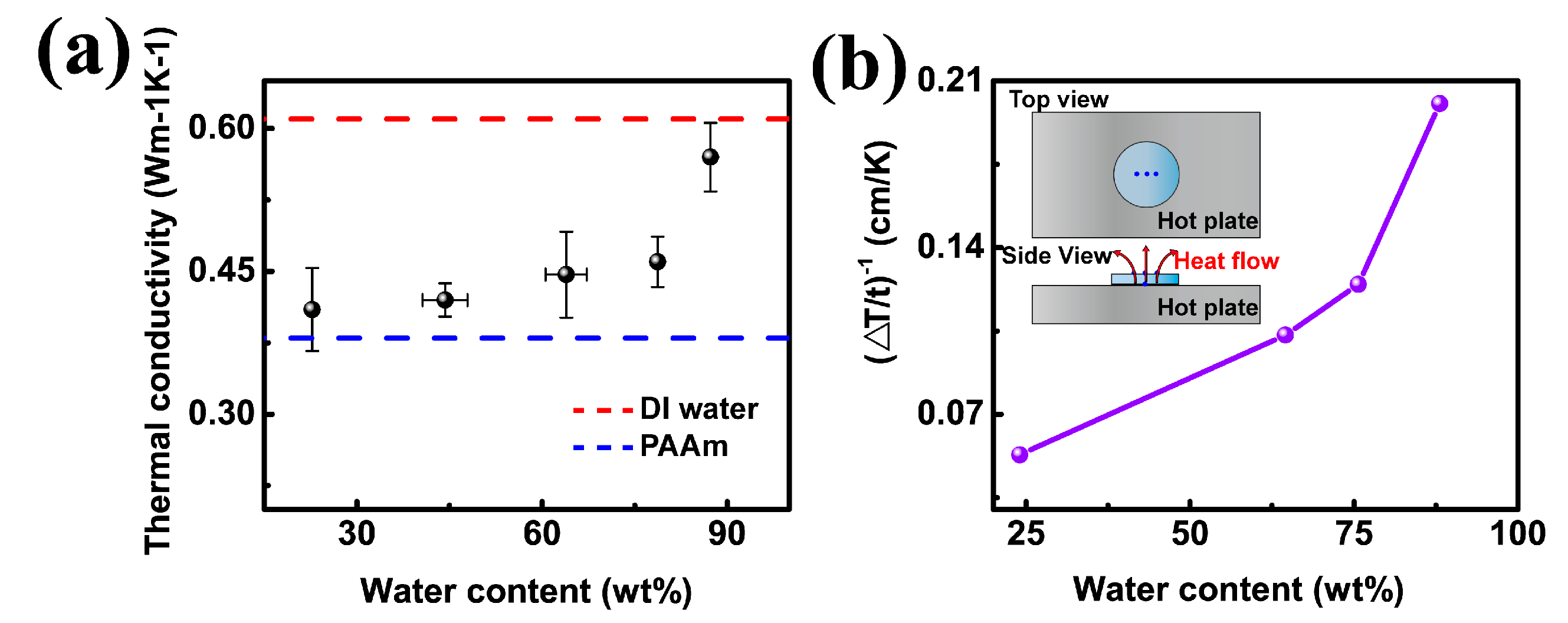
3.2. The Effect of Water Content
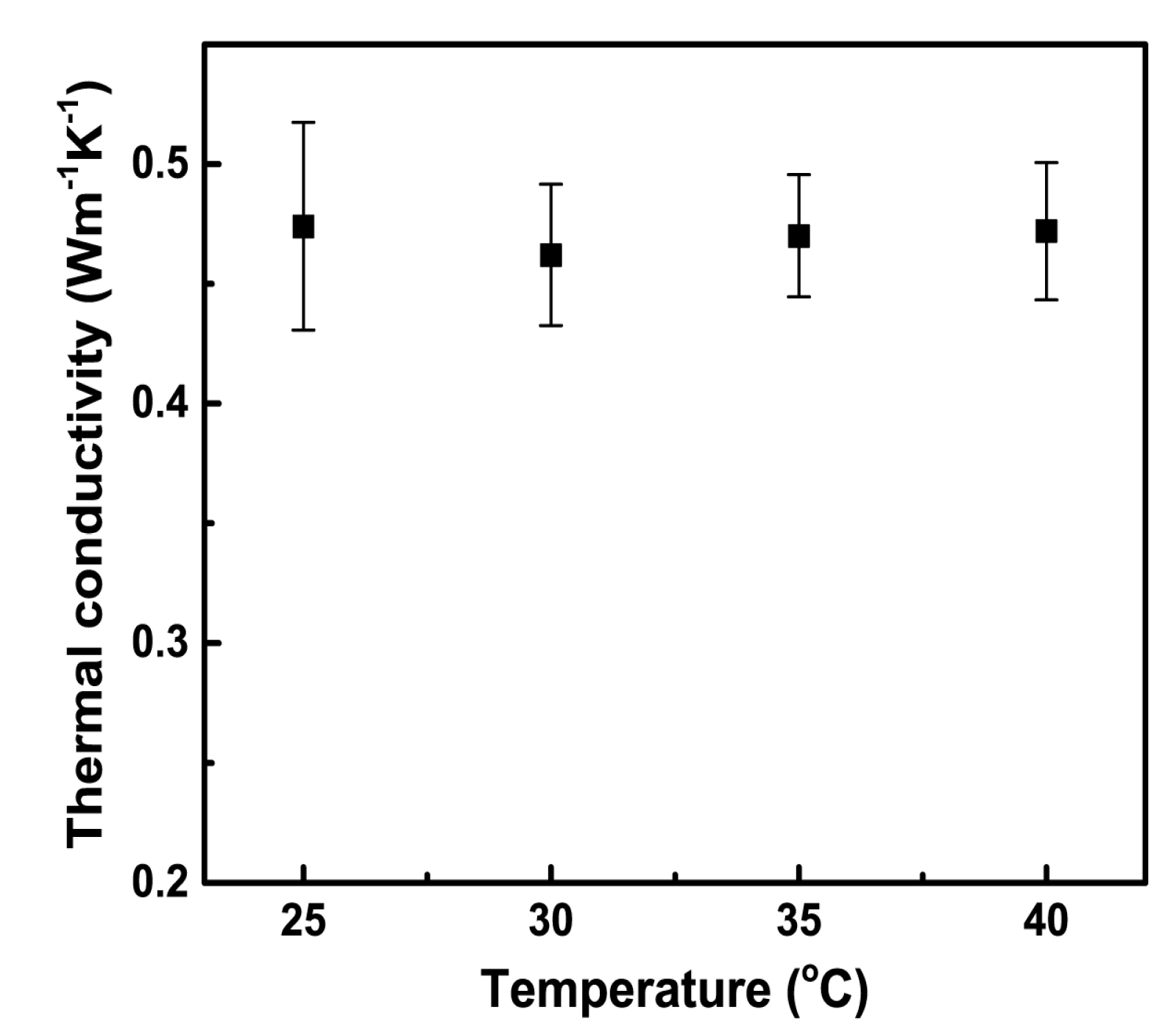
3.3. The Effect of Temperature
3.4. Temperature Distribution in Hydrogel-Based Electronics
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gerlach, G.; Arndt, K.F. Hydrogel Sensors and Biosensors; Springer: Berlin, Germany, 2010. [Google Scholar]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Calvert, P. Hydrogels for soft machines. Adv. Mater. 2009, 21, 743–756. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Suo, Z.; Vlassak, J.J. Stiff, strong, and tough hydrogels with good chemical stability. J. Mater. Chem. B 2014, 2, 6708–6713. [Google Scholar] [CrossRef]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D Printing: 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Adv. Mater. 2015, 27, 4035–4040. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-C.; Lee, H.-H.; Oh, K.H.; Sun, J.-Y. Highly stretchable, transparent ionic touch panel. Science 2016, 353, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.; Takahata, K. A steerable smart catheter tip realized by flexible hydrogel actuator. In Proceedings of the 2016 IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS), Shanghai, China, 24–28 January 2016; pp. 161–164. [Google Scholar]
- Guo, J.; Liu, X.; Jiang, N.; Yetisen, A.K.; Yuk, H.; Yang, C.; Khademhosseini, A.; Zhao, X.; Yun, S.H. Highly Stretchable, Strain Sensing Hydrogel Optical Fibers. Adv. Mater. 2016, 28, 10244–10249. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Ballerini, L.; Prato, M. Nanomaterials for stimulating nerve growth. Science 2017, 356, 1010–1012. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, G.; Liang, Y.; Cheng, T.; Dai, J.; Yang, X.; Liu, B.; Zeng, Z.; Huang, Z.; Luo, Y.; et al. Fast-moving soft electronic fish. Sci. Adv. 2017, 3, e1602045. [Google Scholar] [CrossRef] [PubMed]
- Wirthl, D.; Pichler, R.; Drack, M.; Kettlguber, G.; Moser, R.; Gerstmayr, R.; Hartmann, F.; Bradt, E.; Kaltseis, R.; Siket, C.M.; et al. Instant tough bonding of hydrogels for soft machines and electronics. Sci. Adv. 2017, 3, e1700053. [Google Scholar] [CrossRef] [PubMed]
- Tél, A.; Bauer, R.A.; Varga, Z.; Zrínyi, M. Heat conduction in poly(N-isopropylacrylamide) hydrogels. Int. J. Therm. Sci. 2014, 85, 47–53. [Google Scholar] [CrossRef]
- Iglesias, D.; Bosi, S.; Melchionna, M.; Da Ros, T.; Marchesan, S. The Glitter of Carbon Nanostructures in Hybrid/Composite Hydrogels for Medicinal Use. Curr. Top. Med. Chem. 2016, 16, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Mehrali, M.; Thakur, A.; Pennisi, C.P.; Talebian, S.; Arpanaei, A.; Nikkhah, M.; Dolatshahi-Pirouz, A. Nanoreinforced Hydrogels for Tissue Engineering: Biomaterials That Are Compatible with Load-Bearing and Electroactive Tissues. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fang, R.; Rong, Q.; Liu, M. Bioinspired Nanocomposite Hydrogels with Highly Ordered Structures. Adv. Mater. 2017, 29, 1703045. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, X.; Liu, Y.; Zhao, G.; Jiang, Y. Effect of magnetic nanoparticles on the properties of magnetic rubber. Mater. Res. Bull. 2010, 45, 878–881. [Google Scholar] [CrossRef]
- Koob, T.J.; Hernandez, D.J. Mechanical and thermal properties of novel polymerized NDGA-gelatin hydrogels. Biomaterials 2003, 24, 1285–1292. [Google Scholar] [CrossRef]
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.B.H.; Akil, H.M.; Rasib, S.Z.M.; Khan, A.; Ahmad, Z. Thermal properties and kinetic investigation of chitosan-PMAA based dual-responsive hydrogels. Ind. Crops Prod. 2015, 66, 178–187. [Google Scholar] [CrossRef]
- Zaragoza, J.; Babhadiashar, N.; O’Brien, V.; Chang, A.; Blanco, M.; Zabalegui, A.; Lee, H.; Asuri, P. Experimental investigation of mechanical and thermal properties of silica nanoparticle-reinforced poly(acrylamide) nanocomposite hydrogels. PLoS ONE 2015, 10, e0136293. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Naficy, S.; Casillas, G.; Khan, M.H.; Katkus, T.; Jiang, L.; Liu, H.; Li, H.; Huang, Z. Edge-Hydroxylated Boron Nitride Nanosheets as an Effective Additive to Improve the Thermal Response of Hydrogels. Adv. Mater. 2015, 27, 7196–7203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Z.; Geng, H.; Song, X.; Zeng, H.; Zhi, C. Highly Flexible and Self-Healable Thermal Interface Material Based on Boron Nitride Nanosheets and a Dual Cross-linked Hydrogel. ACS Appl. Mater. Interfaces 2017, 9, 10078–10084. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 9.1; Natl Std. Ref. Data Series (NIST NSRDS); National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2010. [Google Scholar]
- Xu, X.; Pereira, L.F.C.; Wang, Y.; Wu, J.; Zhang, K.; Zhao, X.; Bae, S.; Tinh Bui, C.; Xie, R.; Thong, J.T.L.; et al. Length-dependent thermal conductivity in suspended single-layer graphene. Nat. Commun. 2014, 5, 3689. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Victoria, P.D.; David, A.R.; Wolff, J.; Genest, M. Structure and Energetics of Ligand Binding to Proteins: Escherichia coli Dihydrofolate Reductase-Trimethoprim, a Drug-Receptor System. Protein Struct. Funct. Bioinform. 1988, 47, 31–47. [Google Scholar]
- Varshney, V.; Patnaik, S.S.; Roy, A.K.; Farmer, B.L. Heat transport in epoxy networks: A molecular dynamics study. Polymer 2009, 50, 3378–3385. [Google Scholar] [CrossRef]
- Cahill, D.G. Thermal conductivity measurement from 30 to 750 K: The 3ω method. Rev. Sci. Instrum. 1990, 61, 802–808. [Google Scholar] [CrossRef]
- Yusibani, E.; Woodfield, P.L.; Fujii, M.; Shinzato, K.; Zhang, X.; Takata, Y. Application of the Three-Omega Method to Measurement of Thermal Conductivity and Thermal Diffusivity of Hydrogen Gas. Int. J. Thermophys. 2009, 30, 397–415. [Google Scholar] [CrossRef]
- Oh, D.W.; Jain, A.; Eaton, J.K.; Goodson, K.E.; Lee, J.S. Thermal conductivity measurement and sedimentation detection of aluminum oxide nanofluids by using the 3ω method. Int. J. Heat Fluid Flow 2008, 29, 1456–1461. [Google Scholar] [CrossRef]
- Cahill, D.G.; Katiyar, M.; Abelson, J.R. Thermal-conductivity of α-SiH thin-films. Phys. Rev. B 1994, 50, 1456–1461. [Google Scholar] [CrossRef]
- Baysal, B.M.; Kayaman, N. Swelling of Polyacrylamide Gels in Polyacrylamide Solutions. J. Polym. Sci. B Polym. Phys. Ed. 1998, 36, 1313–1320. [Google Scholar]
- Ni, B.; Watanabe, T.; Phillpot, S.R. Thermal transport in polyethylene and at polyethylene-diamond interfaces investigated using molecular dynamics simulation. J. Phys. Condens. Matter. 2009, 21, 084219. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, G.; Desai, T.G.; Keblinski, P.; Ohara, T. Effect of crosslink formation on heat conduction in amorphous polymers. J. Appl. Phys. 2013, 114, 034302. [Google Scholar] [CrossRef]
- Shen, S.; Henry, A.; Tong, J.; Zheng, R.; Chen, G. Polyethylene nanofibres with very high thermal conductivities. Nat. Nanotechnol. 2010, 5, 251–255. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, N.; Peng, Z.; Guo, R.; An, M.; Chen, X.; Li, X.; Yang, N.; Zang, J. Thermal Transport in Soft PAAm Hydrogels. Polymers 2017, 9, 688. https://doi.org/10.3390/polym9120688
Tang N, Peng Z, Guo R, An M, Chen X, Li X, Yang N, Zang J. Thermal Transport in Soft PAAm Hydrogels. Polymers. 2017; 9(12):688. https://doi.org/10.3390/polym9120688
Chicago/Turabian StyleTang, Ni, Zhan Peng, Rulei Guo, Meng An, Xiandong Chen, Xiaobo Li, Nuo Yang, and Jianfeng Zang. 2017. "Thermal Transport in Soft PAAm Hydrogels" Polymers 9, no. 12: 688. https://doi.org/10.3390/polym9120688
APA StyleTang, N., Peng, Z., Guo, R., An, M., Chen, X., Li, X., Yang, N., & Zang, J. (2017). Thermal Transport in Soft PAAm Hydrogels. Polymers, 9(12), 688. https://doi.org/10.3390/polym9120688