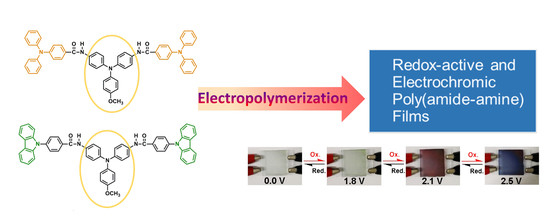
Electrosynthesis of Aromatic Poly(amide-amine) Films from Triphenylamine-Based Electroactive Compounds for Electrochromic Applications
Abstract
:1. Introduction
2. Results and Discussion
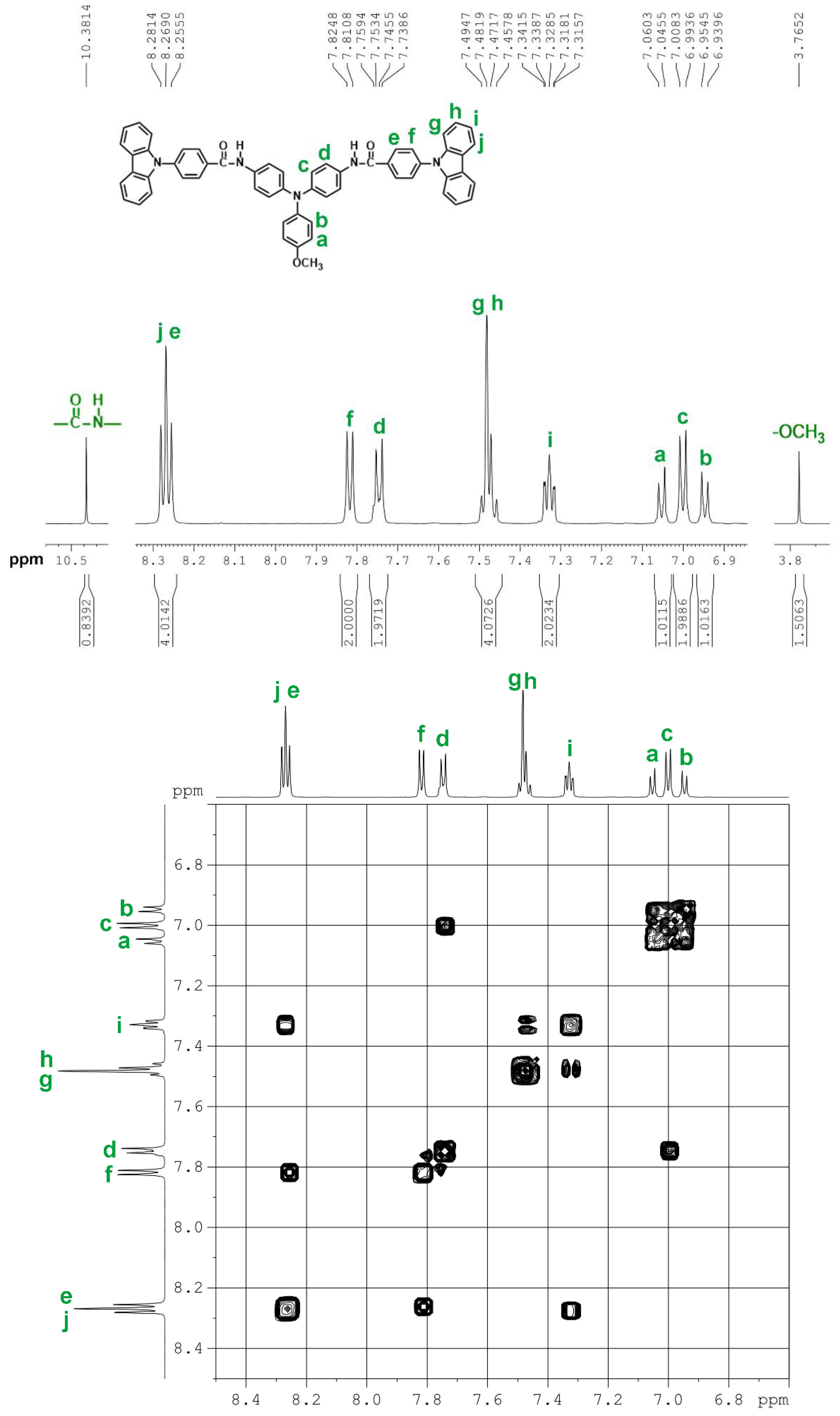
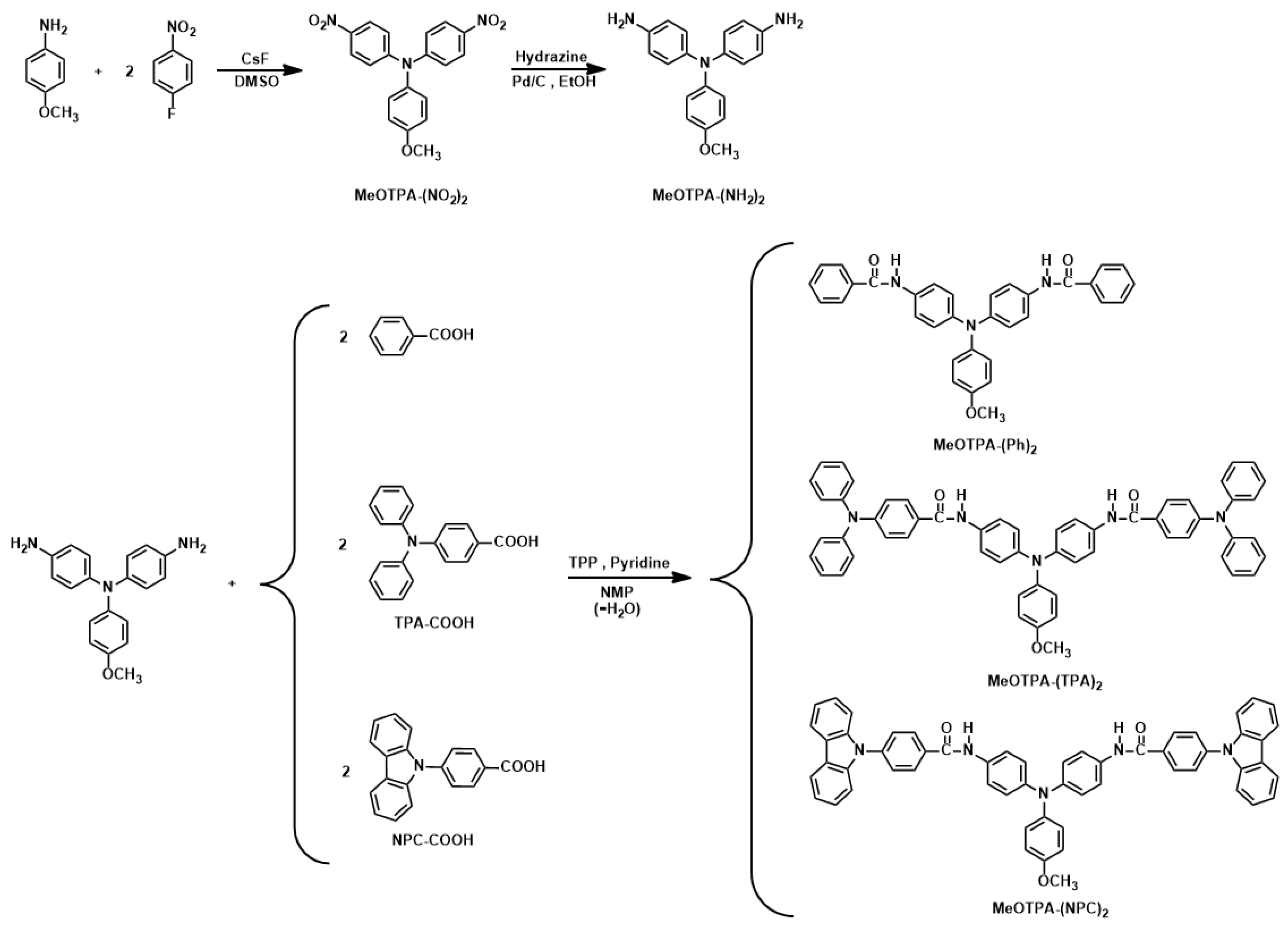
2.1. Monomer Synthesis
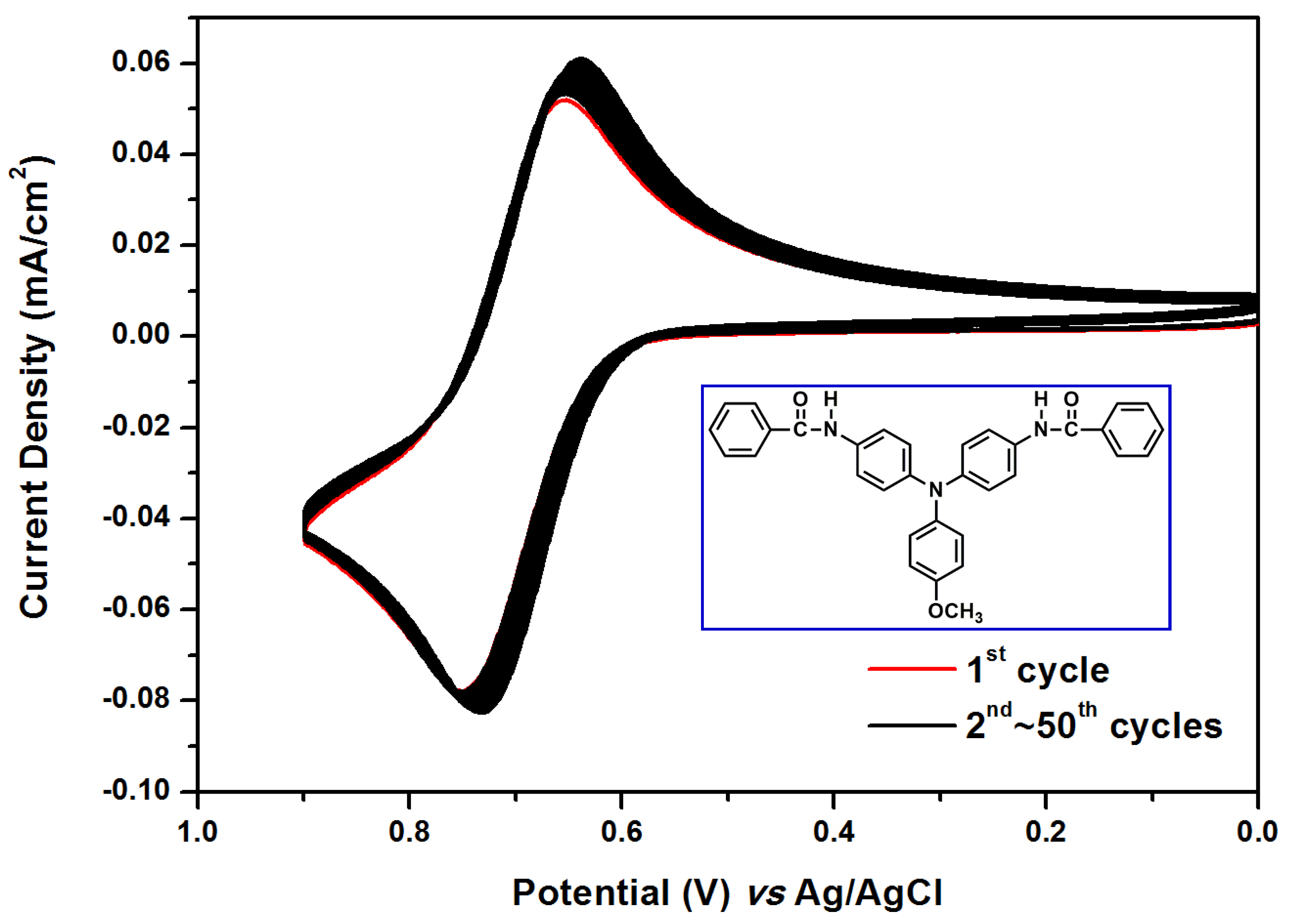
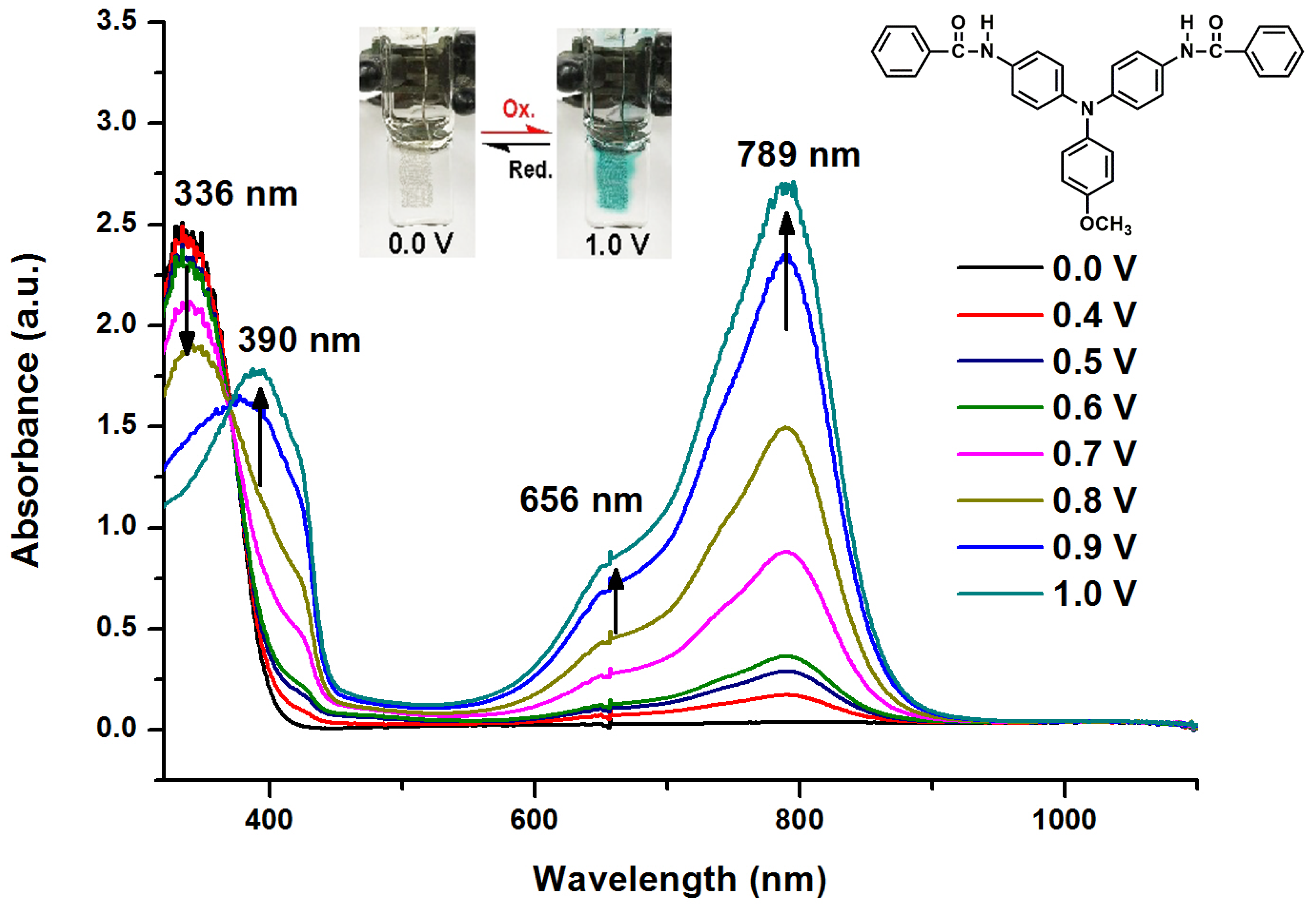
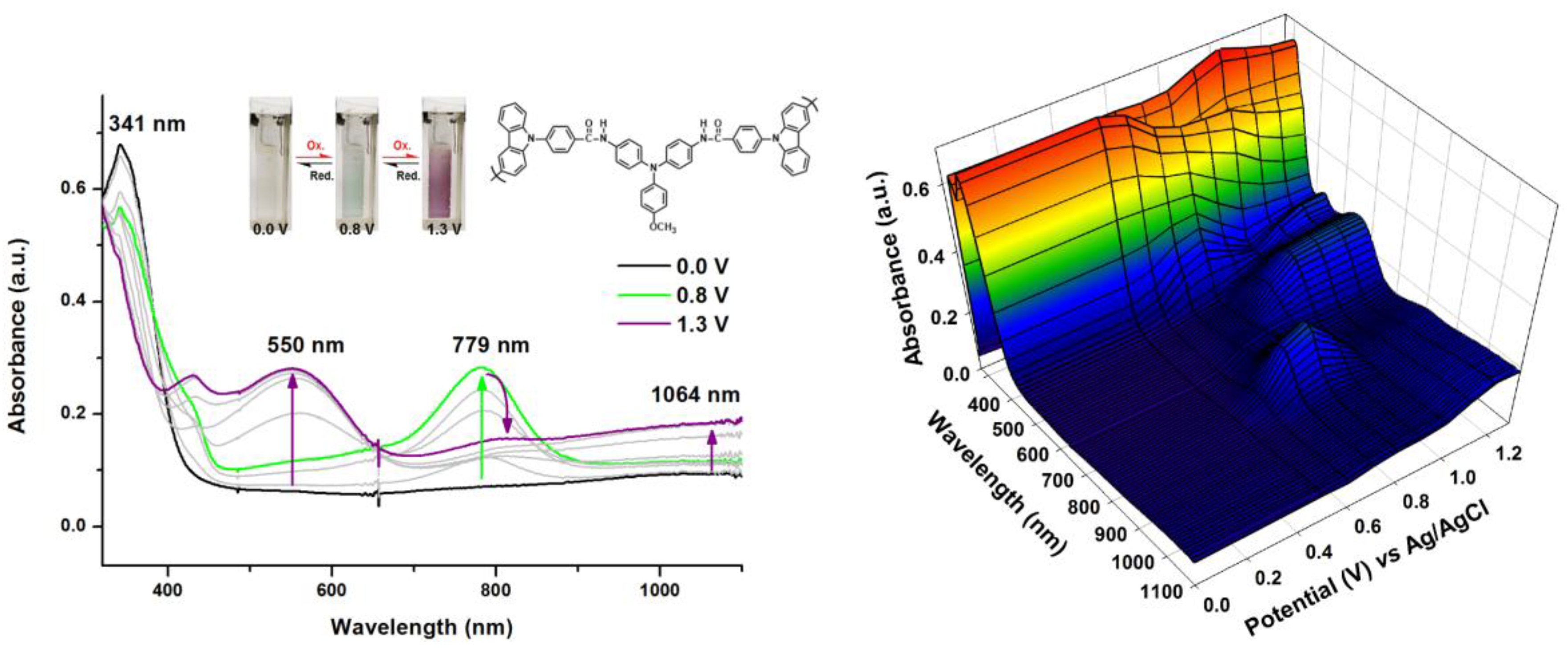
2.2. Electrochemical and Spectroelectrochemical Properties of Model Compound
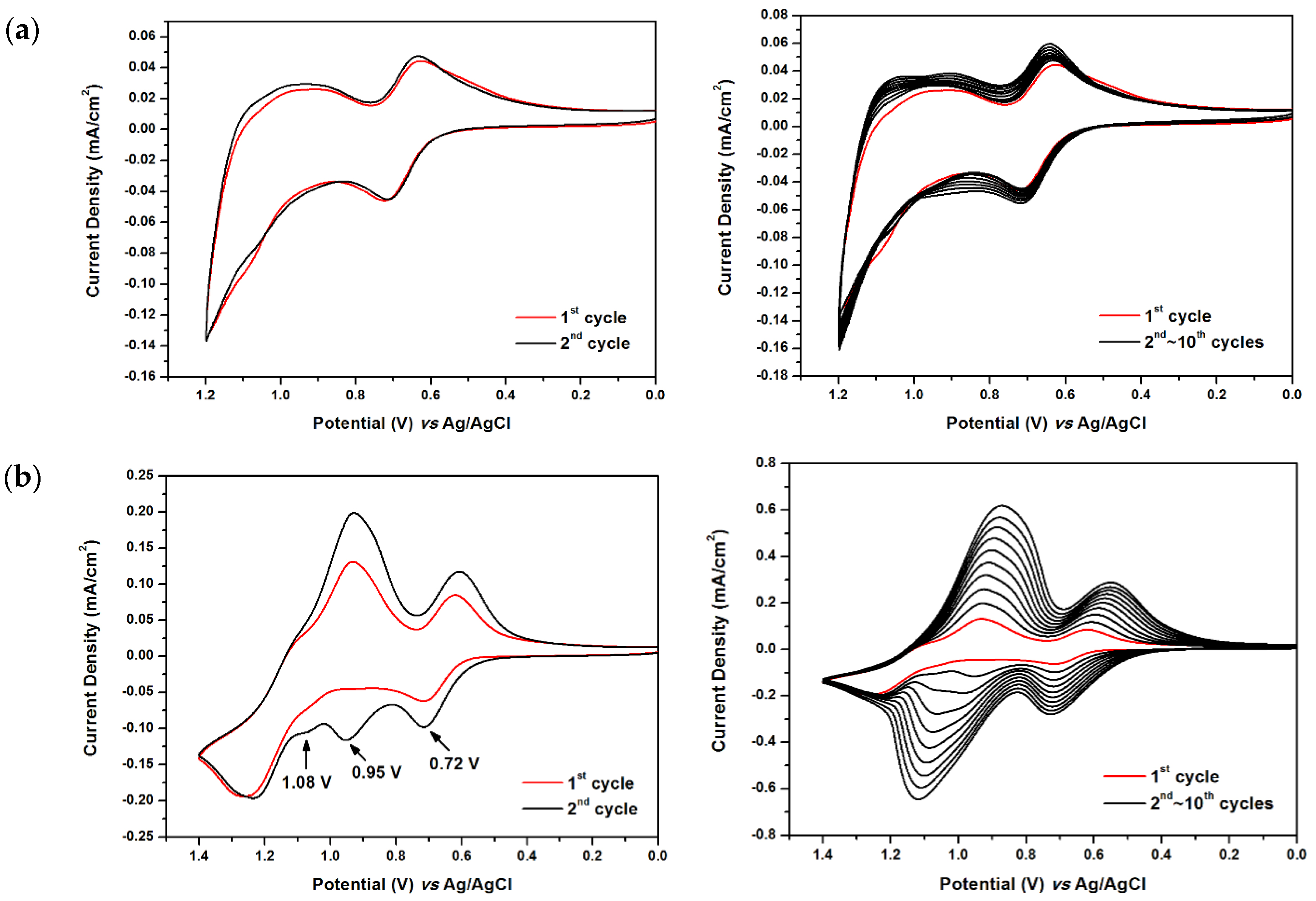
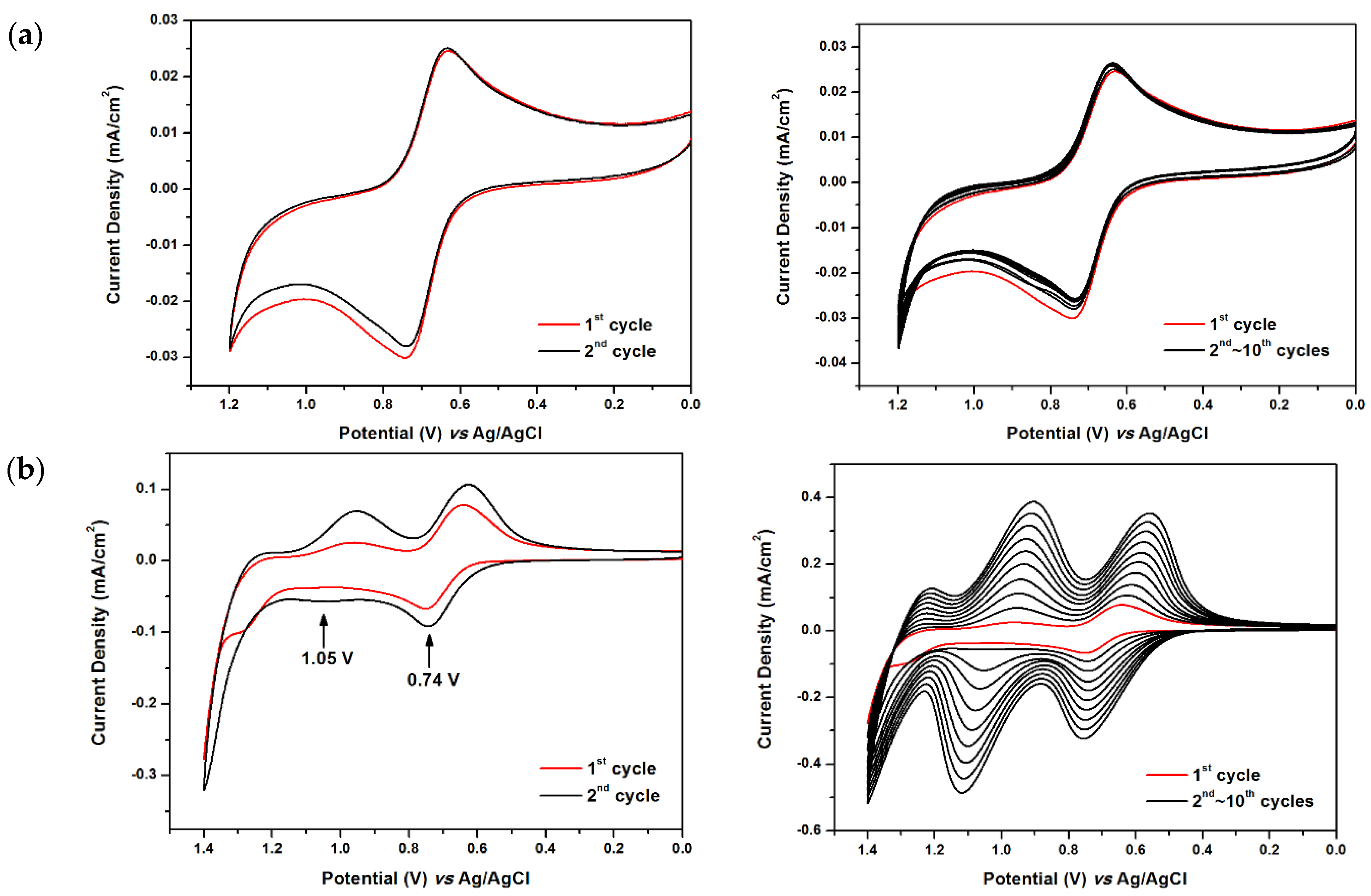
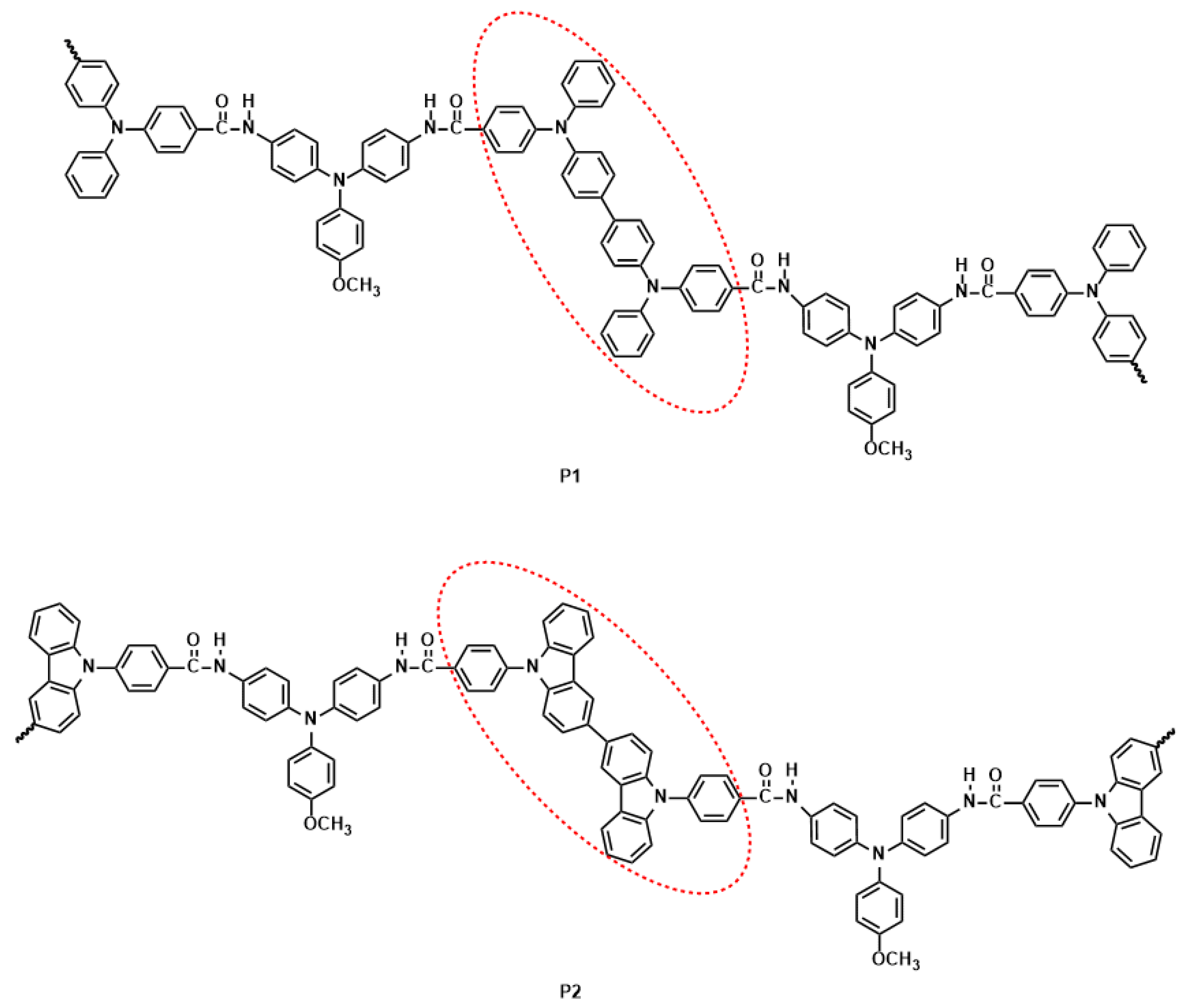
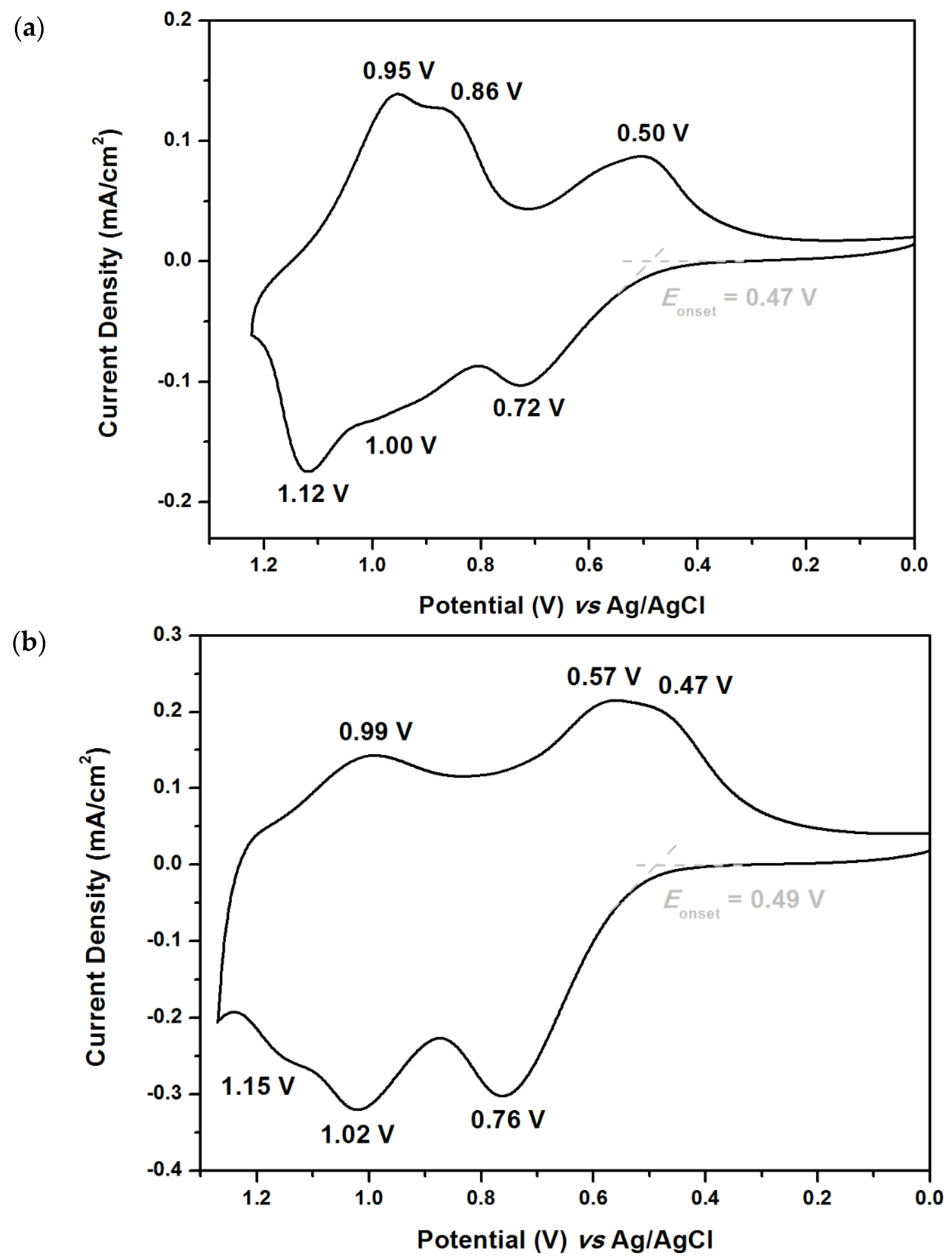
2.3. Electrochemical Polymerization of Monomers
2.4. Optical and Electrochemical Properties of Polymers
2.5. Spectroelectrochemical Properties of Polymers
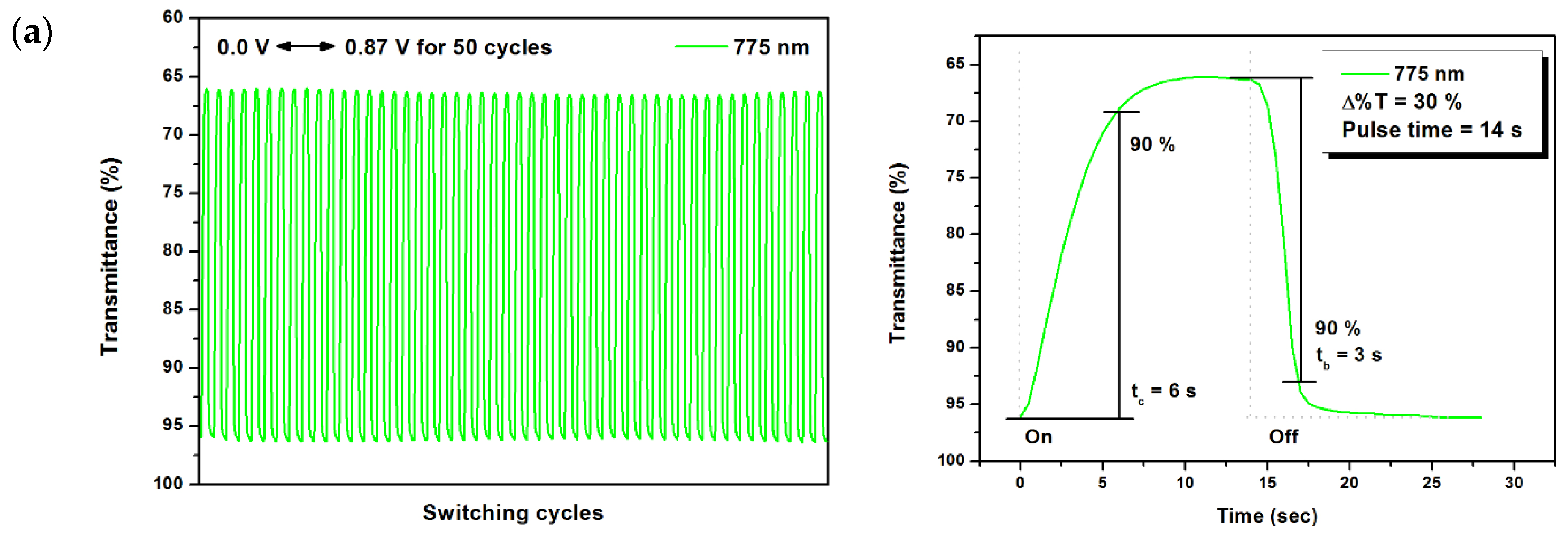
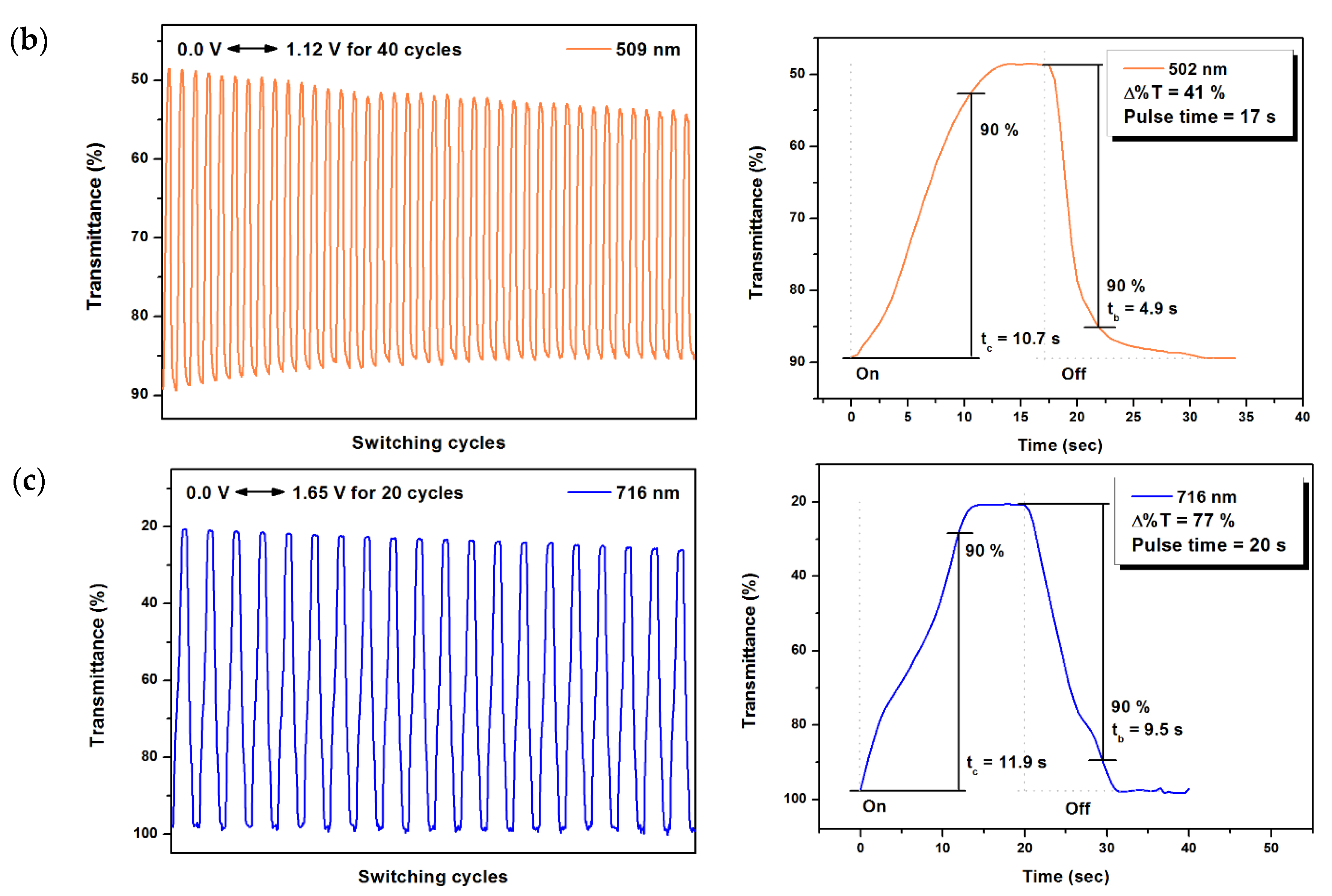
2.6. Electrochromic Switching
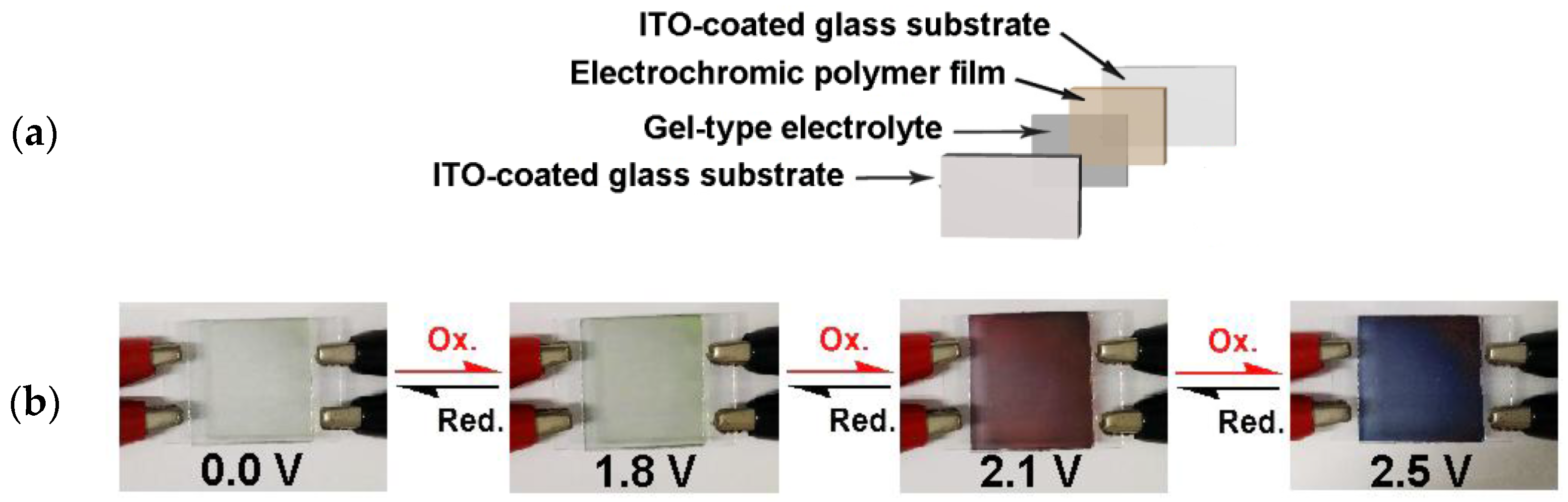
2.7. Electrochromic Devices
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Monk, P.M.S.; Mortimer, R.J.; Rosseinsky, D.R. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Kobayashi, N.; Miura, S.; Nishimura, M.; Urano, H. Organic electrochromism for a new color electronic paper. Sol. Energy Mater. Sol. Cells 2008, 92, 136–139. [Google Scholar] [CrossRef]
- Tehrani, P.; Hennerdal, L.-O.; Dyer, A.L.; Reynolds, J.R.; Berrgren, M. Improving the contrast of all-printed electrochromic polymer on paper displays. J. Mater. Chem. 2009, 19, 1799–1802. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in build ings: A state-of-the-art review. Sol. Energy Mater. Sol. Cells 2010, 94, 87–105. [Google Scholar] [CrossRef]
- Beaupre, S.; Breton, A.-C.; Dumas, J.; Leclerc, M. Multicolored electrochromic cells base on poly(2,7-carbazole) derivatives for adaptive camouflage. Chem. Mater. 2009, 21, 1504–1513. [Google Scholar] [CrossRef]
- Yu, H.; Shao, S.; Yan, L.; Meng, H.; He, Y.; Yao, C.; Xu, P.; Zhang, X.; Hu, W.; Huang, W. Side-chain engineering of green color electrochromic polymer materials: Toward adaptive camouflage application. J. Mater. Chem. C 2016, 4, 2269–2273. [Google Scholar] [CrossRef]
- Ma, C.; Taya, M.; Xu, C. Smart sunglasses based on electrochromic polymers. Polym. Eng. Sci. 2008, 48, 2224–2228. [Google Scholar] [CrossRef]
- Österholm, A.M.; Shen, D.E.; Kerszulis, J.A.; Bulloch, R.H.; Kuepfert, M.; Dyer, A.L.; Reynolds, J.R. Four shades of brown: Tuning of electrochromic polymer blends toward high-contrast eyewear. ACS Appl. Mater. Interfaces 2015, 7, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Sun, P.; Mai, W. Electrochromic energy storage devices. Mater. Today 2016, 19, 394–402. [Google Scholar] [CrossRef]
- Tong, Z.; Tian, Y.; Zhang, H.; Li, X.; Ji, J.; Qu, H.; Li, N.; Zhao, J.; Li, Y. Recent advances in multifunctional electrochromic energy storage devices and photoelectrochromic devices. Sci. China Chem. 2017, 60, 13–37. [Google Scholar] [CrossRef]
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic systems and the prospects for devices. Adv. Mater. 2001, 13, 783–793. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic organic and polymeric materials for display applications. Displays 2006, 27, 2–18. [Google Scholar] [CrossRef]
- Higuchi, M. Electrochromic organic-metallic hybrid polymers: Fundamentals and device applications. Polym. J. 2009, 41, 511–520. [Google Scholar] [CrossRef]
- Gillapie, D.T.; Tenent, R.C.; Dillon, A.C. Metal-oxide films for electrochromic applications: Present technology and future directions. J. Mater. Chem. 2010, 20, 9585–9592. [Google Scholar] [CrossRef]
- Mortimer, R.J. Electrochromic materials. Annu. Rev. Mater. Res. 2011, 41, 241–268. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Gunbas, G.; Toppare, L. Electrochromic conjugated polyheterocycles and derivatives—Highlights from the last decade towards realization of long lived aspirations. Chem. Commun. 2012, 48, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Beverina, L.; Pagani, G.A.; Sassi, M. Multichromophoric electrochromic polymers: Color tuning of conjugated polymers through the side chain functionalization approach. Chem. Commun. 2014, 50, 5413–5430. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Hӧsel, M.; Dyer, A.L.; Krebs, F.C. Development and manufacture of polymer-based electrochromic devices. Adv. Funct. Mater. 2015, 25, 2073–2090. [Google Scholar] [CrossRef]
- Neo, W.T.; Ye, Q.; Chua, S.-J.; Xu, J. Conjugated polymer-based electrochromics: Materials, device fabrication and application prospects. J. Mater. Chem. C 2016, 4, 7364–7376. [Google Scholar] [CrossRef]
- Thelakkat, M. Star-shaped, dendrimeric and polymeric triarylamines as photoconductors and hole transport materials for electro-optical applications. Macromol. Mater. Eng. 2002, 287, 442–461. [Google Scholar] [CrossRef]
- Shirota, Y.; Kageyama, H. Charge carrier transporting molecular materials and their applications in devices. Chem. Rev. 2007, 107, 953–1010. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Tian, H. Triarylamine: A promising core unit for efficient photovoltaic materials. Chem. Commun. 2009, 5483–5495. [Google Scholar] [CrossRef] [PubMed]
- Iwan, A.; Sek, D. Polymers with triphenylamine units: Photonic and electroactive materials. Prog. Polym. Sci. 2011, 36, 1277–1325. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-H.; Hsiao, S.-H.; Su, T.-H.; Liou, G.-S. Novel aromatic poly(amine-imide)s bearing a pendent triphenylamine group: Synthesis, thermal, photophysical, electrochemical, and electrochromic characteristics. Macromolecules 2005, 38, 307–316. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liou, G.-S.; Kung, Y.-C.; Yen, H.-J. High contrast ratio and rapid switching electrochromic polymeric films based on 4-(dimethylamino)triphenylamine-functionalized aromatic polyamides. Macromolecules 2008, 41, 2800–2808. [Google Scholar] [CrossRef]
- Kung, Y.-C.; Hsiao, S.-H. Fluorescent and electrochromic polyamides with pyrenylamine chromophore. J. Mater. Chem. 2010, 20, 5481–5492. [Google Scholar] [CrossRef]
- Kung, Y.-C.; Hsiao, S.-H. Solution-processable, high-Tg, ambipolar polyimide electrochromics bearing pyrenylamine units. J. Mater. Chem. 2011, 21, 1746–1754. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Solution-processable triarylamine-based electroactive high performance polymers for anodically electrochromic applications. Polym. Chem. 2012, 3, 255–264. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Ambipolar, multi-electrochromic polypyromellitimides and polynaphthalimides containing di(tert-butyl)-substituted bis(triarylamine) units. J. Mater. Chem. C 2014, 2, 1553–1564. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Cheng, S. New electroactive and electrochromic aromatic polyamides with ether-linked bis(triphenylamine) units. J. Polym. Sci. A 2015, 53, 496–510. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Hsiao, Y.-H.; Kung, Y.-R.; Leu, C.-M.; Lee, T.-M. Triphenylamine-based redox-active aramids with 1-piperidinyl substituent as an auxiliary donor: Enhanced electrochemical stability and electrochromic performance. React. Funct. Polym. 2016, 108, 54–62. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Han, J.-S. Solution-processable transmissive-to-green switching electrochromic polyamides bearing 2,7-bis(diphenylamino)naphthalene units. J. Polym. Sci. A 2017, 55, 1409–1421. [Google Scholar] [CrossRef]
- Chang, C.-W.; Liou, G.-S.; Hsiao, S.-H. Highly stable anodic green electrochromic aromatic polyamides: Synthesis and electrochromic properties. J. Mater. Chem. 2007, 17, 1007–1015. [Google Scholar] [CrossRef]
- Seo, E.T.; Nelson, R.F.; Fritsch, J.M.; Marcoux, L.S.; Leedy, D.W.; Adams, R.N. Anodic oxidation pathways of aromatic amines. Electrochemical and electron paramagnetic resonance studies. J. Am. Chem. Soc. 1966, 88, 3498–3503. [Google Scholar] [CrossRef]
- Nelson, R.F.; Adams, R.N. Anodic oxidation pathways of substituted triphenylamines. II. Quantitative studies of benzidine formation. J. Am. Chem. Soc. 1968, 90, 3925–3930. [Google Scholar] [CrossRef]
- Leung, M.-K.; Chou, M.-Y.; Su, Y.-O.; Chiang, C.-L.; Chen, H.-L.; Yang, C.-F.; Yang, C.-C.; Lin, C.-C.; Chen, H.-T. Diphenylamino group as an effective handle to conjugated donor-acceptor polymers through electropolymerization. Org. Lett. 2003, 5, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Natera, J.; Otero, L.; Sereno, L.; Fungo, F.; Wang, N.S.; Tsai, Y.-M.; Hwu, T.-Y.; Wong, K.-T. A novel electrochromic polymer synthesized through electropolymerization of a new donor-acceptor bipolar system. Macromolecules 2007, 40, 4456–4463. [Google Scholar] [CrossRef]
- Chiang, C.-C.; Chen, H.-C.; Lee, C.-S.; Leung, M.-K.; Lin, K.-R.; Hsieh, K.-H. Electrochemical deposition of bis(N,N′-diphenylaminoaryl) substituted ferrocenes, and their application as a hole-injection layer on polymeric light-emitting diodes. Chem. Mater. 2008, 20, 540–552. [Google Scholar] [CrossRef]
- Natera, J.; Otero, L.; D’Eramo, F.; Sereno, L.; Fungo, F.; Wang, N.-S.; Tsai, Y.-M.; Wong, K.-T. Synthesis and properties of a novel cross-linked electroactive polymer formed from a bipolar starburst monomer. Macromolecules 2009, 42, 626–635. [Google Scholar] [CrossRef]
- Mangione, M.I.; Spanevello, R.A.; Rumbero, A.; Heredia, D.; Marzari, G.; Fernandez, L.; Otero, L.; Fungo, F. Electrogenerated conductive polymers from triphenylamine end-capped dendrimers. Macromolecules 2013, 46, 4754–4763. [Google Scholar] [CrossRef]
- Ma, H.; Li, F.; Li, P.; Wang, H.; Zhang, M.; Zhang, G.; Baumgarten, M.; Mullen, K. A dendrimer-based electropolymerized microporus film: Multifunctional, reversible, and highly sensitive fluorescent probe. Adv. Funct. Mater. 2016, 26, 2025–2031. [Google Scholar] [CrossRef]
- Ambrose, J.F.; Nelson, R.F. Anodic oxidation pathways of carbazoles I. Carbazole and N-substituted derivatives. J. Electrochem. Soc. 1968, 115, 1159–1164. [Google Scholar] [CrossRef]
- Ambrose, J.F.; Carpenter, L.L.; Nelson, R.F. Electrochemical and spectroscopic properties of cation radicals III. Reaction pathways of carbazolium radical ions. J. Electrochem. Soc. 1975, 122, 876–894. [Google Scholar] [CrossRef]
- Morin, J.F.; Leclerc, M.; Ades, D.; Siove, A. Polycarbazoles: 25 years of progress. Macromol. Rapid Commun. 2005, 26, 761–778. [Google Scholar] [CrossRef]
- Koyuncu, S.; Gultekin, B.; Zafer, C.; Bilgili, H.; Can, M.; Demic, S.; Kaya, I.; Icli, S. Electrochemical and optical properties of biphenyl bridged-dicarbazole oligomer films: Electropolymerization and electrochromism. Electrochim. Acta 2009, 54, 5694–5702. [Google Scholar] [CrossRef]
- Usluer, O.; Koyuncu, S.; Demic, S.; Janssen, R.A. A novel high-contrast ration electrochromic material from spiro (cyclododecane-1,9′-fluorene) bicarbazole. J. Polym. Sci. B 2011, 49, 333–341. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Hsueh, J.-C. Electrochemical synthesis and electrochromic properties of new conjugated polycarbazoles from di(carbazol-9-yl)-substituted triphenylamine and N-phenylcarbazole derivatives. J. Electroana. Chem. 2015, 758, 100–110. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lin, S. The electrochemical fabrication of electroactive polymer films from diamide- or diimide-cored N-phenylcarbazole dendrons for electrochromic applications. J. Mater. Chem. C 2016, 4, 1271–1280. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wu, L.-C. Fluorescent and electrochromic polymers from 2,8-di(carbazol-9-yl)dibenzothiophene and its S,S-dioxide derivative. Dyes Pigments 2016, 134, 51–63. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liao, Y.-C. Facile synthesis of electroactive and electrochromic triptycene poly(ether-imide)s containing triarylamine units via oxidative electro-coupling. Polymers 2017, 9, 497. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Scherf, U. Electrochemically generated thin films of microporous polymer networks: Synthesis, properties, and applications. Macromol. Chem. Phys. 2016, 217, 827–841. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chiu, Y.-T. Electrosynthesis and electrochromic properties of poly(amide-triarylamine)s containing triptycene units. RSC Adv. 2015, 5, 90941–90951. [Google Scholar] [CrossRef]




| Polymers | UV-Vis Absorption (nm) a | Oxidation Potential (V) b | Eg (eV) c | HOMO (eV) d | LUMO (eV) d | ||||
|---|---|---|---|---|---|---|---|---|---|
| λmax | λonset | Eonset | E1/2Ox1 | E1/2Ox2 | E1/2Ox3 | ||||
| P1 | 361 | 454 | 0.47 | 0.61 | 0.93 | 1.04 | 2.73 | 4.83 | 2.10 |
| P2 | 338 | 449 | 0.49 | 0.62 | 0.80 | 1.07 | 2.76 | 4.85 | 2.09 |
| Polymers | λmax a (nm) | Δ% T | Response Time b | ΔOD c | Qd d (mC/cm2) | CE e (cm2/C) | |
|---|---|---|---|---|---|---|---|
| tc (s) | tb (s) | ||||||
| P1 | 775 | 30 | 6.0 | 3.0 | 0.16 | 2.21 | 73 |
| 502 | 41 | 10.7 | 4.9 | 0.26 | 4.56 | 57 | |
| 716 | 77 | 11.9 | 9.5 | 0.68 | 10.39 | 65 | |
| P2 | 779 | 36 | 6.0 | 5.8 | 0.38 | 3.09 | 123 |
| 550 | 33 | 7.1 | 6.4 | 0.18 | 4.25 | 42 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, S.-H.; Lu, H.-Y. Electrosynthesis of Aromatic Poly(amide-amine) Films from Triphenylamine-Based Electroactive Compounds for Electrochromic Applications. Polymers 2017, 9, 708. https://doi.org/10.3390/polym9120708
Hsiao S-H, Lu H-Y. Electrosynthesis of Aromatic Poly(amide-amine) Films from Triphenylamine-Based Electroactive Compounds for Electrochromic Applications. Polymers. 2017; 9(12):708. https://doi.org/10.3390/polym9120708
Chicago/Turabian StyleHsiao, Sheng-Huei, and Hsing-Yi Lu. 2017. "Electrosynthesis of Aromatic Poly(amide-amine) Films from Triphenylamine-Based Electroactive Compounds for Electrochromic Applications" Polymers 9, no. 12: 708. https://doi.org/10.3390/polym9120708