Bio-Inspired Polymeric Structures with Special Wettability and Their Applications: An Overview
Abstract
:1. Introduction
2. Theoretical Background
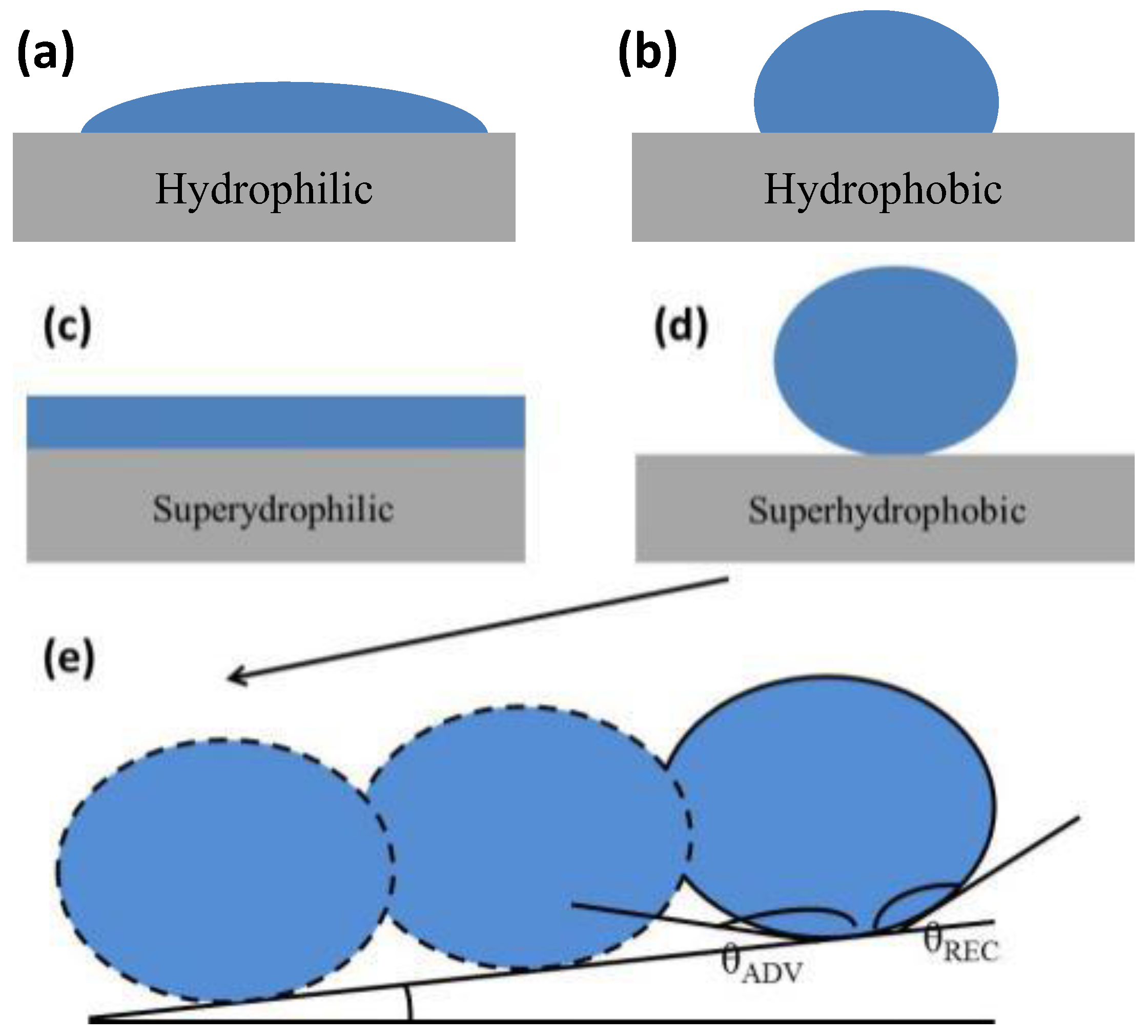
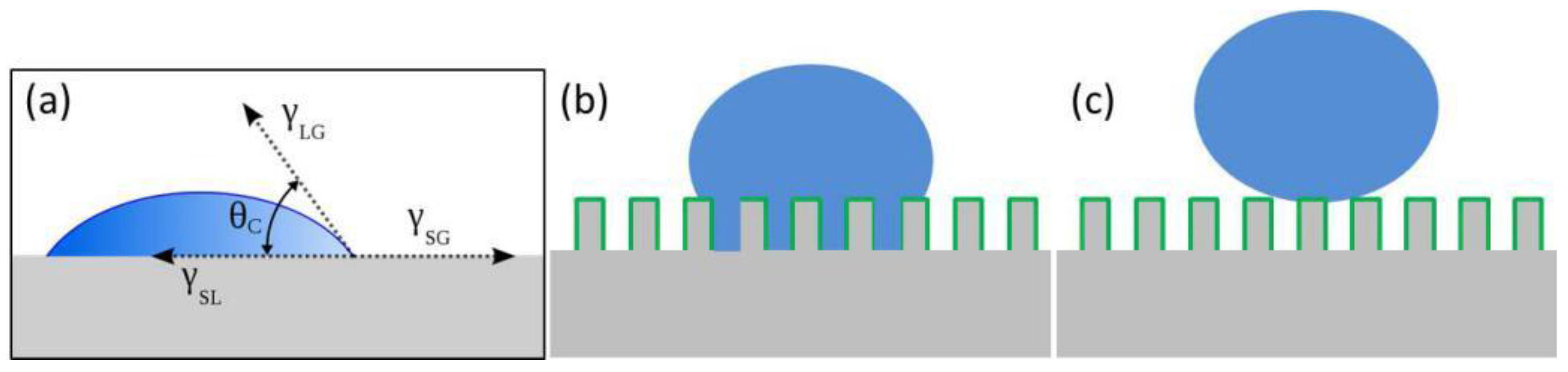
2.1. Young’s Equation
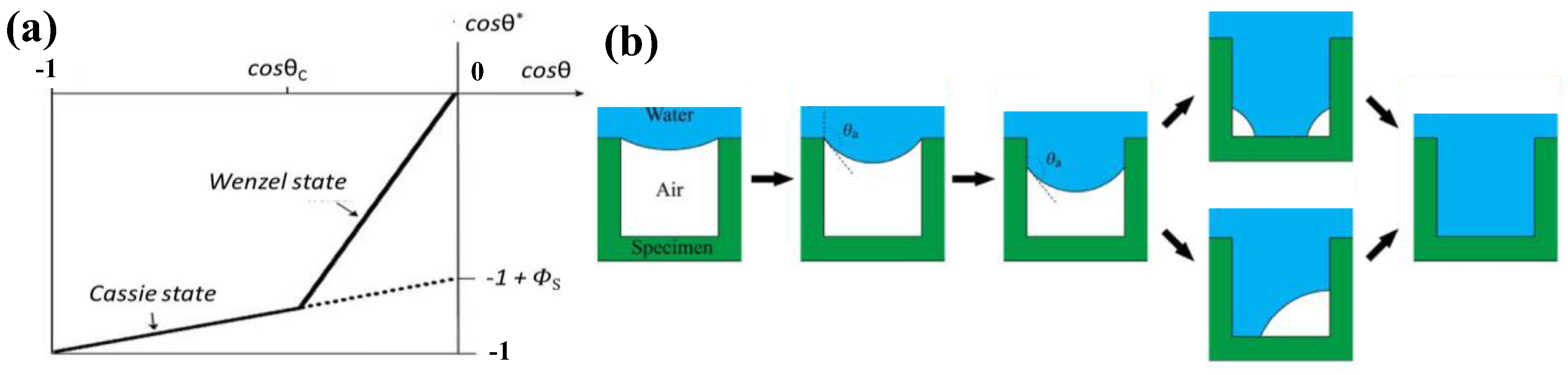
2.2. The Wenzel Model
2.3. The Cassie–Baxter Model
2.4. Wetting Transition
2.5. Superoleophobic Surface Design
3. Creatures with Special Wettability in Nature
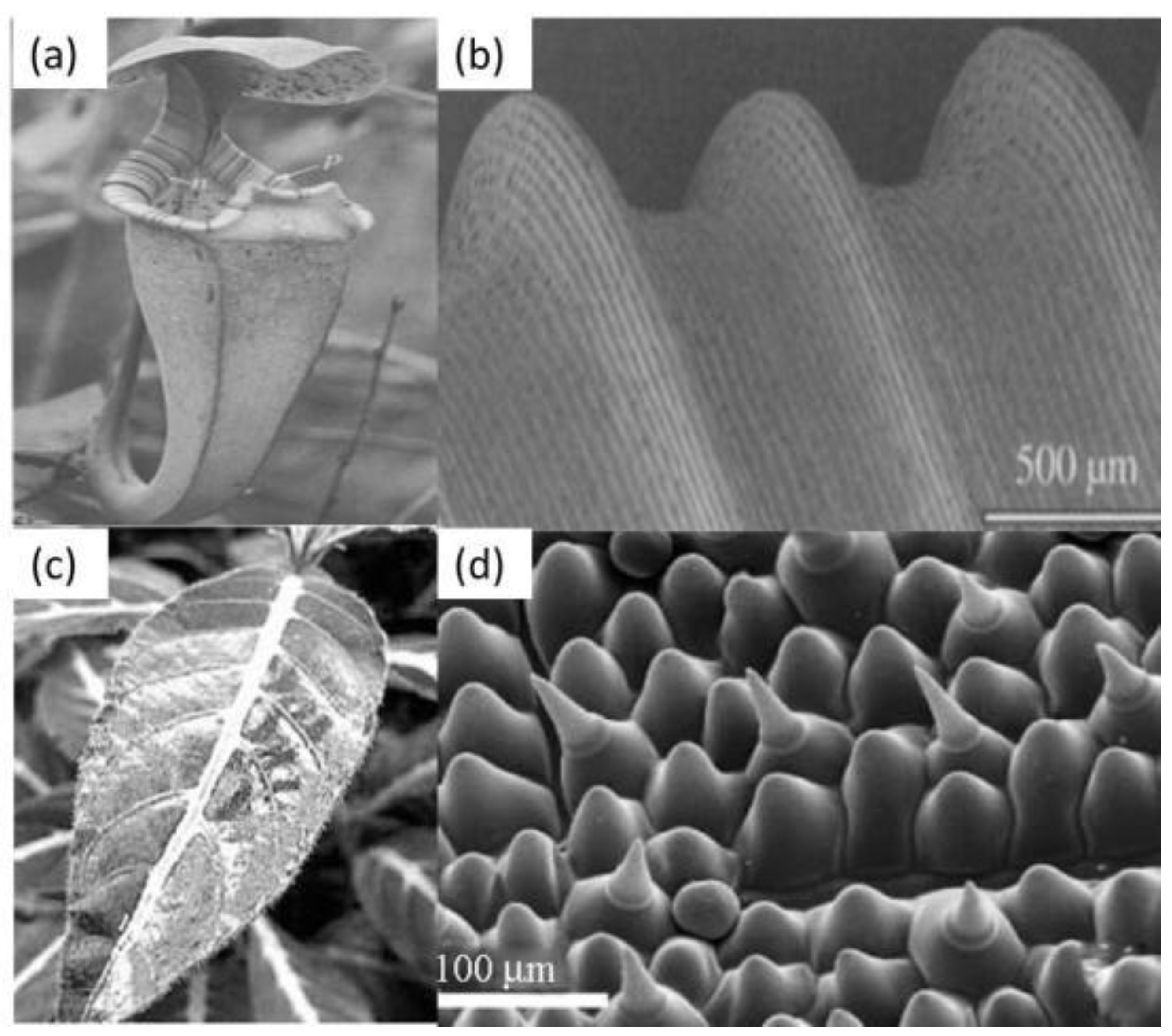
3.1. Superhydrophilic Structures
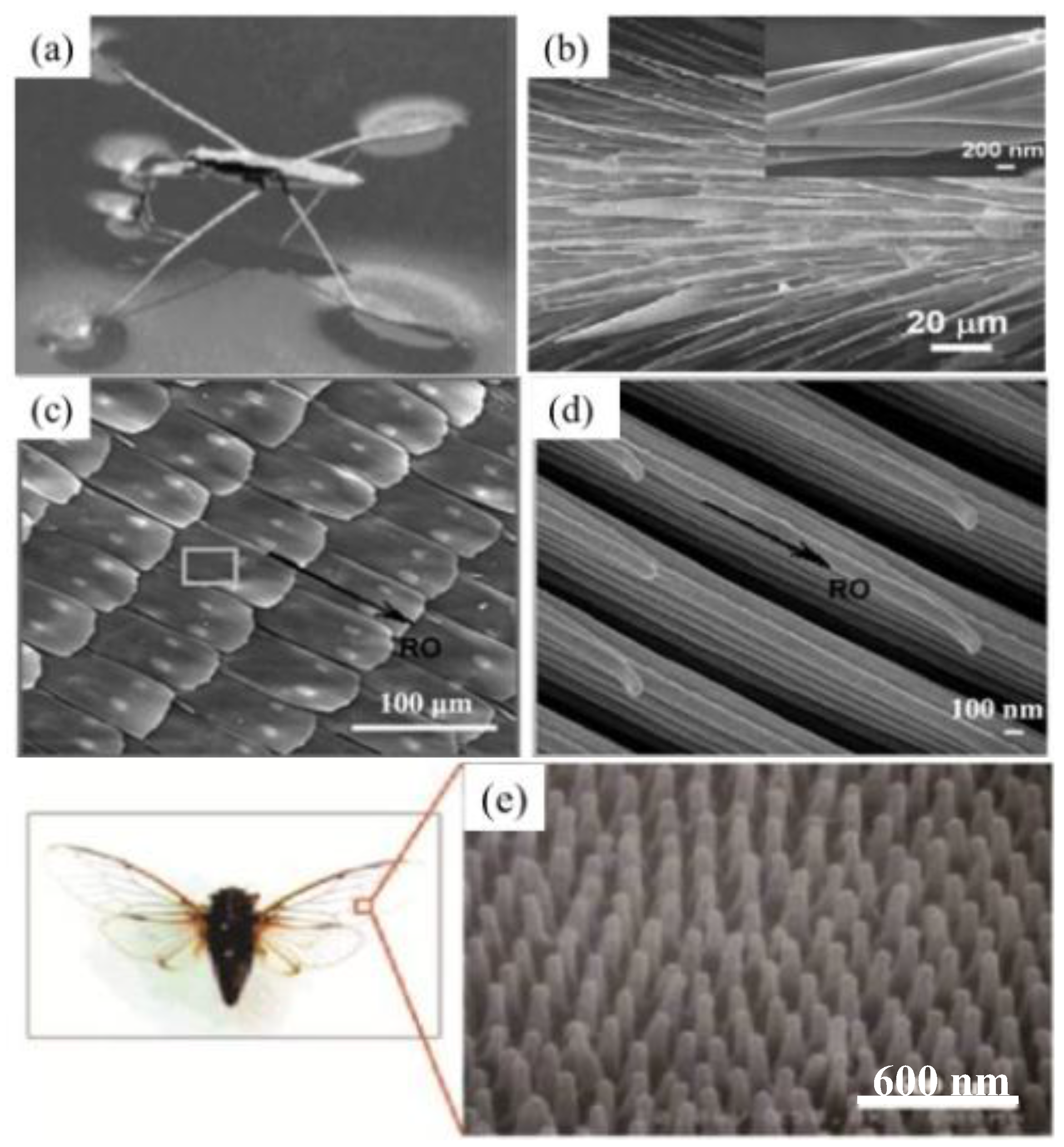
3.2. Natural Superhydrophobic Structures
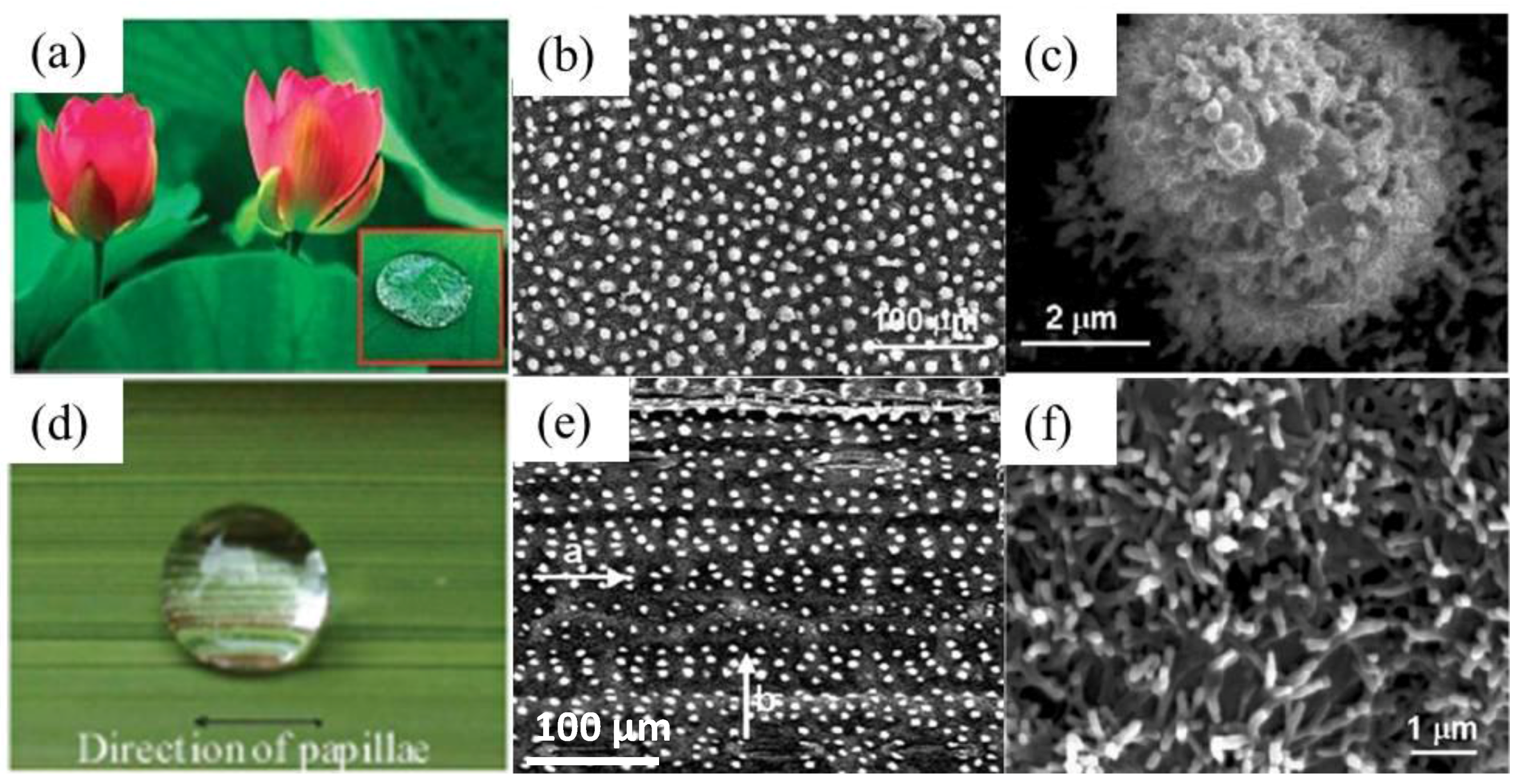
3.2.1. Natural Structures with the “Lotus Effect”
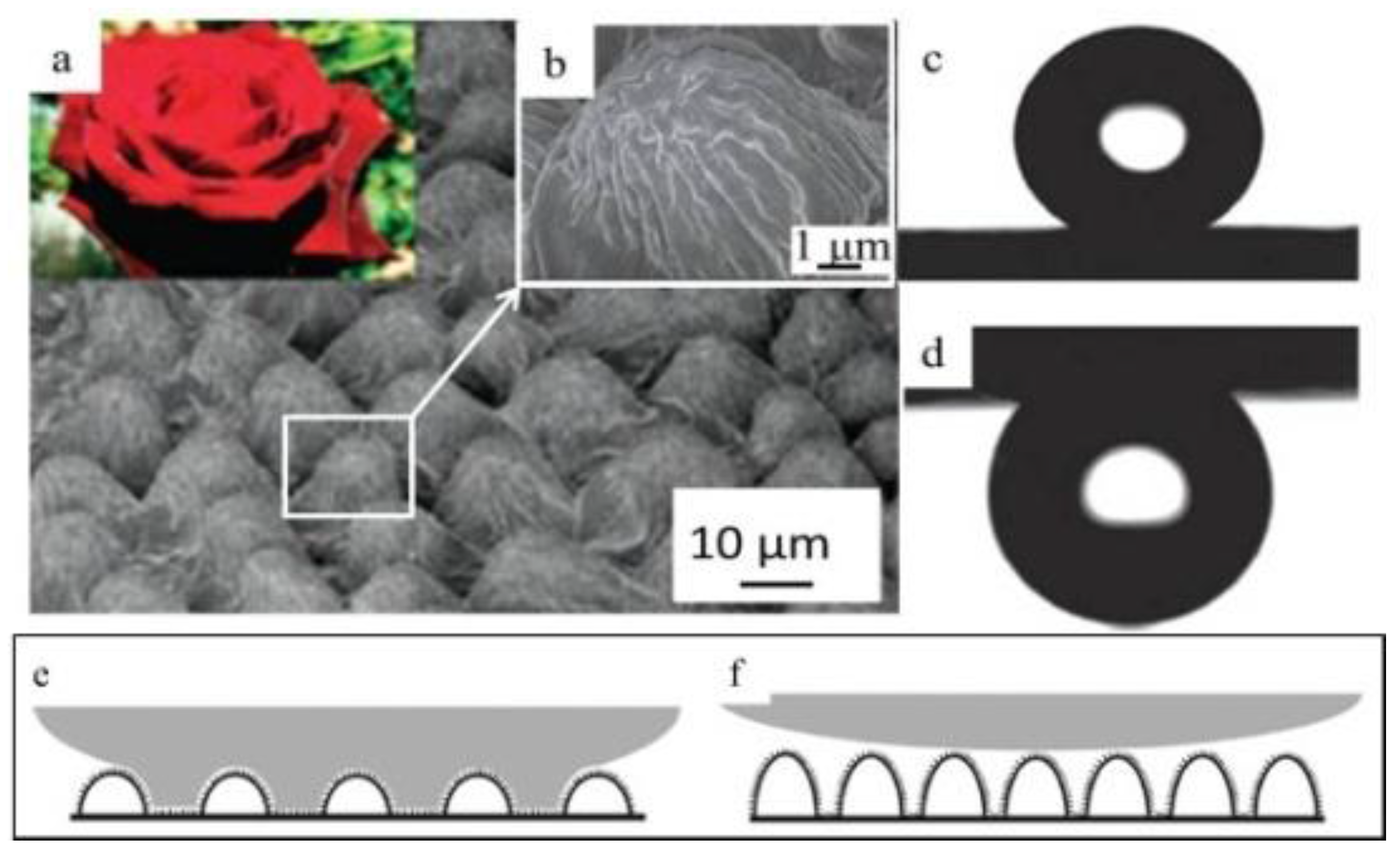
3.2.2. “Petal Effect”
4. Bio-Inspired Superhydrophobic, Superhydrophilic, and Superoleophobic Structures and Their Applications
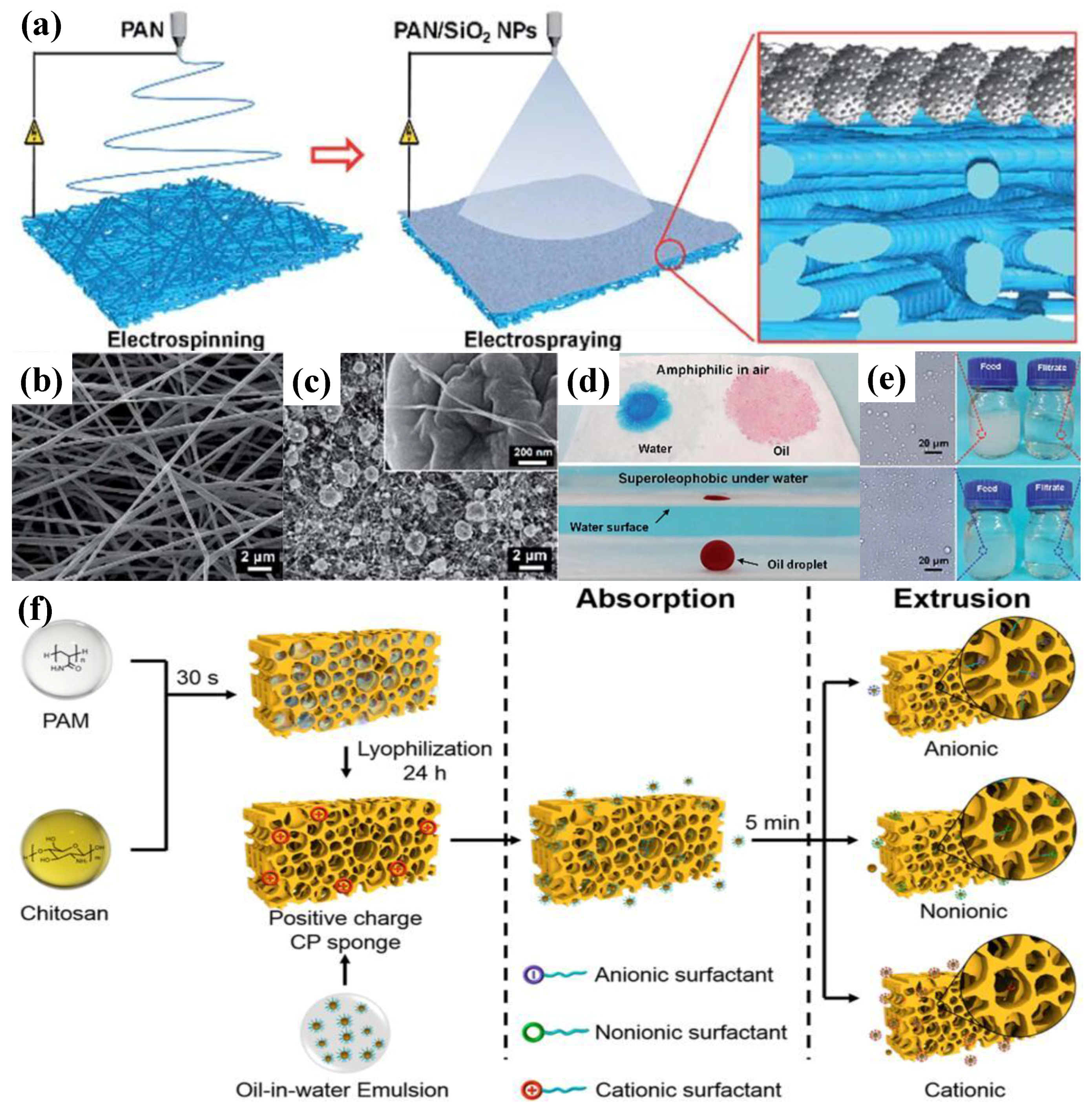
4.1. Bio-Inspired Superhydrophilic Structures and Their Applications
4.2. Bio-Inspired Superhydrophobic Structures and Their Applications
4.3. Bio-Inspired Superoleophobic Structures and Their Applications
Bio-Inspired Superoleophobic Structures
5. Conclusions and Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bonn, D.; Eggers, J.; Indekeu, J.; Meunier, J.; Rolley, E. Wetting and spreading. Rev. Mod. Phys. 2009, 81, 739–805. [Google Scholar] [CrossRef]
- Wenzel, R.D.M. Resistance of solid surfaces. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
- Chen, D.; Tan, L.; Liu, H.; Tang, F.; Hu, J.; Li, Y. Fabrication of fast-absorbing and quick-drying wool fabrics with good washing durability. ChemSusChem 2010, 3, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.X.H.; Yu, H. Preparation of transparent superhydrophobic glass slides: Demonstration of surface chemistry characteristics. J. Chem. Educ. 2013, 90, 1203–1206. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; He, Y.; Xu, B.; Wei, S.; Xiao, F. Solvothermal synthesis of nanoporous polymer chalk for painting superhydrophobic surfaces. Langmuir 2011, 27, 12585–12590. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Batra, A.; Rego, J.M.; Macosko, C.W. Progress in organic coatings polyethylene/polyurethane blends for improved paint adhesion. Prog. Org. Coat. 2011, 72, 492–497. [Google Scholar] [CrossRef]
- Chem, J.M. Switchable wettability of vertical Si nanowire array surface by simple contact-printing of siloxane oligomers and chemical washing. J. Mater. Chem. 2012, 22, 10625–10630. [Google Scholar]
- Park, H.Y.; Kang, B.J.; Lee, D.; Oh, J.H. Control of surface wettability for inkjet printing by combining hydrophobic coating and plasma treatment. Thin Solid Films 2013, 546, 162–166. [Google Scholar] [CrossRef]
- Tian, D.; Song, Y.; Jiang, L. Patterning of controllable surface wettability for printing techniques. Chem. Soc. Rev. 2013, 42, 5184–5209. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Alcaraz, M.L.; Rubner, M.F.; Cohen, R.E. Zwitter-wettability and antifogging coatings with frost-resisting capabilities. ACS Nano 2013, 3, 2172–2185. [Google Scholar] [CrossRef] [PubMed]
- Raut, H.K.; Dinachali, S.S.; Loke, Y.C.; Ganesan, R.; Ansah-Antwi, K.K.; Góra, A.; Khoo, E.H.; Ganesh, V.A.; Saifullash, M.S.M.; Remakrishna, S. Multiscale ommatidial arrays with broadband and omnidirectional antire flection and antifogging properties by sacrificial layer mediated nanoimprinting. ACS Nano 2015, 9, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Introzzi, L.; Mar, J.; Cozzolino, C.A.; Trabattoni, S.; Tavazzi, S.; Biamchi, C.L.; Schiraldi, A.; Piergiovanni, L.; Farris, F. “Wetting enhancer” pullulan coating for antifog packaging applications. ACS Appl. Mater. Interfaces 2012, 4, 3692–3700. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, W.; Liu, Y.; Hu, H.; Pei, X. The effect of wetting property on anti-fouling/foul-release performance under quasi-static/hydrodynamic conditions. Prog. Org. Coat. 2016, 95, 64–71. [Google Scholar] [CrossRef]
- Wouters, M.; Rentrop, C.; Willemsen, P. Coatings surface structuring and coating performance novel biocidefree nanocomposite coatings with anti-fouling and fouling-release properties. Prog. Org. Coat. 2010, 68, 4–11. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Li, B. Excellent wetting resistance and anti-fouling performance of PVDF membrane modified with superhydrophobic papillae-like surfaces. J. Membr. Sci. 2017, 540, 401–410. [Google Scholar] [CrossRef]
- Lu, X.; Peng, Y.; Ge, L.; Lin, R.; Zhu, Z.; Liu, S. Amphiphobic PVDF composite membranes for anti-fouling direct contact membrane distillation. J. Membr. Sci. 2016, 505, 61–69. [Google Scholar] [CrossRef]
- Smith, M.K.; Singh, V.; Kalaitzidou, K.; Cola, B.A.; Al, S.E.T. Array surfaces with tunable wetting and contact thermal energy transport. ACS Nano 2015, 2, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Bavlère, R.; Boutet, J.; Fouillet, Y. Dynamics of droplet transport induced by electrowetting actuation. Microfluid. Nanofluid. 2008, 4, 287–294. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Fei, B.; Hu, H.; Lai, C.; Xin, J.H. Superhydrophobic surface with directional wetting, adhesion, and transport of water. Adv. Funct. Mater. 2015, 25, 5047–5056. [Google Scholar] [CrossRef]
- Hancock, M.J.; Sekeroglu, K.; Demirel, M.C. Bioinspired directional surfaces for adhesion, wetting, and transport. Adv. Funct. Mater. 2012, 22, 2223–2234. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Wang, J.; Wang, X.; Niu, S.; Li, S.; Liao, Q.; Xu, Y.; You, Z.; Wang, Z. Stretchable and waterproof self-charging power system for harvesting energy from diverse deformation and powering wearable electronics. ACS Nano 2016, 10, 6519–6525. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wei, Z.; Lin, X.; Huang, T.; Yu, A. Polyaniline membranes as waterproof barriers for lithium air batteries. Electrochim. Acta 2012, 78, 195–199. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Luo, W.; Zhou, W. A novel surface waterproof geopolymer derived from metakaolin by hydrophobic modification. Mater. Lett. 2016, 164, 172–175. [Google Scholar] [CrossRef]
- Aspenes, E.; Ersland, G.; Graue, A.; Stevens, J.; Baldwin, B.A. Wetting phase bridges establish capillary continuity across open fractures and increase oil recovery in mixed-wet fractured chalk. Transp. Porous Med. 2008, 74, 35–47. [Google Scholar] [CrossRef]
- Sohal, M.A.; Søgaard, E.G. Effect of the temperature on wettability and optimum wetting conditions for maximum oil recovery in a carbonate reservoir system. Energy Fuels 2017, 31, 3557–3566. [Google Scholar] [CrossRef]
- Hua, Z.; Li, M.; Ni, X.; Wang, H.; Yang, Z.; Lin, M. Effect of injection brine composition on wettability and oil recovery in sandstone reservoirs. Fuel 2016, 182, 687–695. [Google Scholar] [CrossRef]
- Chen, T.; Chen, R.; Jin, Z.; Liu, J. Engineering hollow mesoporous silica nanocontainers with molecular switches for continuous self-healing anticorrosion coating. J. Mater. Chem. A 2015, 3, 9510–9516. [Google Scholar] [CrossRef]
- Ramachandran, R.; Nosonovsky, M. Coupling of surface energy with electric potential makes superhydrophobic surfaces. Phys. Chem. Chem. Phys. 2015, 17, 24988–24997. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yuan, J.; Huang, J.; Ren, K.; Liu, Y.; Lu, S.; Li, H. Large-scale fabrication of superhydrophobic polyurethane/nano-Al2O3 coatings by suspension flame spraying for anti-corrosion applications. Appl. Surf. Sci. 2014, 311, 864–869. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Colloid Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4414–4419. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Bhushan, B. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv. 2013, 3, 671–690. [Google Scholar] [CrossRef]
- Dorrer, C.; Rühe, J. Some thoughts on superhydrophobic wetting. Soft Matter 2009, 5, 51–61. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, M.; Jiang, L. Recent developments in polymeric superoleophobic surfaces. J. Polym. Sci. Pol. Phys. 2012, 50, 1209–1224. [Google Scholar] [CrossRef]
- Nakajima, A.; Hashimoto, H.; Watanabe, T. Recent studies on super-hydrophobic films. Monateshefte Chem. 2001, 41, 31–41. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Wetting behavior of water and oil droplets in three-phase interfaces for hydrophobicity/philicity and oleophobicity/philicity. Langmuir 2009, 25, 14165–14173. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Khorshid, M.; Li, J.; Markey, K. Fabrication of optomicrofluidics for real-time bioassays based on hollow sphere colloidal photonic crystals with wettability patterns. J. Mater. Chem. C 2016, 4, 7853–7858. [Google Scholar] [CrossRef]
- Chang, T.; Tsai, T.; Yang, H.; Huang, J. Microelectronic engineering effect of ultra-fast laser texturing on surface wettability of microfluidic channels. Microelectron. Eng. 2012, 98, 684–688. [Google Scholar] [CrossRef]
- Tsougeni, K.; Petrou, P.S.; Papageorgiou, D.P.; Kakabakos, S.E.; Tserepi, A.; Gogolides, E. Chemical controlled protein adsorption on microfluidic channels with engineered roughness and wettability. Sens. Actuators B Chem. 2012, 161, 216–222. [Google Scholar] [CrossRef]
- Vafaei, S.; Tuck, C.; Ashcroft, I.; Wildman, R. Surface microstructuring to modify wettability for 3D printing of nano-filled inks Surface microstructuring to modify wettability for 3D printing of nano-filled inks. Chem. Eng. Res. Des. 2016, 109, 414–420. [Google Scholar] [CrossRef]
- Penner, R.M. Rapid, wafer-scale laser nanoprinting of polymer surfaces. ACS Nano 2011, 5, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Korsmeyer, T. Principles of droplet electrohydrodynamics for lab-on-a-chip. Lab Chip 2004, 4, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; Papautsky, I.; Heikenfeld, J. Investigation of Laplace barriers for arrayed electrowetting lab-on-a-chip. Langmuir 2014, 30, 5349–5356. [Google Scholar] [CrossRef] [PubMed]
- Cassie, B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 5, 546–551. [Google Scholar] [CrossRef]
- Jin, B.M.; Feng, X.; Feng, L.; Sun, T.; Zhai, J.; Li, T.; Jiang, L. Superhydrophobic aligned polystyrene nanotube films with high adhesive force. Adv. Mater. 2005, 17, 1977–1981. [Google Scholar] [CrossRef]
- Feng, L.; Song, Y.; Zhai, J.; Liu, B.; Xu, J.; Jiang, L.; Zhu, D. Creation of a superhydrophobic surface from an amphiphilic polymer. Angew. Chem. 2003, 115, 824–826. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.; Chen, Z.; Chen, G.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. Robust translucent superhydrophobic PDMS/PMMA film by facile one-step spray for self-cleaning and efficient emulsion separation. Chem. Eng. J. 2017, 330, 26–35. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, J.; Zhang, Q.; Zhan, X.; Chen, F. Robust liquid-repellent coatings based on polymer nanoparticles with excellent self-cleaning andantibacterial performances. J. Mater. Chem. 2017, 5, 275–284. [Google Scholar] [CrossRef]
- Marabotti, I.; Morelli, A.; Orsini, L.M.; Martinelli, E.; Galli, G.; Chiellini, E.; Lien, E.M.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; et al. Fluorinated/siloxane copolymer blends for fouling release: Chemical characterisation and biological evaluation with algae and barnacles. Biofouling 2009, 25, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Suffredini, M.; Galli, G.; Glisenti, A.; Pettitt, M.E.; Callow, M.E.; Callow, J.A.; Williams, D.; Lyall, G. Amphiphilic block copolymer/poly (dimethylsiloxane) (PDMS) blends and nanocomposites for improved fouling-release. Biofouling 2011, 27, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Momen, G.; Farzaneh, M. Facile approach in the development of icephobic hierarchically textured coatings as corrosion barrier. Appl. Surf. Sci. 2014, 299, 41–46. [Google Scholar] [CrossRef]
- Jafari, R.; Menini, R.; Farzaneh, M. Superhydrophobic and icephobic surfaces prepared by RF-sputtered polytetrafluoroethylene coatings. Appl. Surf. Sci. 2010, 257, 1540–1543. [Google Scholar] [CrossRef]
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of anti-icing surfaces: Smooth, textured or slippery? Nat. Rev. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Wang, E.; Yuan, R.; Gao, D.; Zhang, X.; Zhu, Y. A robust superhydrophobic PVDF composite coating with wear/corrosion-resistance properties. Appl. Surf. Sci. 2015, 332, 518–524. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, L.; Liu, X.; Weng, R.; Wang, Y.; Wu, Z. Wetting behavior and drag reduction of superhydrophobic layered double hydroxides films on aluminum. Appl. Surf. Sci. 2016, 380, 178–184. [Google Scholar] [CrossRef]
- Ishizaki, T.; Saito, N.; Takai, O. Correlation of cell adhesive behaviors on superhydrophobic, superhydrophilic, and micropatterned superhydrophobic/superhydrophilic surfaces to their durface chemistry. Langmuir 2010, 26, 8147–8154. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, A.; Choi, W.; Ma, M.; Mabry, J.M.; Mazella, S.A.; Rutledge, G.C.; McKinley, G.H.; Cohen, R.E. Designing superoleophobic surfaces. Science 2007, 318, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Guo, Z.; Liu, W. Biomimetic superoleophobic surfaces : Focusing on their fabrication and applications. J. Mater. Chem. A 2015, 3, 1811–1827. [Google Scholar] [CrossRef]
- Wu, Y.; Su, B.; Jiang, L.; Heeger, A.J. “Liquid-Liquid-Solid” type superoleophobic surfaces to pattern polymeric semiconductors towards high-quality organic field-effect Transistors. Adv. Mater. 2013, 25, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, Z.; Zhu, X.; Ge, B.; Wang, K.; Xu, X.; Men, X.; Zhou, X. A facile method for imparting superoleophobicity to polymer substrates. Appl. Phys. A 2014, 114, 1129–1133. [Google Scholar] [CrossRef]
- Brown, P.S.; Bhushan, B. Durable superoleophobic polypropylene surfaces. Philos. Trans. R. Soc. A 2016, 374, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.K.; Li, Y.; Mabry, J.M.; Tuteja, A. Hierarchically structured superoleophobic surfaces with ultralow contact angle hysteresis. Adv. Mater. 2012, 24, 5838–5843. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, H.; Darmanin, T.; Ta, E.; Guittard, F. Influence of intrinsic oleophobicity and surface structuration on the superoleophobic properties of PEDOP films bearing two fluorinated tails. J. Mater. Chem. A 2013, 1, 2896–2903. [Google Scholar] [CrossRef]
- Das, A.; Schutzius, T.M.; Bayer, I.S.; Megaridis, C.M. Superoleophobic and conductive carbon nanofiber/fluoropolymer composite films. Carbon 2011, 50, 1346–1354. [Google Scholar] [CrossRef]
- Zhao, H.; Law, K. Directional self-cleaning superoleophobic surface. Langmuir 2012, 28, 11812–11818. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Gao, J.; Song, Y.; Jiang, L. Superoleophobic surfaces with controllable oil adhesion and their application in oil transportation. Adv. Funct. Mater. 2011, 21, 4270–4276. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F.; Amigoni, S.; Givenchy, T.D.; Noblin, X. Superoleophobic behavior of fluorinated conductive polymer films combining electropolymerization and lithography. Soft Matter 2011, 7, 1053–1057. [Google Scholar] [CrossRef]
- Steele, A.; Bayer, I.; Loth, E. Inherently superoleophobic nanocomposite coatings by spray satomization. Nano Lett. 2009, 9, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Ellinas, K.; Tsougeni, K.; Petrou, P.S.; Boulousis, G.; Tsoukleris, D.; Pavlatou, E.; Tserepi, A.; Kakabakos, S.E.; Gogolides, E. Three-dimensional plasma micro-nanotextured cyclo-olefin-polymer surfaces for biomolecule immobilization and environmentally stable superhydrophobic and superoleophobic behavior. Chem. Eng. J. 2016, 300, 394–403. [Google Scholar] [CrossRef]
- Zhao, H.; Law, K. Super toner and ink repellent superoleophobic surface. ACS Appl. Mater. Interfaces 2012, 4, 4288–4295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Law, K.; Sambhy, V. Fabrication, surface properties, and origin of superoleophobicity for a model textured surface. Langmuir 2011, 27, 5927–5935. [Google Scholar] [CrossRef] [PubMed]
- Im, M.; Im, H.; Lee, J.; Yoon, J.; Choi, Y. A robust superhydrophobic and superoleophobic surface with inverse- trapezoidal microstructures on a large transparent flexible substrate. Soft Matter 2010, 6, 1401–1404. [Google Scholar] [CrossRef]
- Kang, S.M.; Kim, M.; Kim, N.; Kwak, K.; Tahk, H.; Suh, K.Y. Robust superomniphobic surfaces with mushroom-like micropillar arrays. Soft Matter 2012, 8, 8563–8568. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Liu, X.; Zhou, F. Engineering a titanium durface with controllable oleophobicity and Switchable oil adhesion. J. Phys. Chem. C 2010, 114, 9938–9944. [Google Scholar] [CrossRef]
- Hoefnagels, H.F.; Wu, D.; With, G.D.; Ming, W. Biomimetic superhydrophobic and highly oleophobic cotton textiles. Langmuir 2007, 23, 13158–13163. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, X.; Wang, D.; Chen, M.; Zhou, F. Alumina nanowire forests via unconventional anodization and super-repellency plus low adhesion to diverse liquids. Chem. Commun. 2009, 9, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.W.; Tuteja, A.; Chhatre, S.; Mabry, J.M.; Cohen, R.E.; Mckinley, G.H. Fabrics with Tunable Oleophobicity. Adv. Mater. 2009, 21, 2190–2195. [Google Scholar] [CrossRef]
- Coffinier, Y.; Galopin, E.; Boukherroub, R. Preparation of superhydrophobic and oleophobic diamond nanograss array. J. Mater. Chem. A 2010, 20, 10671–10675. [Google Scholar] [CrossRef]
- Liu, Y.; Xiu, Y.; Hess, D.W.; Wong, C.P.; Drive, F. Silicon surface structure-controlled oleophobicity. Langmuir 2010, 26, 8908–8913. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Shibuichi, S. Super oil-repellent surfaces. Angew. Chem. Int. Ed. Engl. 1997, 36, 1011–1012. [Google Scholar]
- Krumpfer, J.W.; Mccarthy, T.J.; Audry, M. Wetting dynamics of hydrophobic and structured surfaces. Faraday Discuss. 2010, 146, 57–65. [Google Scholar]
- Zimmermann, J.; Rabe, M.; Artus, G.R.J.; Seeger, S. Patterned superfunctional surfaces based on a silicone nanofilament coating. Soft Matter 2008, 4, 450–452. [Google Scholar] [CrossRef]
- Kong, L.; Chen, X.; Yu, L.; Wu, Z.; Zhang, P. Superhydrophobic cuprous oxide nanostructures on phosphor-copper meshes and their oil−water separation and oil spill cleanup. ACS Appl. Mater. Interfaces 2015, 7, 2616–2625. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Z.; Xu, X.; Men, X. Superoleophobic textured aluminum surfaces. New J. Chem. 2011, 35, 2422–2426. [Google Scholar] [CrossRef]
- Vengatesh, P.; Kulandainathan, M.A. Hierarchically ordered self-lubricating superhydrophobic anodized aluminum surfaces with enhanced corrosion resistance. ACS Appl. Mater. Interfaces 2015, 7, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Innovations, S.; Columbia, B. Superhydrophobic laser ablated stainless steel substrates and their wettability superhydrophobic laser-ablated stainless steel substrates and their wettability. Surf. Innov. 2015, 1–27. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, Y.; Jin, J.; Tian, L.; Han, Z.; Ren, L. Facile fabrication of biomimetic superhydrophobic surface with anti-frosting on stainless steel substrate. Appl. Surf. Sci. 2015, 355, 1238–1244. [Google Scholar] [CrossRef]
- Roig, A.; Molins, E.; Rodrı, E.; Martı, S.; Vallribera, A. Superhydrophobic silica aerogels by fluorination at the gel stage. Chem. Commun. 2004, 20, 2316–2317. [Google Scholar] [CrossRef] [PubMed]
- Baldacchini, T.; Carey, J.E.; Zhou, M.; Mazur, E. Superhydrophobic surfaces prepared by microstructuring of silicon using a femtosecond laser. Langmuir 2006, 22, 4917–4919. [Google Scholar] [CrossRef] [PubMed]
- Hoshian, S.; Jokinen, V.; Somerkivi, V.; Lokanathan, A.R.; Franssila, S. Robust Superhydrophobic Silicon without a Low Surface-Energy Hydrophobic Coating. ACS Appl. Mater. Interfaces 2015, 7, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.; Guo, Z.; Liu, H.; Huang, X. Bioinspired multifunctional hetero-hierarchical micro/nanostructure tetragonal array with self-cleaning, anticorrosion, and concentrators for the SERS detection. ACS Appl. Mater. Interfaces 2013, 5, 10633–10642. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Lin, C.; Cheng, T.; Shieh, J.; Lin, H. Nanoimprinting of flexible polycarbonate sheets with a flexible polymer mold and application to superhydrophobic surfaces. Adv. Mater. Interfaces 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.B.; Shi, Z.; Wang, D.; Jin, J.; Jiang, L. Nanowire-haired inorganic membranes with superhydrophilicity and underwater ultralow adhesive superoleophobicity for high-efficiency oil/water separation. Adv. Mater. 2013, 25, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Jung, Y.C. Wetting, adhesion and friction of superhydrophobic and hydrophilic leaves and fabricated micro/nanopatterned. J. Phys. Condens. Matter. 2008, 20, 1–24. [Google Scholar] [CrossRef]
- Wang, B.J.; Wen, Y.; Hu, J.; Song, Y.; Jiang, L. Fine Control of the wettability transition temperature of colloidal-crystal films: From superhydrophilic to superhydrophobic. Adv. Mater. Funct. 2007, 17, 219–225. [Google Scholar] [CrossRef]
- Huh, C.; Mason, S.G. Effects of surface roughness on wetting (theoretical). J. Colloid Interface Sci. 1977, 60, 7–10. [Google Scholar] [CrossRef]
- Whyman, G.; Bormashenko, E.; Stein, T. The rigorous derivation of Young, Cassie-Baxter and Wenzel equations and the analysis of the contact angle hysteresis phenomenon. Chem. Phys. Lett. 2008, 450, 355–359. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Farzaneh, M. Effect of contact angle hysteresis on water droplet evaporation from super-hydrophobic surfaces. Appl. Surf. Sci. 2009, 255, 4056–4060. [Google Scholar] [CrossRef]
- Reyssat, M.; Que, D. Contact angle hysteresis generated by strong dilute defects. J. Phys. Chem. B 2009, 113, 3906–3909. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B. Bioinspired structured surfaces. Langmuir 2012, 28, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef] [PubMed]
- Bartolo, D.; Bouamrirene, F.; Verneuil, É.; Buguin, A.; Silberzan, P.; Moulinet, S. Bouncing or sticky droplets: Impalement transitions on superhydrophobic micropatterned surfaces. Europhys. Lett. 2006, 74, 299–305. [Google Scholar] [CrossRef]
- Liu, B.; Lange, F.F. Pressure induced transition between superhydrophobic states : Configuration diagrams and effect of surface feature size. J. Colloid Interface Sci. 2006, 298, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Ran, C.; Ding, G.; Liu, W.; Deng, Y.; Hou, W. Wetting on nanoporous alumina surface: Transition between Wenzel and Cassie states controlled by surface Structure. Langmuir 2008, 24, 9952–9955. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Xue, Y.; Liu, H.; Shi, Y.; Xi, P.; Lin, H.; Duan, H. Symmetric and asymmetric meniscus collapse in wetting transitionon submerged structured surfaces. Langmuir 2015, 31, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Nam, Y.; Lastakowski, H.; Hur, J.I. Two types of Cassie-to-Wenzel wetting transitions on superhydrophobic surfaces during drop impact. Soft Matter 2015, 11, 4592–4599. [Google Scholar] [CrossRef] [PubMed]
- Pashos, G.; Kokkoris, G.; Boudouvis, A.G. Minimum energy paths of wetting transitions on grooved surfaces. Langmuir 2015, 31, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Tay, B.; Tan, C.; Shakerzadeh, M.; Ostrikov, K. Electrowetting control of Cassie-to-Wenzel transitions in superhydrophobic carbon nanotube-based nanocomposites. ACS Nano 2009, 3, 3031–3036. [Google Scholar] [CrossRef] [PubMed]
- Rwei, S.P.; Ku, F.H.; Cheng, K.C. Dispersion of carbon black in a continuous phase: Electrical, rheological, and morphological studies. Colloid Polym. Sci. 2002, 280, 1110–1115. [Google Scholar]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Philos. Trans. R. Soc. A 2009, 367, 1487–1509. [Google Scholar] [CrossRef] [PubMed]
- Drelich, J.; Chibowski, E. Superhydrophilic and superwetting surfaces: Definition and mechanisms of control. Langmuir 2010, 26, 18621–18623. [Google Scholar] [CrossRef] [PubMed]
- Bauer, U.; Bohn, H.F.; Federle, W. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. Proc. Biol. Soc. 2008, 275, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, L. Water-repellent legs of water striders. Nature 2004, 432, 36. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef]
- Lee, W.; Jin, M.; Yoo, W.; Lee, J. Nanostructuring of a polymeric substrate with well-defined nanometer-scale topography and tailored surface wettability. Langmuir 2004, 24, 7665–7669. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Bhushan, B. Biomimetic structures for fluid drag reduction in laminar and turbulent flows. J. Phys. Condens. Matter 2010, 22, 035104. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Hwang, J.; Lee, H. Replication of cicada wing’s nano-patterns by hot embossing and UV nanoimprinting. Nanotechnology 2009, 20, 385303. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Her, E.K. Fabrication of Superhydrophobic Surfaces with High and Low Adhesion Inspired from Rose Petal. Langmuir 2010, 26, 8207–8217. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Bhushan, B. Wear-resistant rose petal-effect surfaces with superhydrophobicity and high droplet adhesion using hydrophobic and hydrophilic nanoparticles. J. Colloid Interface Sci. 2012, 384, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, Y.; Li, M.; Zheng, Y.; Shen, W.; Jiang, L. The structural color of red Rose petals and their duplicates. Lagmuir 2010, 26, 14885–14888. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Yang, H.; Song, S.; Lu, B.; Chen, R. A magnetic superhydrophilic/oleophobic sponge for continuous oil-water separation. Chem. Eng. J. 2017, 309, 366–373. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Liu, N.; Zhang, W.; Yang, Y.; Cao, Y.; Lin, X.; Wei, Y.; Feng, L. Breathing demulsi fication: Athree-dimensional (3D) free-dtanding duperhydrophilic sponge. ACS Appl. Mater. Interfaces 2015, 7, 22264–22271. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yang, M.; Jeong, H.; Chang, M.; Hong, J. Durable superhydrophilic coatings formed for anti-biofouling and oil-water separation. J. Membr. Sci. 2016, 506, 22–30. [Google Scholar] [CrossRef]
- Park, J.T.; Kim, H.; Lee, D. Excellent anti-fogging dye-sensitized solar cells based on superhydrophilic nanoparticle coatings. Nanoscale 2014, 6, 7362–7368. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Nishimoto, S.; Kubo, A.; Tryk, D. Fabrication and application of TiO2-based superhydrophilic-superhydrophobic patterns on titanium substrates for offset printing. Chem. Asian J. 2009, 4, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, S.; Wu, L. Preparation of waterborne self-cleaning nanocomposite coatings based on TiO2/PMMA latex. Prog. Org. Coat. 2015, 85, 208–215. [Google Scholar] [CrossRef]
- Sasaki, K.; Tenjimbayashi, M.; Manabe, K.; Shiratori, S. Asymmetric superhydrophobic/superhydrophilic cotton fabrics designed by spraying polymer and nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Qi, G.; Xiao, K.; Sun, J.; Giannelis, E.P.; Huang, X.; Elimelech, M. Organic fouling behavior of superhydrophilic polyvinylidene fluoride (PVDF) ultra fi ltration membranes functionalized with surface-tailored nanoparticles: Implications for organic fouling in membrane bioreactors. J. Membr. Sci. 2014, 463, 94–101. [Google Scholar] [CrossRef]
- Goetz, L.A.; Jalvo, B.; Rosal, R.; Mathew, A.P. Superhydrophilic anti-fouling electrospun cellulose acetate membranes coated with chitin nanocrystals for water fi ltration. J. Membr. Sci. 2016, 510, 238–248. [Google Scholar] [CrossRef]
- Tiraferri, A.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Superhydrophilic thin-film composite forward osmosis membranes for organic fouling control: Fouling behavior and antifouling mechanisms. Environ. Sci. Technol. 2012, 46, 11135–11144. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Lee, J.; Hwang, W. Durable superhydrophilic/phobic surfaces based on green patina with corrosion resistance. Phys. Chem. Chem. Phys. 2015, 17, 6786–6793. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wen, B.; Tu, Y.; Wan, R.; Fang, H. Friction reduction at a superhydrophilic surface: Role of ordered water. J. Phys. Chem. C 2015, 119, 11679–11684. [Google Scholar] [CrossRef]
- Wang, J.; Kaplan, J.A.; Colson, Y.L.; Grinstaff, M.W. Stretch-induced drug delivery from superhydrophobic polymer composites: Use of crack propagation failure modes for controlling release rates. Angew. Chem. 2016, 128, 2846–2850. [Google Scholar] [CrossRef]
- Zahner, D.; Abagat, J.; Svec, F.; Fréchet, J.M.J.; Levkin, P.A. A Facile Approach to Superhydrophilic–Superhydrophobic Patterns in Porous Polymer Films. Adv. Mater. 2011, 23, 3030–3034. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Ji, Y.; Ma, R.; Zhao, F.; An, Q.; Gao, C. Superhydrophilic and antibacterial zwitterionic polyamide nano filtration membranes for antibiotics separation. J. Membr. Sci. 2016, 510, 122–130. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, J.; Wang, F.; Li, Z.; Ding, B. Superhydrophilic and underwater superoleophobic nano fibrous membrane with hierarchical structured skin for effective oil-in-water emulsion separation. J. Mater. Chem. A 2016, 5, 497–502. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, M.; Wang, D.; Wang, X.; Liu, J.; Huang, L. Superhydrophilic molecularly imprinted polymers based on a water-soluble functional monomer for the recognition of gastrodin in water media. J. Chromatogr. A 2015, 1425, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Levkin, B.P.A.; Svec, F.; Fre, J.M.J. Porous polymer coatings: A versatile approach to superhydrophobic surfaces. Adv. Funct. Mater. 2009, 19, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Liu, S.; Chen, X.; Wang, Y.; Yang, W. High-yield synthesis of superhydrophilic polypyrrole nanowire networks. Macromolecules 2006, 39, 3224–3230. [Google Scholar] [CrossRef]
- Xiao, X.; Bai, W.; Lin, J. Strategy for self-assembly of the poly(9,9-dihexylfluorene) to microspheres: Optimizing the self-assembling conditions. Polym. Bull. 2014, 71, 2103–2112. [Google Scholar] [CrossRef]
- Siqueira, I.A.W.B.; Corat, M.A.F.; Cavalcanti, N.; Neto, W.A.R.; Martin, A.A.; Bretas, R.E.S.; Marciano, F.R.; Lobo, A.O. In Vitro and in vivo studies of novel Poly (d,l-lactic acid), superhydrophilic carbon nanotubes, and nanohydroxyapatite scaff olds for bone regeneration. ACS Appl. Mater. Interfaces 2015, 7, 9385–9398. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.V.M.; Silva, A.S.; Melo, G.F.S.; Vasconscellos, L.M.R.; Marciano, F.R.; Lobo, A.O. Influence of low contents of superhydrophilic MWCNT on the properties and cell viability of electrospun poly (butylene adipate-co-terephthalate) fibers. Mater. Sci. Eng. C 2016, 59, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dong, Z.; Zhang, P.; Zhong, Q.; Xu, Z. Preparation and characterization of superhydrophilic PVDF electrospun nanofibrous membrane based on in situ free radical polymerization. Mater. Lett. 2015, 156, 58–61. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Liu, Y.; Hu, H.; Li, Y.; Yan, P.; Yu, B.; Zhou, F. One-step modification of fabrics with bioinspired polydopamine@octadecylamine nanocapsules for robust and healable self-cleaning performance. Small 2015, 11, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tian, Y.; Jiang, L. Bio-inspired superoleophobic and smart materials: Design, fabrication, and application. Prog. Mater. Sci. 2013, 58, 503–564. [Google Scholar] [CrossRef]
- Carlborg, C.F.; van der Wijngaart, W. Sustained superhydrophobic friction reduction at high liquid pressures and large flows. Langmuir 2011, 27, 12812–12818. [Google Scholar] [CrossRef] [PubMed]
- Acatay, K.; Simsek, E.; Ow-yang, C.; Menceloglu, Y.Z. Tunable, superhydrophobically stable polymeric durfaces by electrospinning. Angew. Chem. 2004, 43, 5210–5213. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Hsu, S.H.; Chung, Y.C. Thermal imprint techniques for preparation of superhydrophobic polymer coatings. Surf. Coat. Technol. 2013, 231, 501–506. [Google Scholar] [CrossRef]
- Hurst, S.M.; Farshchian, B.; Choi, J.; Kim, J.; Park, S. A universally applicable method for fabricating superhydrophobic polymer surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2012, 407, 85–90. [Google Scholar] [CrossRef]
- Manna, U.; Lynn, D.M. Restoration of superhydrophobicity in crushed polymer films by treatment with water: Self-healing and recovery of damaged topographic features aided by an unlikely source. Adv. Mater. 2013, 25, 5104–5108. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; You, I.; Cho, W.K.; Shon, K.; Lee, T.G.; Choi, I.S.; Karp, J.M.; Lee, H. One-step modification of superhydrophobic surfaces by a mussel-inspired polymer coating. Angew. Chem. Int. Ed. 2010, 49, 9401–9404. [Google Scholar] [CrossRef] [PubMed]
- Erbil, H.Y.; Demirel, A.L.; Avci, Y.; Mert, O. Transformation of a simpleplastic into a superhydrophobic surface. Science 2003, 299, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- Letters, X.P.; Chemical, K.C.E. UV-curable nanocasting technique to prepare bioinspired superhydrophobic organic-inorganic composite anticorrosion coatings. Polym. Lett. 2015, 9, 143–153. [Google Scholar]
- Li, R.; Chen, C.; Li, J.; Xu, L.; Xiao, M.; Yan, D. A facile approach to superhydrophobic and superoleophilic graphene/polymer aerogels. J. Mater. Chem. A 2014, 2, 3057–3064. [Google Scholar] [CrossRef]
- Yoo, Y.; You, J.B.; Choi, W.; Im, S.G. A stacked polymer film for robust superhydrophobic fabrics. Polym. Chem. 2013, 4, 1664–1671. [Google Scholar] [CrossRef]
- Zhu, B.Y.; Hu, D.; Wan, M.; Jiang, L.; Wei, Y. Conducting and superhydrophobic rambutan-like hollow spheres of polyaniline. Adv. Mater. 2007, 19, 2092–2096. [Google Scholar] [CrossRef]
- Darmanin, T.; Nicolas, M. Electrodeposited polymer films with both superhydrophobicity and superoleophilicity. Phys. Chem. Chem. Phys. 2008, 10, 4322–4326. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Deva, D.; Kumar, R.; Sharma, A. Exceptionally robust and conductive superhydrophobic free-standing films of mesoporous carbon nanocapsule/polymer composite for multifunctional applications. Carbon 2015, 93, 492–501. [Google Scholar] [CrossRef]
- Xu, M.; Lu, N.; Qi, D.; Xu, H.; Wang, Y.; Shi, S.; Chi, L. Fabrication of superhydrophobic polymer films with hierarchical silver microbowl array structures. J. Colloid Interface Sci. 2011, 360, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Ye, M.; Boonkerd, K.; Xin, Z. Fabrication of superhydrophobic surface by a laminating exfoliation method. J. Mater. Chem. A 2014, 60, 1268–1271. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Yuan, X. Facile preparation of superhydrophobic coating by spraying a fluorinated acrylic random copolymer micelle. Soft Matter 2013, 9, 1005–1009. [Google Scholar] [CrossRef]
- Tung, P.; Kuo, S.; Jeong, K.; Cheng, S.Z.D.; Huang, C.; Chang, F. Formation of honeycomb structures and superhydrophobic surfaces by casting a block copolymer from selective solvent mixtures. Macromol. Rapid Commun. 2007, 28, 271–275. [Google Scholar] [CrossRef]
- Cardoso, M.R.; Tribuzi, V.; Balogh, D.T.; Misoguti, L.; Mendonc, C.R. Laser microstructuring for fabricating superhydrophobic polymeric surfaces. Appl. Surf. Sci. 2011, 257, 3281–3284. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, T.; Zhang, X.; Xin, Z.; Deng, X.; Prakashan, K. Mechanically stable superhydrophobic polymer films by a simple hot press lamination and peeling. RSC Adv. 2016, 6, 12530–12536. [Google Scholar] [CrossRef]
- Kato, S.; Sato, A. Micro/nanotextured polymer coatings fabricated by UV curing-induced phase separation: Creation of superhydrophobic surfaces. J. Mater. Chem. 2012, 22, 8613–8621. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Ge, B.; Men, X.; Xue, Q. One-pot, template-free synthesis of a robust superhydrophobic polymer monolith with an adjustable hierarchical porous structure. Green Chem. 2016, 18, 5266–5272. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Wang, E.; Zhang, X.; Yuan, R.; Zhu, Y. Superhydrophobic poly (vinylidene fluoride) membranes with controllable structure and tunable wettability prepared by one-step electrospinning. Polymer 2016, 82, 105–113. [Google Scholar] [CrossRef]
- Feng, W.; Li, L.; Ueda, E.; Li, J.; Heißler, S.; Welle, A.; Trapp, O.; Levkin, P.A. Surface patterning via Thiol-Yne click chemistry: An extremely fast and versatile approach to superhydrophilic-superhydrophobic micropatterns. Adv. Mater. Interfaces 2014, 1, 1400269. [Google Scholar] [CrossRef]
- Godeau, G.; Darmanin, T.; Guittard, F. Staudinger–Vilarrasa reaction to develop novel monomers with amide bonds for superhydrophobic properties. Prog. Org. Coat. 2016, 90, 431–437. [Google Scholar] [CrossRef]
- Godeaua, G.; N’Na, J.; Kout, E.E.; Trad, R.B.; Darmanin, T.; Kateb, M.B.; Bejic, M.; Guittard, F. Staudinger–Vilarassa reaction versus Huisgen reaction for the control of surface hydrophobicity and water adhesion. Polym. Adv. Technol. 2016. [Google Scholar] [CrossRef]
- Gong, D.; Long, J.; Fan, P.; Jiang, D.; Zhang, H.; Zhong, M. Thermal stability of micro–nano structures and superhydrophobicity of polytetrafluoroethylene films formed by hot embossing via a picosecond laser ablated template. Appl. Surf. Sci. 2015, 331, 437–443. [Google Scholar] [CrossRef]
- Falah, S.; Moradi, S.; Kamal, S.; Hatzikiriakos, S.G. Superhydrophobic laser ablated PTFE substrates. Appl. Surf. Sci. 2015, 349, 715–723. [Google Scholar] [CrossRef]
- Yilg, E.; Yilg, I. Simple processes for the preparation of superhydrophobic polymer surfaces. Polymer 2016, 99, 580–593. [Google Scholar]
- Zhou, C.; Chen, Z.; Yang, H.; Hou, K.; Zeng, X.; Zheng, Y.; Cheng, J. Nature-inspired strategy toward superhydrophobic fabrics for versatile oil/water separation. ACS Appl. Mater. Interface 2017, 9, 9184–9194. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gong, Q.; Zhan, S.; Jiang, L.; Zheng, Y. Robust anti-icing performance of a flexible superhydrophobic surface. Adv. Mater. 2016, 28, 7729–7735. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, Y.; Li, H.; Qi, H.; Li, B.; Yuan, X. Preparation and evaluation of hydrophobic surfaces of polyacrylate-polydimethylsiloxane copolymers for anti-icing. Prog. Org. Coat. 2013, 76, 1435–1444. [Google Scholar] [CrossRef]
- Zhao, J.; Song, L.; Yin, J.; Ming, W. Anti-bioadhesion on hierarchically structured, superhydrophobic surfaces. Chem. Commun. 2013, 49, 9191–9193. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jia, Z.; Li, Z.; Wei, X.; Guan, Z.; Macalpine, M. Anti-icing performance of RTV coatings on porcelain insulators by controlling the leakage current. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 760–766. [Google Scholar] [CrossRef]
- Laforte, J.L.; Allaire, M.A.; Laflamme, J. State-of-the-art on power line de-icing. Atoms. Res. 1998, 46, 143–158. [Google Scholar] [CrossRef]
- Parent, O.; Ilinca, A. Anti-icing and de-icing techniques for wind turbines: Critical review. Cold Reg. Sci. Technol. 2011, 65, 88–96. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, S.; Zhang, Z.; Sun, C.; Shu, L. Simulation and experimental investigation of DC ice-melting process on an iced conductor. IEEE Trans. Power Deliv. 2010, 25, 919–929. [Google Scholar] [CrossRef]
- Petrenko, V.F.; Sullivan, C.R.; Kozlyuk, V. Variable-resistance conductors (VRC) for power-line de-icing. Cold Reg. Sci. Technol. 2011, 65, 23–28. [Google Scholar] [CrossRef]
- Yilgor, I.; Bilgin, S.; Isik, M.; Yilgor, E. Facile preparation of superhydrophobic polymer surfaces. Polymer 2012, 53, 1180–1188. [Google Scholar] [CrossRef]
- Manna, U.; Kratochvil, M.J.; Lynn, D.M. Superhydrophobic polymer multilayers that promote the extended, long-term release of embedded water-soluble agents. Adv. Mater. 2013, 25, 6405–6409. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Cheng, Z.; Zhang, E.; Kang, H.; Liu, Y.; Jiang, L. Self-restoration of superhydrophobicity on shape memory polymer arrays with both crushed microstructure and damaged surface chemistry. Small 2017, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Superhydrophobic, superoleophobic coatings for the protection of silk textiles. Prog. Org. Coat. 2016, 97, 44–52. [Google Scholar] [CrossRef]
- Victor, J.J.; Facchini, D.; Erb, U. A low-cost method to produce superhydrophobic polymer surfaces. J. Mater. Sci. 2012, 47, 3690–3697. [Google Scholar] [CrossRef]
- Bharathidasan, T.; Narayanan, T.N.; Sathyanaryanan, S.; Sreejakumari, S.S. Above 170° water contact angle and oleophobicity of fluorinated graphene oxide based transparent polymeric films. Carbon 2014, 84, 207–213. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Wu, P.; Zhang, S.; Geng, B. Facile preparation of superhydrophobic and superoleophilic porous polymer membranes for oil/water separation from a polyarylester polydimethylsiloxane block copolymer. J. Mater. Sci. 2016, 51, 3211–3218. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Boinovich, L.B.; Bezdomnikov, A.A.; Chulkova, E.V.; Emelyanenko, K.A. Reinforced superhydrophobic coating on silicone rubber for longstanding anti-icing performance in severe conditions. ACS Appl. Mater. Interfaces 2017, 9, 24210–24219. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zheng, Y.; Wen, M.; Song, C.; Lin, Y.; Jiang, L. Icephobic/anti-icing properties of micro/nanostructured surfaces. Adv. Mater. 2012, 24, 2642–2648. [Google Scholar] [CrossRef] [PubMed]
- Fukada, K.; Nishizawa, S.; Shiratori, S.; Fukada, K.; Nishizawa, S.; Shiratori, S. Antifouling property of highly oleophobic substrates for solar cell surfaces. J. Appl. Phys. 2014, 115, 103516. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Z.; Yang, F.K.; Zhao, B. A facile in situ approach to polypyrrole functionalization through bioinspired catechols. Adv. Funct. Mater. 2015, 25, 1588–1597. [Google Scholar] [CrossRef]
- Links, D.A. Facile creation of hierarchical PDMS microstructures with extreme underwater superoleophobicity for anti-oil application in microfluidic. Lab Chip 2011, 11, 3873–3879. [Google Scholar]
- Vilčnik, A.; Jerman, I.; Vuk, A.Š.; Koželj, M.; Orel, B.; Tomšič, B.; Simončič, B.; Kovač, J. Structural properties and antibacterial effects of hydrophobic and oleophobic sol-gel coatings for cotton fabrics. Langmuir 2009, 25, 5869–5880. [Google Scholar] [CrossRef] [PubMed]
- Burdallo, I.; Fern, C. Integration of microelectronic chips in microfluidic systems on printed circuit board. J. Micromech. Microeng. 2012, 22, 105022. [Google Scholar] [CrossRef]
- Taleb, S.; Darmanin, T.; Guittard, F. Superoleophobic/superhydrophobic PEDOP conducting copolymers with dual-responsivity by voltage and ion exchange. Mater. Today Commun. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Bellanger, H.; Darmanin, T.; de Givenchy, E.T.; Guittard, F. Influence of long alkyl spacers in the elaboration of superoleophobic surfaces with short fluorinated chains. RSC Adv. 2013, 3, 5556–5562. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, J.; Su, B.; Liu, X.; Peng, J.; Liu, Y.; Liu, H.; Yang, G.; Jiang, L.; Wen, Y.; et al. An ion-induced low-oil-adhesion organic/inorganic hybrid film for stable superoleophobicity in seawater. Adv. Mater. 2013, 25, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Bodas, D.; Khan-malek, C. Formation of more stable hydrophilic surfaces of PDMS by plasma and chemical treatments. Microelectron. Eng. 2006, 83, 1277–1279. [Google Scholar] [CrossRef]
- Bodas, D.; Khan-malek, C. Hydrophilization and hydrophobic recovery of PDMS by oxygen plasma and chemical treatment–An SEM investigation. Sens. Actuators B 2007, 123, 368–373. [Google Scholar] [CrossRef]
- Coclite, A.M.; Shi, Y.; Gleason, K.K. Grafted crystalline poly-perfluoroacrylate structures for superhydrophobic and oleophobic functional coatings. Adv. Mater. 2012, 24, 4534–4539. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, H.; Morrow, N.R. Effect of brine composition on recovery of Moutray crude oil by waterflooding. J. Petrol. Sci. Eng. 1996, 14, 159–168. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.K.; Pan, Z.; Zhang, J.; Zhao, B. Bio-inspired dopamine functionalization of polypyrrole for improved adhesion and conductivity. Macromol. Rapid Commun. 2014, 35, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Li, M.; Yang, F.; Liu, M.; Li, L.; Wang, S.; Jiang, L. An underwater pH-responsive superoleophobic surface with reversibly switchable oil-adhesion. Soft Matter. 2012, 8, 6740–6743. [Google Scholar] [CrossRef]
- Buckley, J.S.; Liu, Y. Some mechanisms of crude oil/brine/solid interactions. J. Petrol. Sci. Eng. 1998, 20, 155–160. [Google Scholar] [CrossRef]
- Foglia, T.A.; Nelson, L.A.; Dunn, R.O.; Marmer, W.N. Low-temperature properties of alkyl esters of tallow and grease. JAOCS 1997, 74, 951–955. [Google Scholar] [CrossRef]
- Martínez-palou, R.; Lourdes, M.D.; Zapata-rendón, B.; Mar-Juárez, E.; Bernal-Huicochea, C.; de la Clavel-López, J.; Aburto, J. Transportation of heavy and extra-heavy crude oil by pipeline: A review. J. Petrol. Sci. Eng. 2011, 75, 274–282. [Google Scholar] [CrossRef]
- Prowse, T.D.; Furgal, C.; Chouinard, R.; Melling, H.; Milburn, D.; Smith, S.L. Implications of climate change for economic development in northern canada: Energy, resource, and transportation sectors. Ambio 2009, 38, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Jayadas, N.H.; Nair, K.P. Coconut oil as base oil for industrial lubricants-evaluation and modification of thermal, oxidative and low temperature properties. Tribol. Int. 2006, 39, 873–878. [Google Scholar] [CrossRef]
- Pan, Z.; Shahsvan, H.; Zhang, W.; Yang, F.K.; Zhao, B. Superhydro-oleophobic bio-inspired polydimethylsiloxanemicropillared surface via FDTS coating/blending approaches. Appl. Surf. Sci. 2015, 324, 612–620. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, W.; Kowalski, A.; Zhao, B. Oleophobicity of biomimetic micropatterned surface and its effect on the adhesion of frozen oil. Langmuir 2015, 31, 9901–9910. [Google Scholar] [CrossRef] [PubMed]
- Chanda, J.; Ionov, L.; Synytska, A. New insight into icing and de-icing properties of hydrophobic and hydrophilic structured surfaces based on core-shell particles. Soft Matter 2015, 11, 9126–9134. [Google Scholar] [CrossRef] [PubMed]
- Péter, Z.; Farzaneh, M.; Kiss, L.I. Numerical investigations of a new thermal de-icing method for overhead conductors based on high current impulses. IET Gener. Transm. Distrib. 2008, 2, 666–675. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.; Alvarenga, J.; Kreder, M.J.; Adorno-martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Personne, P.; Gayet, L.F. Ice accretion on wires and anti-icing induced by Joule effect. J. Appl. Meteorol. 1988, 27, 101–113. [Google Scholar] [CrossRef]
- Wang, H.; Xue, Y.; Lin, T. One-step vapour-phase formation of patternable, electrically conductive, superamphiphobic coatings on fibrous materials. Soft Matter 2011, 7, 8158–8161. [Google Scholar] [CrossRef]
- Cong, B.H.; Pan, T. Photopatternable conductive PDMS materials for microfabrication. Adv. Mater. 2008, 18, 1912–1921. [Google Scholar]
- Liu, C.; Choi, J. Patterning conductive PDMS nanocomposite in an elastomer using microcontact printing. J. Micromech. Microeng. 2009, 19, 085019. [Google Scholar] [CrossRef]
- Thomas, B.; Hansen, S.; West, K.; Hassager, O.; Larsen, N.B. Highly stretchable and conductive polymer material made from poly (3,4-ethylenedioxythiophene) and polyurethane elastomers. Adv. Funct. Mater. 2007, 17, 3069–3073. [Google Scholar]
- Xu, F.; Zhu, Y. Highly conductive and stretchable silver nanowire conductors. Adv. Mater. 2012, 24, 5117–5122. [Google Scholar] [CrossRef] [PubMed]
- Darmanin, T.; Guittard, F. Superoleophobic surfaces with short fluorinated chains? Soft Matter 2013, 9, 5982–5990. [Google Scholar] [CrossRef]
- Niu, B.X.; Peng, S.; Liu, L.; Wen, W.; Sheng, P. Characterizing and patterning of PDMS-based conducting composites. Adv. Mater. 2007, 19, 2682–2686. [Google Scholar] [CrossRef]
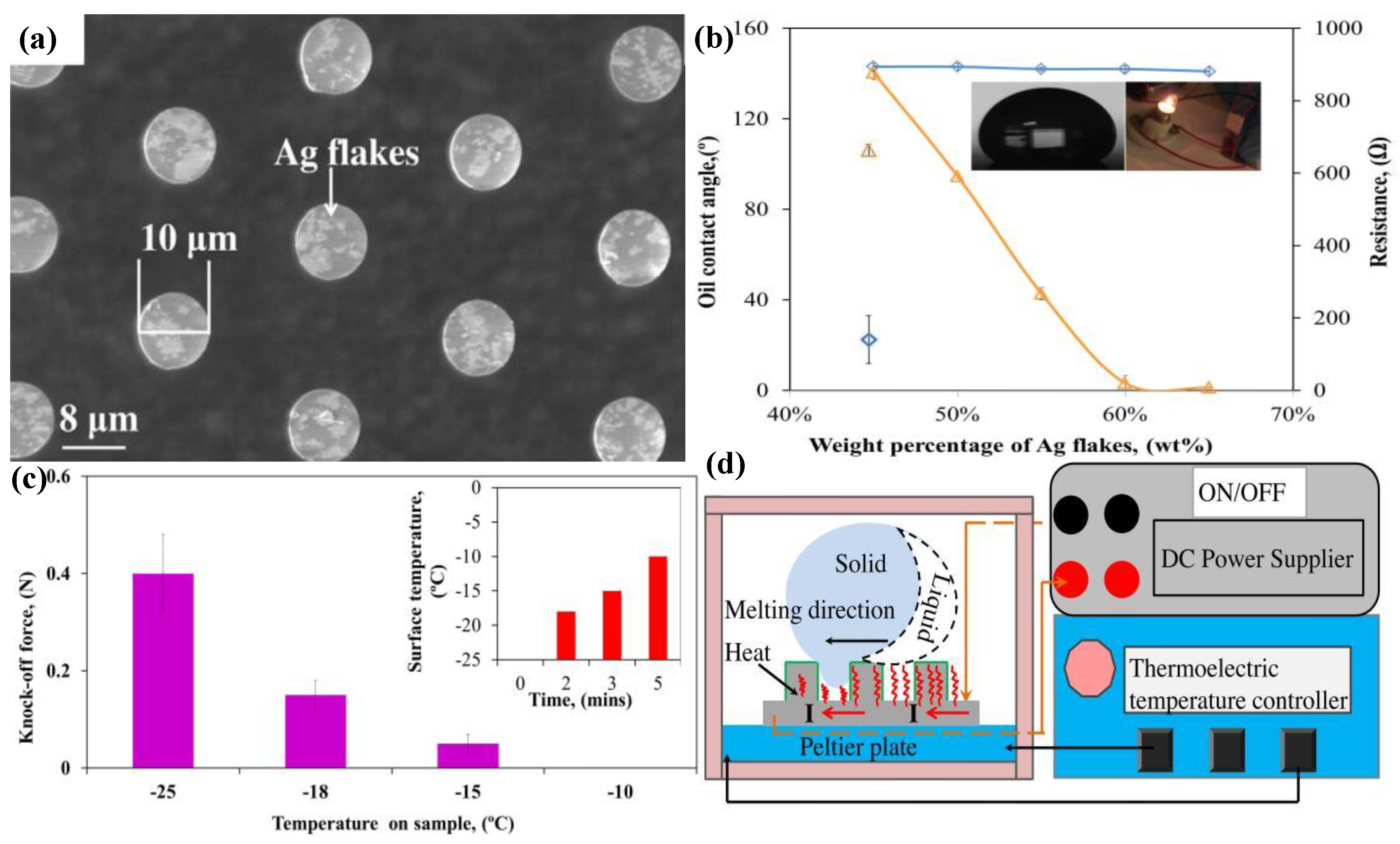
- Pan, Z.; Wang, T.; Zhou, Y.; Zhao, B. Development of electrically conductive-superoleophobic micropillars for reducing surface adhesion of oil at low temperatures. Appl. Surf. Sci. 2016, 389, 623–631. [Google Scholar] [CrossRef]

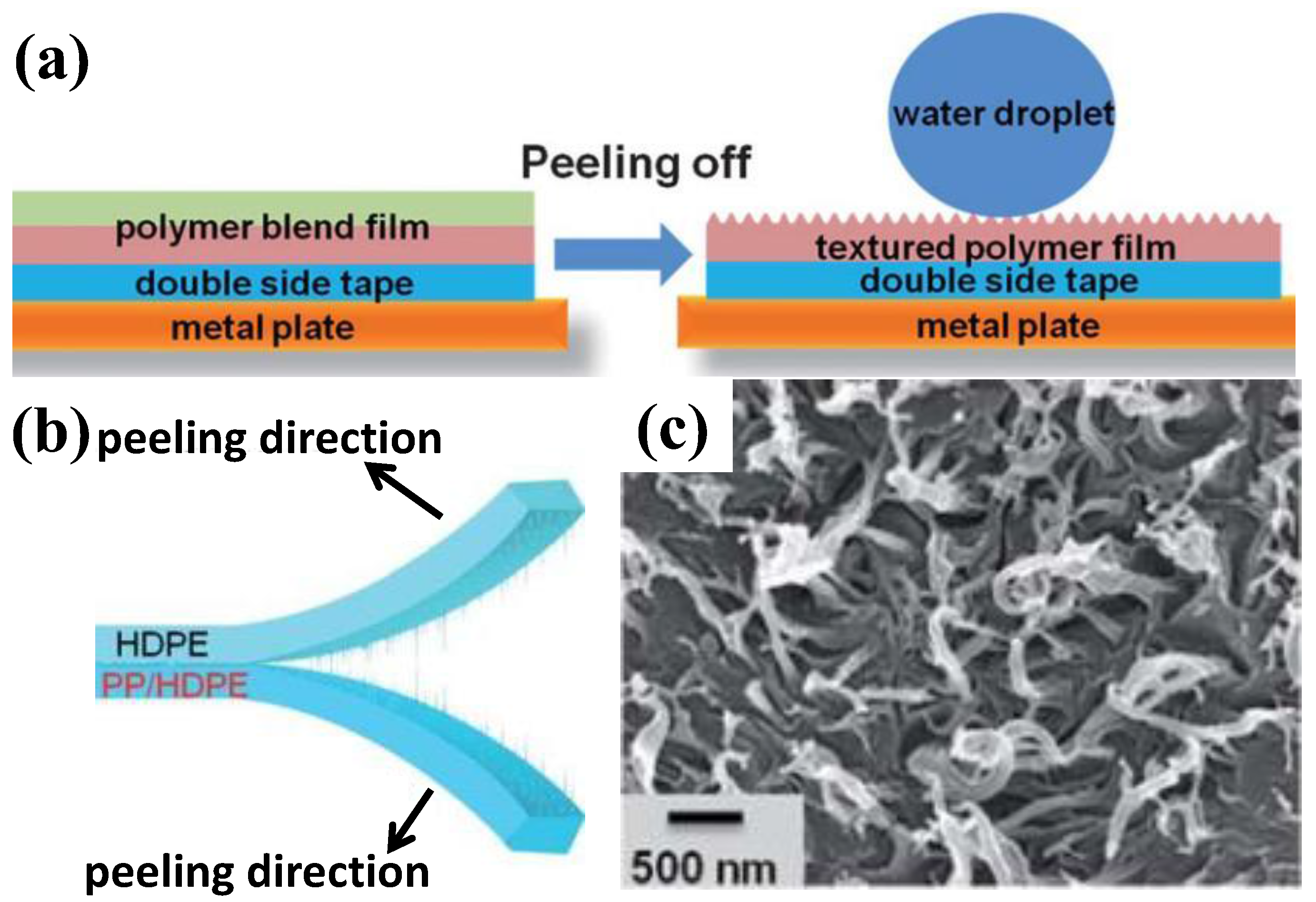
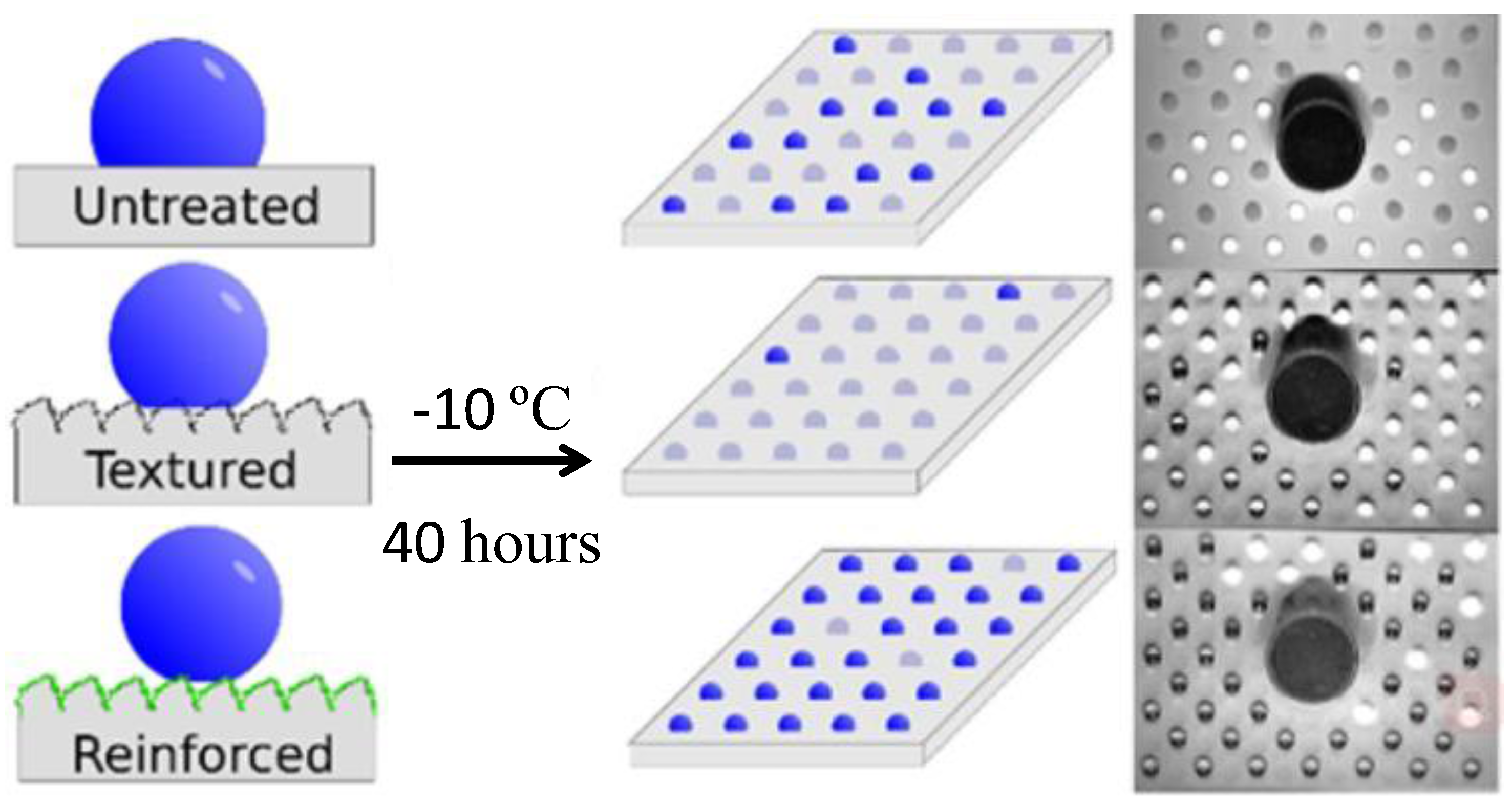

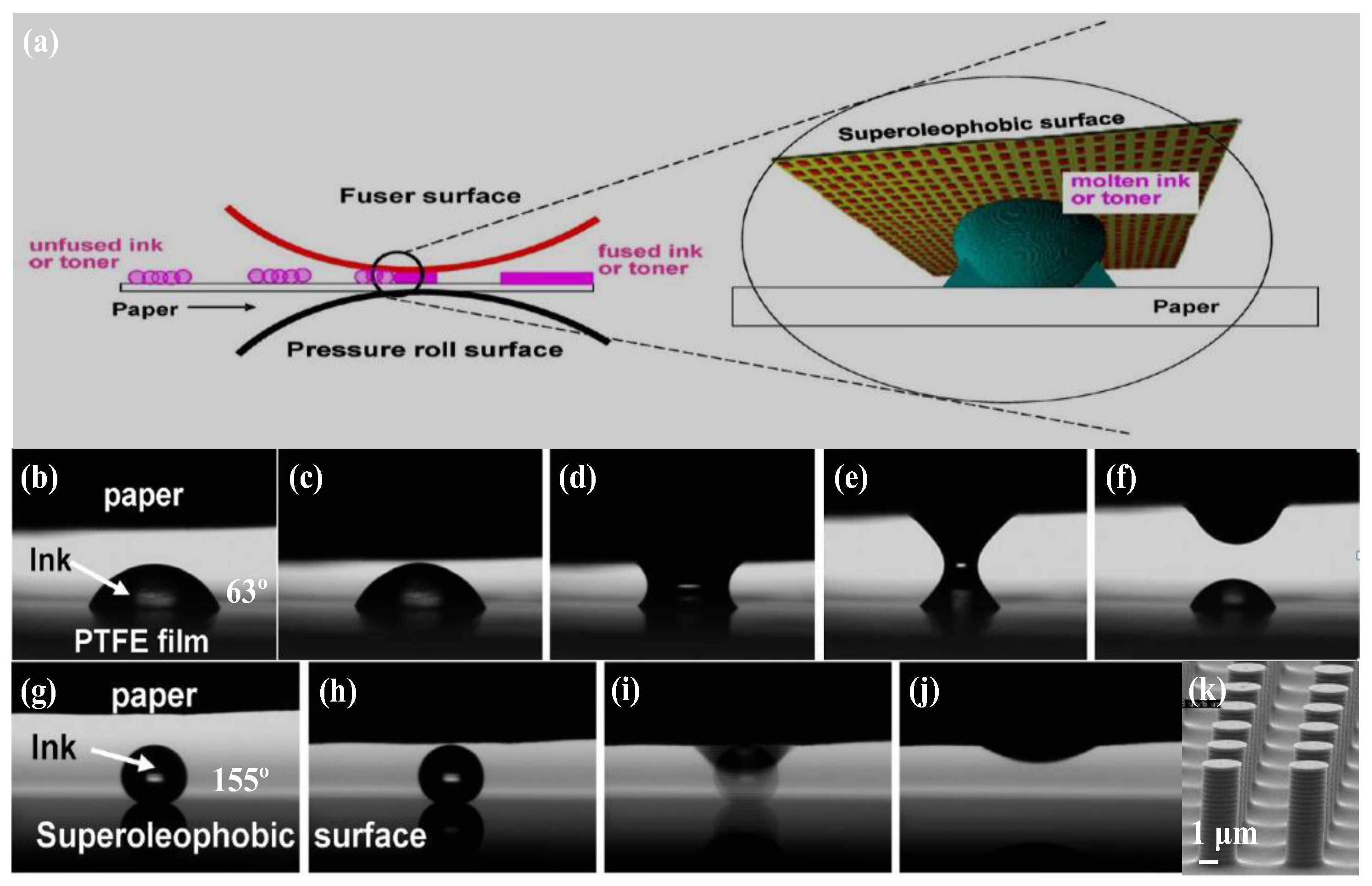
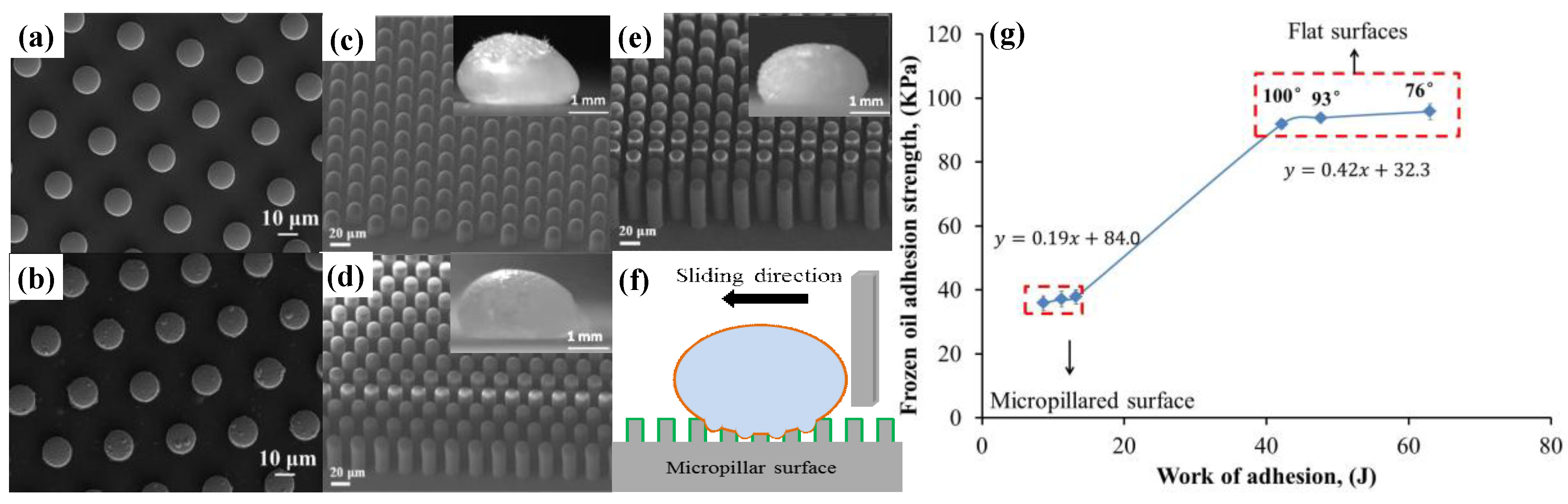
| Structure | Materials | Technique | Reference |
|---|---|---|---|
| porous mesh | polyacrylamide (PAM) | immersed coating | [125] |
| nano-porous structure | poly(2-hydroxyethyl methacryl-ate-co-ethylene dimethacrylate), poly(butyl methacrylate-co-ethylene dimethacrylate) | in situ polymerization | [143] |
| nanowire | polypyrrole (PPy) | chemical oxidative polymerization | [144] |
| carbon nanotubes | poly(d,l-lactide acid, PDLLA) | electrodeposition and immersion | [146] |
| carbon nanotubes/fibers | poly(butylene adipate-co-terephthalate), multiwalled carbon nanotubes | electrospinning | [147] |
| hierarchical membrane | N-aminoethylpiperazine propane sulfonate (AEPPS) monomer, trimesoyl chloride (TMC) monomer | interfacial polymerization | [140] |
| hierarchical nanofibrous membrane | polyacrylonitrile (PAN) (pristine NFM) | electrospinning and electrospraying | [141] |
| Bio-Inspired Structures | Materials | Surface Modification Technique | Water Contact Angle (°) | Rolling-Off Angle (°) | Reference |
|---|---|---|---|---|---|
| polymeric fibers and beads | high-molecular-weight poly(AN-co-TMI) and perfluori-nated linear diol (fluorolink-D) | blending and electrospinning | 166.7 | 4.3° | [152] |
| nanostructure | poly(methyl methacrylate)(PMMA) and polystyrene (PS), perfluorooctyltrichlorosilane (FOTS) | mold transfer | 151 | Sticky | [153] |
| micro-/nanostructure | poly(methyl methacrylate) (PMMA), polycarbonate (PC) and cyclo-olefin copolymer (COC), (heptadecafluoro 1,1,2,2-tetrahydrodecyl)trichlorosilane | plasma etching | 151 | 4 | [154] |
| porous multilayers | poly(ethyleneimine) (PEI), poly(vinyl-4,4-dimethylazlac-tone) (PVDMA) | layer by layer assembly | 156 | 1 | [155] |
| porous layer | poly(styrene-co-divinylbenzene), poly(styrene-co-divinylbenzene) | polymerization | 172 | [143] | |
| nanocapsule-coated fabric | polydopamine, octadecylamine | spontaneously deposition | 145 | Less than 10 | [149] |
| micro-/nano-patterned | polydopamine (PDA) | polymerization and mold transfer | 151 | 180 | [156] |
| porous branched structure | polypropylene, p-xylene | solvent evaporating | 160 | [157] | |
| nanotube | polystyrene | template | 162 | 180 | [48] |
| nanofibers | poly(vinyl alcohol) (PVA) | template extrusion | 171 | [49] | |
| Leaf-like microbumps | poly(methyl methacrylate) (PMMA)/silica | uv-radiation | 163 | 4 | [158] |
| porous aerogel | graphene/polyvinylidene fluoride (G/PVDF) | solvothermal reduction | 153 | [159] | |
| hierarchical fabric films | poly(1,3,5,7-tetravinyl-1,3,5,7-tetramethylcyclotetrasiloxane) (p(V4D4)) layer and poly(1H,1H,2H,2H-perfluorodecylacrylate) (p(PFDA)) layer | initiated chemical vapor deposition (iCVD) | 154 | 2 | [160] |
| rambutan-like hollow sphere | polyaniline, perfluorooctane sulfonic acid (PFOSA) | self-assembly | 164.5 | [161] | |
| hierarchical porous structure | ethylenedioxythiophene (EDOT) | electrodeposition | 155 | [162] | |
| mesoporous/film | polyvinylidene fluoride (PVDF), mesoporous sub-micron carbon capsules (MCC) | dip-coating | 160 | 5 | [163] |
| bowl-like array structure | polyvinyl alcohol (PVA), silver, 1H,1H,2H,2H-perfluorodecanethiol | thermal evaporation, template transfer | 163 | 3 | [164] |
| fibrous texture | polypropylene, polyethylene | laminating exfoliation method | 156 | 5 | [165] |
| nanoscale spherical micelles | fluorinated acrylic copolymer | spray coating | 164 | 1.7 | [166] |
| honeycomb structure | poly(vinyl phenol)-block-polystyrene (PVPh-b-PS) | casting | 159 | [167] | |
| square-shaped pillar patterns | poly(1-methoxy-4-(O-disperse red 1)-2,5-bis(2-methoxyethyl) benzene) (PODR1) | laser microstructuring | 157 | [168] | |
| nanofibrous protrusions | polypropylene (PP), polyethylene (UHMWPE) | hot press lamination and peeling process | 158 | [169] | |
| micro-textured | ethyleneglycoldimethacrylate (EGDMA), tertbutyl methacrylate (TBMA), perfluorooctylethyl methacrylate (FMA) | UV light-triggered micro/ nanofabrication | 163 | 1 | [170] |
| hierarchical porous structure | divinylbenzene (DVB) and SiO2 composites | hydrothermalndolvent evaporation | 161.3 | 4 | [171] |
| nanoroughness-on- nanopillar hierarchical surfaces | polycarbonate (PC), perfluoropolyether (PFPE), C4F8 | nanoimprinting | 170 | 3 | [95] |
| microporous | polydimethylsiloxane (PDMS) and poly(methyl methacrylate) (PMMA) | spray-coating technique | 157.5 | 2.8 | [50] |
| nanofibers | poly(vinylidene fluoride) (PVDF) membranes | electrospinning | 171 | 1.5 | [172] |
| nanoporous | poly(2-hydroxyethyl methacrylate-co-ethylene dimethacrylate) (HEMA-EDMA) and 1H,1H,2H,2H-perfl uorodecanethiol | thiol-yne click-chemistry | 170 | 4.4 | [173] |
| fibrillary structure, cauliflower-like structures | 3,4-ethylenedioxythiophene (EDOT) | Staudinger–Vilarrasa reaction and electrodeposition | 154.5 | [174] | |
| Nano-fibrillary structure | 3,4-ethylenedioxythiophene (EDOT) | Huisgen reaction and electrodeposition | 159 | [175] |
| Biomemetic Structure | Material | Surface Modification Technique | Oils for Testing | Static Contact Angle (°) | Reference |
|---|---|---|---|---|---|
| nanofibers | polymethyl methacrylate (PMMA), fluoro polyhedral oligomeric silsesquioxane (POSS) | electrospin | Hexadecane decane | 110 145 | [60] |
| nanoparticle-covered cotton textiles | silica nano particle, cotton textiles, 1H,1H,2H, 2H-perfluorodecyltrichlorosilane | dip-coating | sunflower oil hexadecane | 140 135 | [78] |
| microfibers | polyester, fluorodecyl polyhedral oligomeric silsesquioxane (POSS) | dip-coating | grapeseed oil | 145 | [80] |
| nanoparticles | silica, sacrificial polystyrene, tridecafluoro-1,1,2,2,-tetrahydrooctyl trichlorosilane | UV-ozone treatment and dip-coating | hexadecane | 70 | [84] |
| diamond nanograss array | polycrystalline boron-doped film, 1H,1H,2H,2H-perfluorodecyltrichlorosilane | dip-coating | hexadecane | 100 | [81] |
| inverse-trapezoidal microstructure | polydimethylsiloxane, 1H,1H,2H,2H-perfluorodecyltrichlorosilane | plasma treatment vapor deposition | methanol | 135 | [75] |
| micropillars roughened with nanoparticels | fluorinated 3,4-ethylenedioxypyrrole | electrodeposition | Hexadecane sunflower oil dodecane | 144 153 135 | [70] |
| microbumps | fluorinated poly(3,4-ethylenedioxypyrrole) (PEDOP) derivatives | electrodeposition | hexadecane | 157 | [75] |
| mushroom-like micropillar | silicon on an insulator wafer, PDMS, perfluoropolyether, octafluorocyclobutane | vapor deposition | ethanol | 150 to 160 | [66] |
| overhang structure | poly (perfluorodecylacrylate)1H,1H,2H,2H-perfluorodecyl acrylate | oxygen plasma treatment vapor deposition | mineral oil | 110 | [207] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Cheng, F.; Zhao, B. Bio-Inspired Polymeric Structures with Special Wettability and Their Applications: An Overview. Polymers 2017, 9, 725. https://doi.org/10.3390/polym9120725
Pan Z, Cheng F, Zhao B. Bio-Inspired Polymeric Structures with Special Wettability and Their Applications: An Overview. Polymers. 2017; 9(12):725. https://doi.org/10.3390/polym9120725
Chicago/Turabian StylePan, Zihe, Fangqin Cheng, and Boxin Zhao. 2017. "Bio-Inspired Polymeric Structures with Special Wettability and Their Applications: An Overview" Polymers 9, no. 12: 725. https://doi.org/10.3390/polym9120725





