Bulk Heterojunction Solar Cells Based on Blends of Conjugated Polymers with II–VI and IV–VI Inorganic Semiconductor Quantum Dots
Abstract
:1. Introduction
2. Operation of a Hybrid Solar Cell
2.1. Overall Energy Conversion
2.2. Equivalent Circuit for Hybrid BHJ Solar Cells
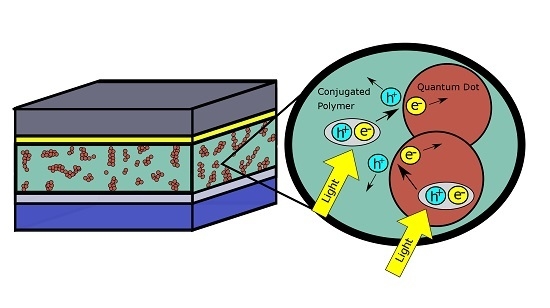
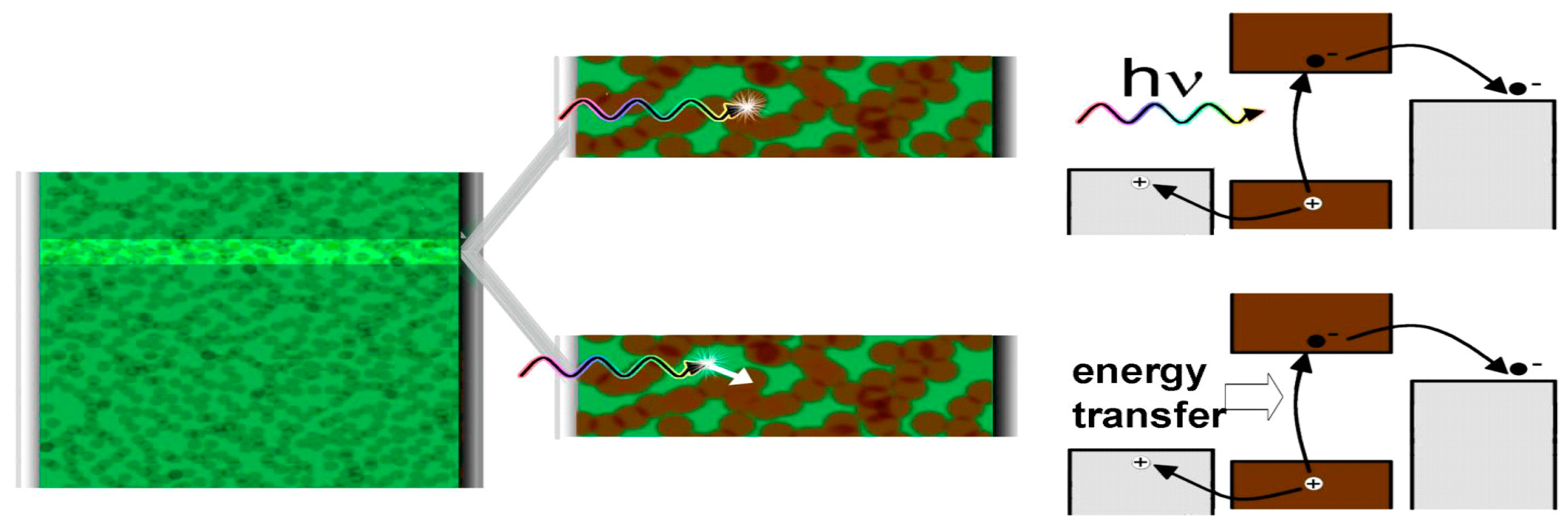
2.3. Photon Absorption and Formation of Excitons
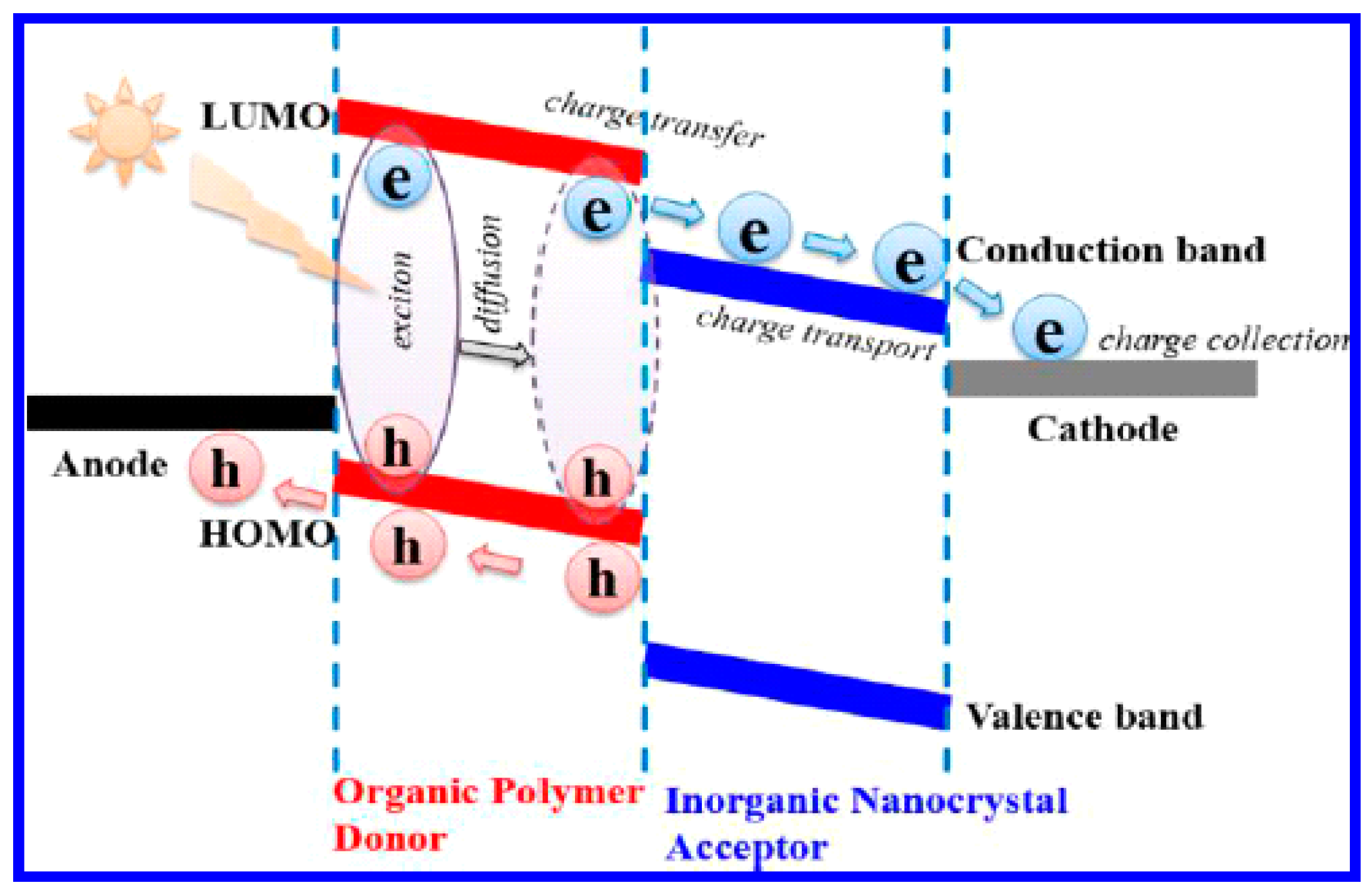
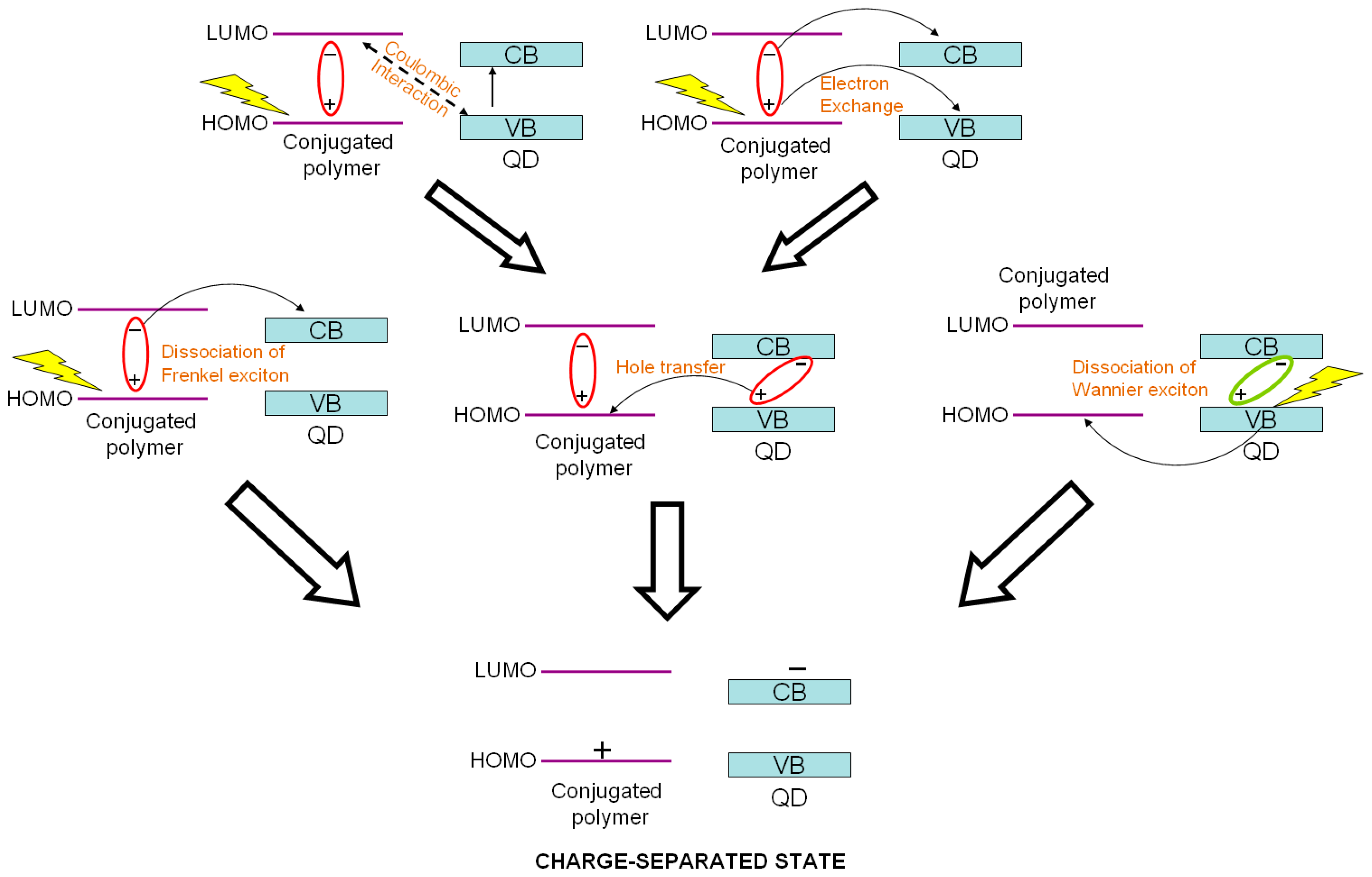
2.4. Charge Separation
2.5. Charge Collection
3. Material Processing and Synthesis-Related Aspects
3.1. Conjugated Polymers
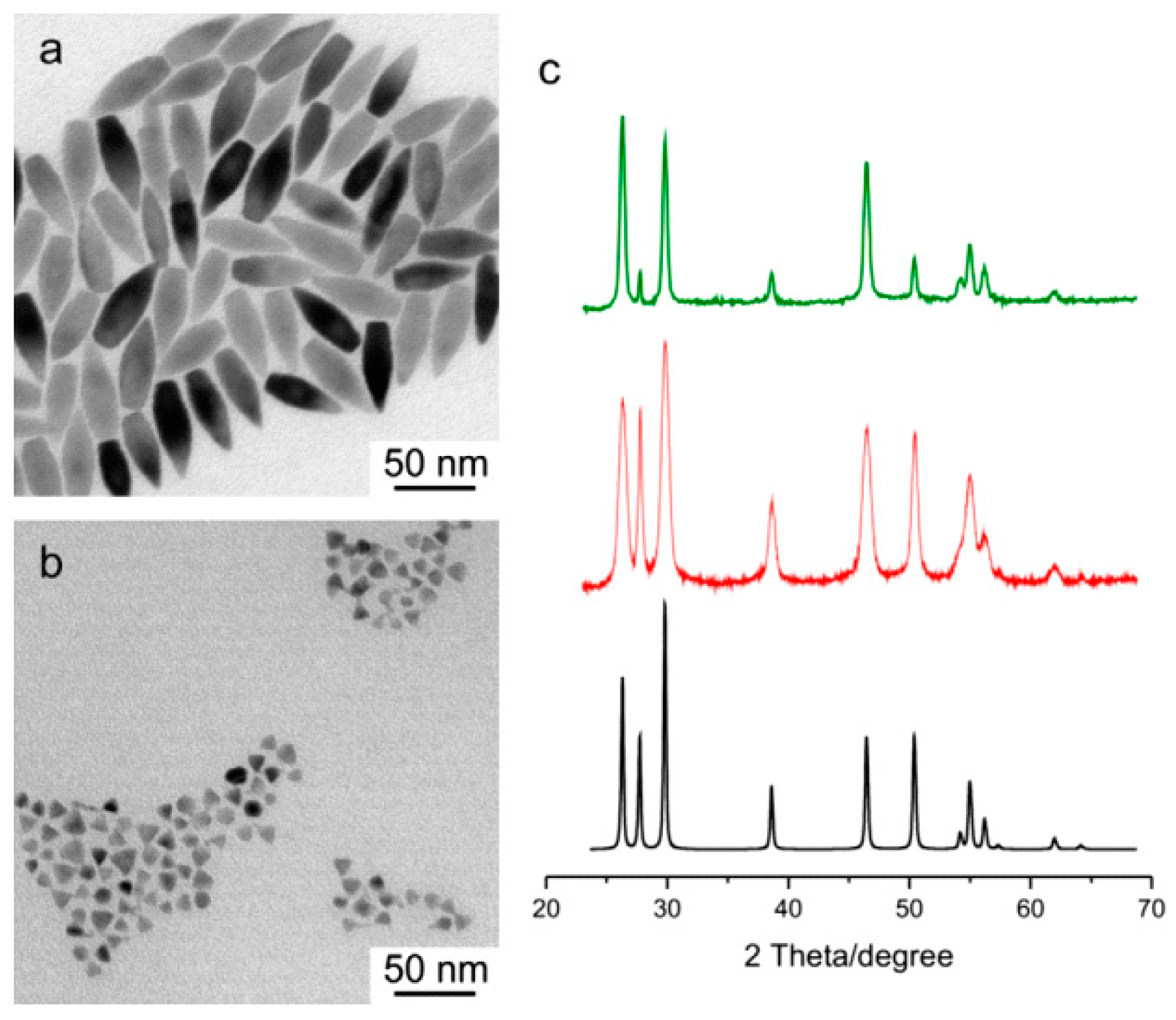
3.2. Quantum Dots
4. Design Considerations
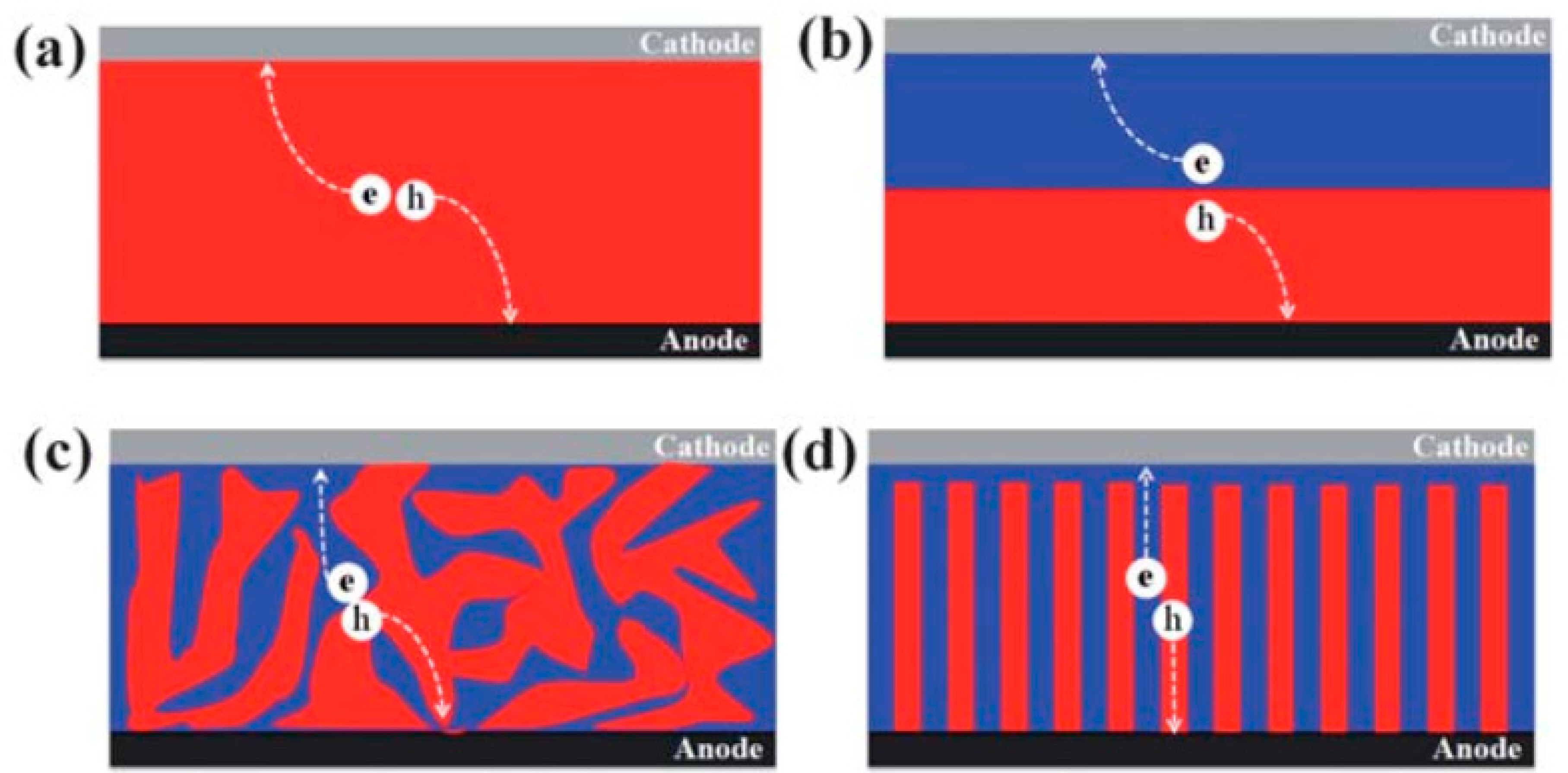
4.1. Film Morphology
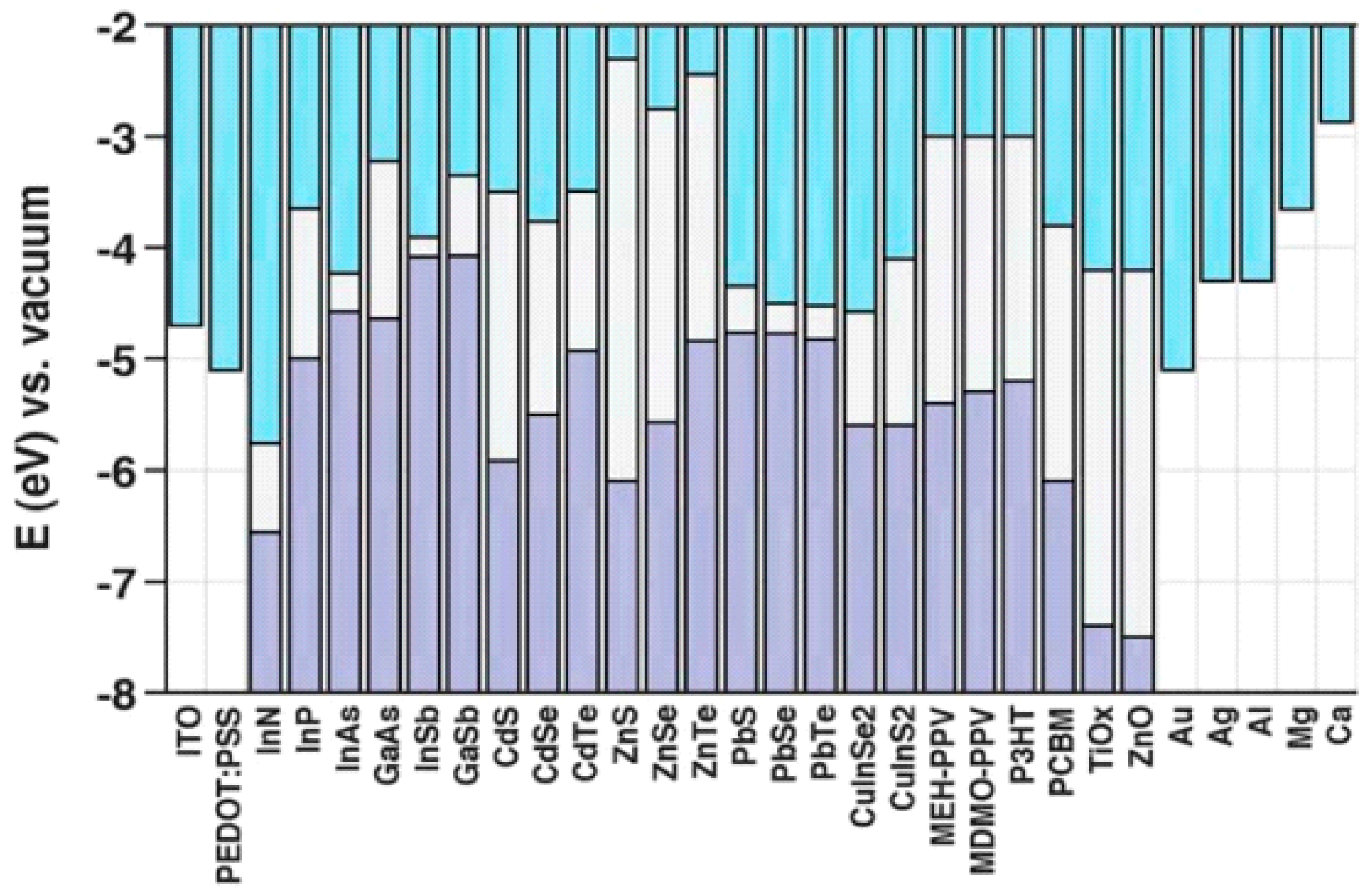
4.2. Material Selection
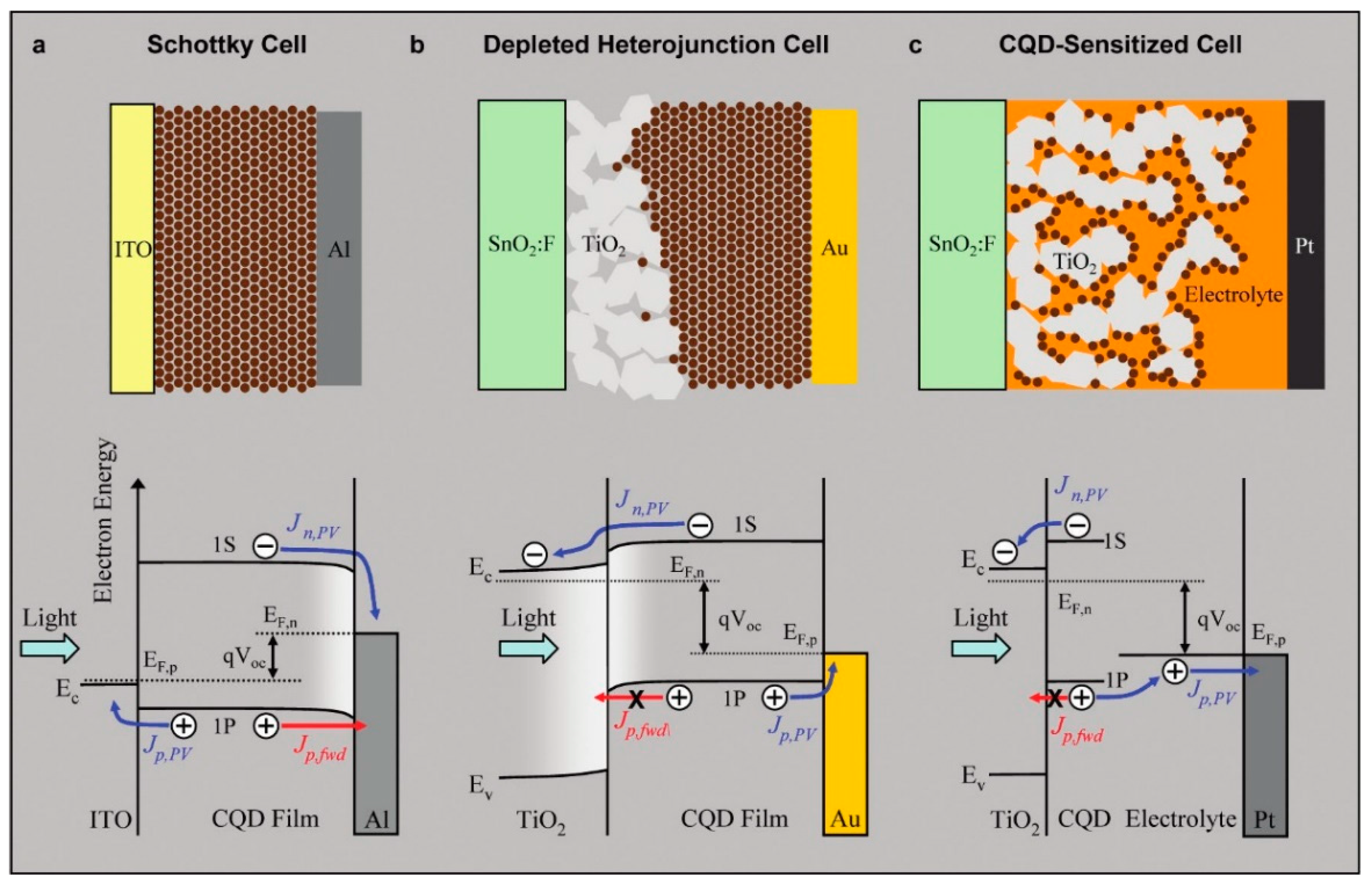
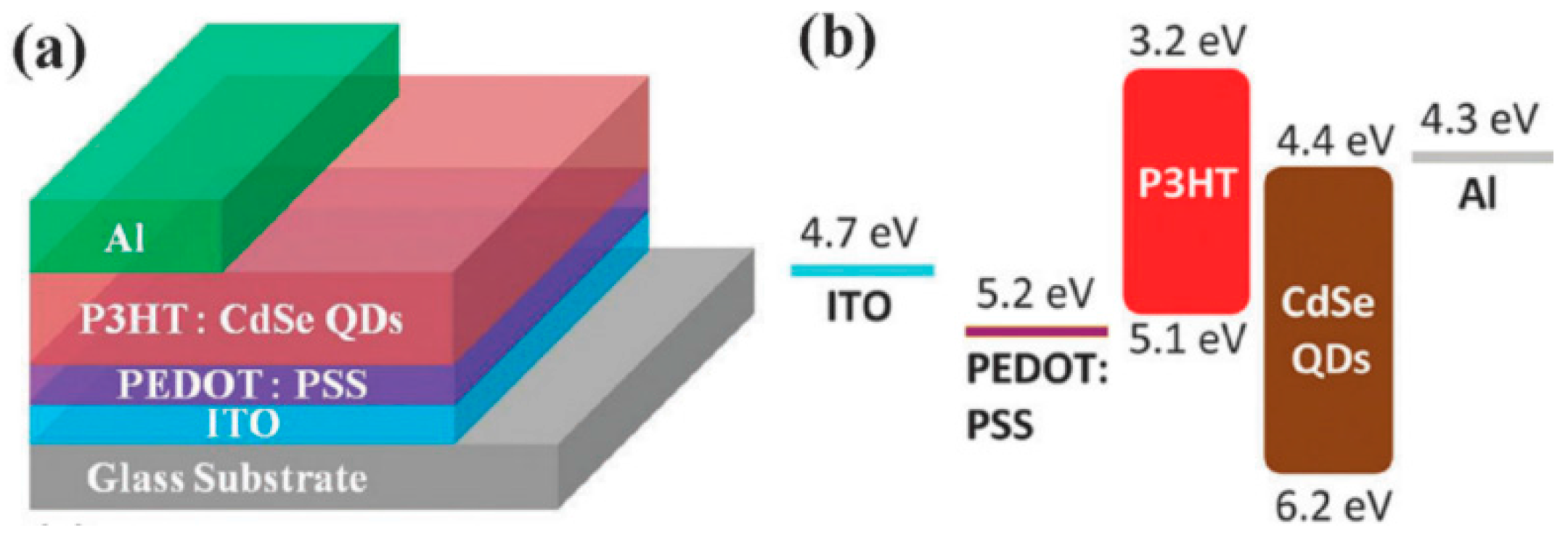
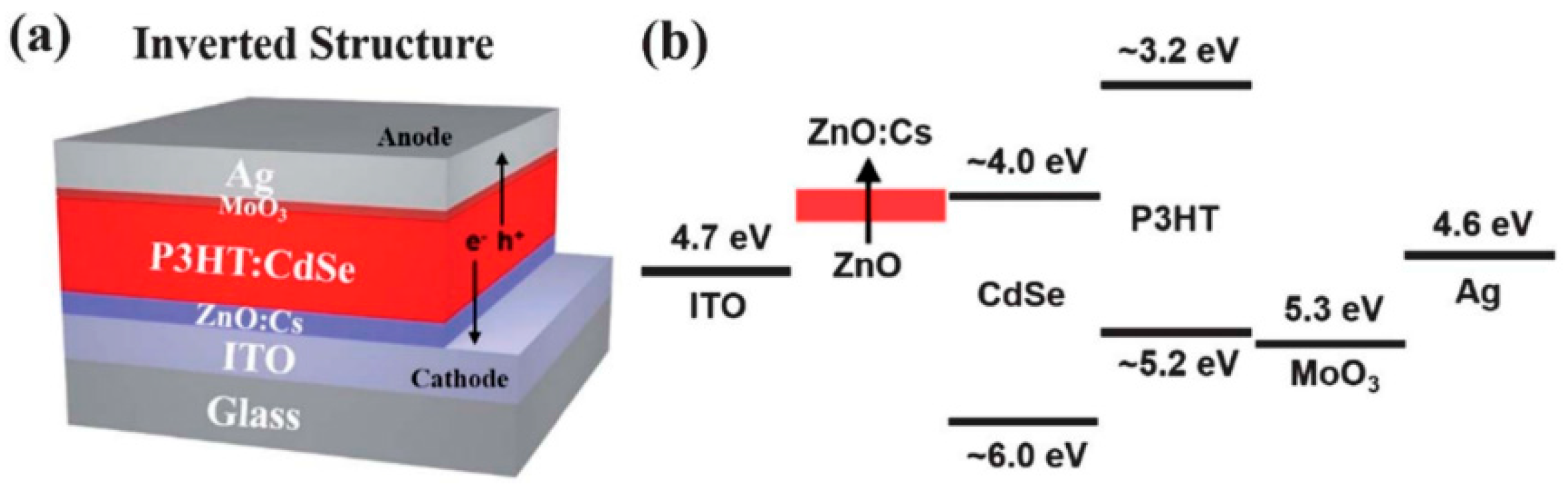
4.3. Device Architecture and Fabrication
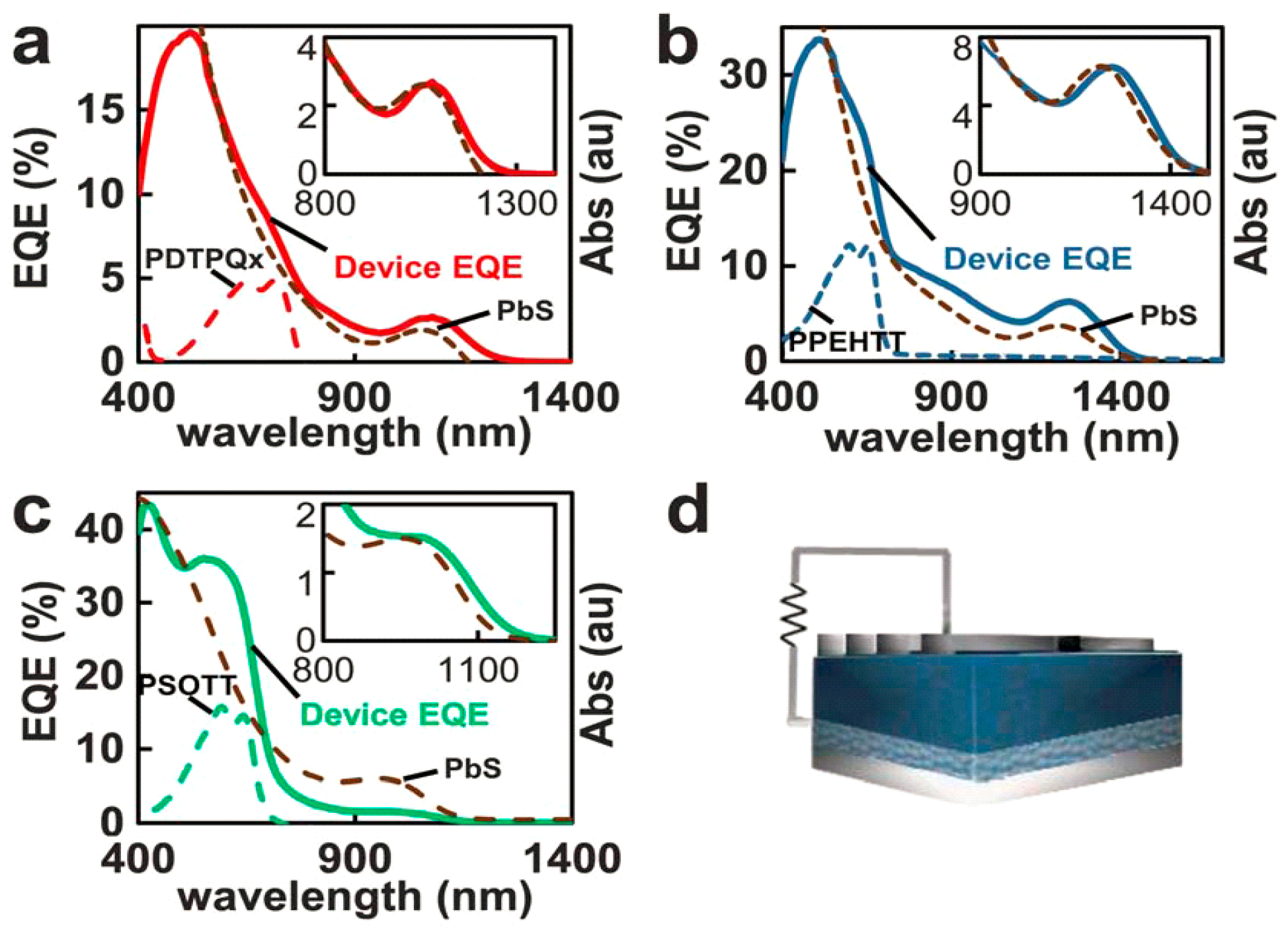
4.4. Device Performance
5. Factors Limiting the Quantum Yield and Overall Power Conversion Efficiency in QD-Polymer Hybrid Solar Cells
5.1. Poor Interfacial Electronic Coupling Due to Stabilizing Ligands
5.2. Local Crystallinity
5.3. Traps
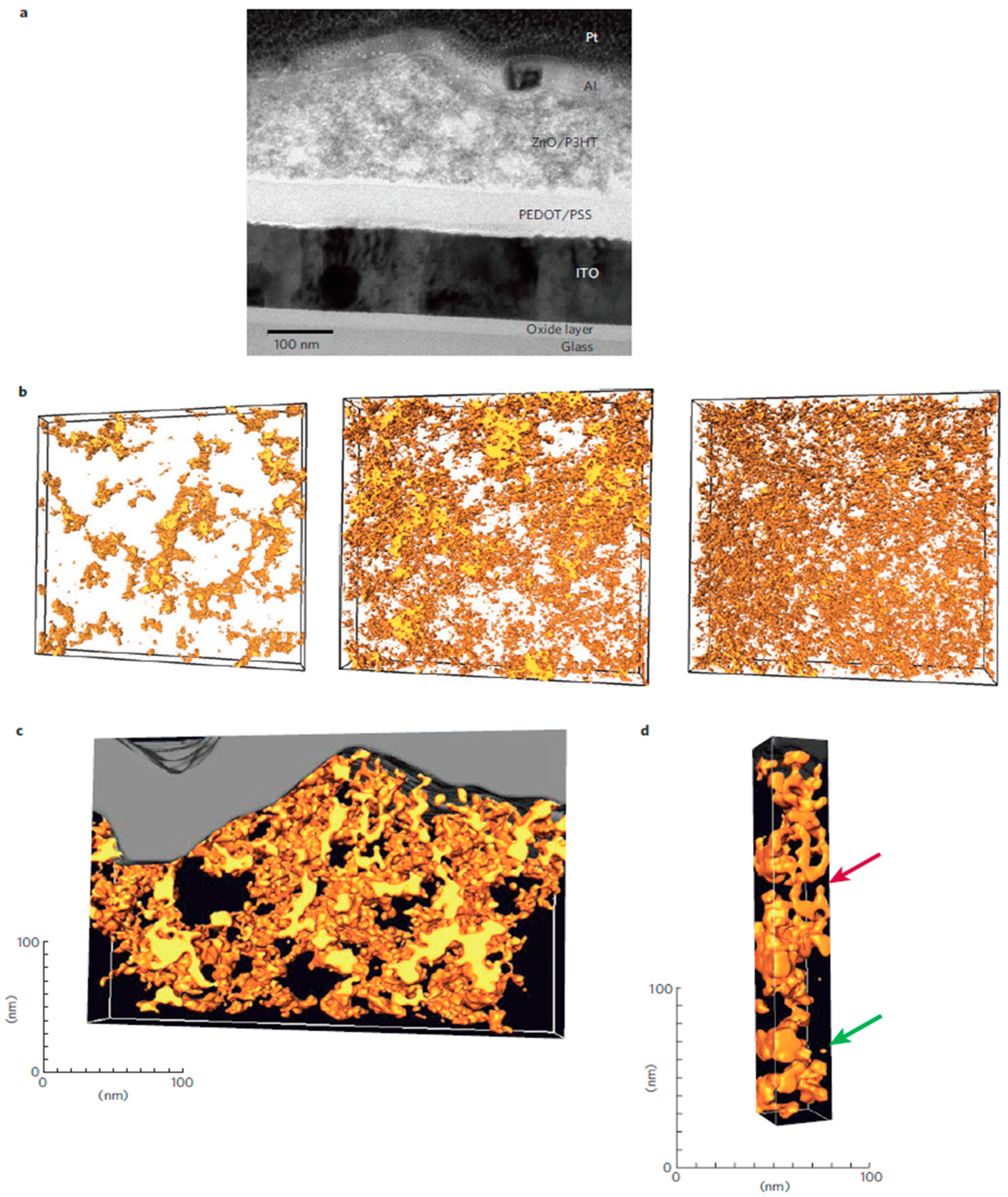
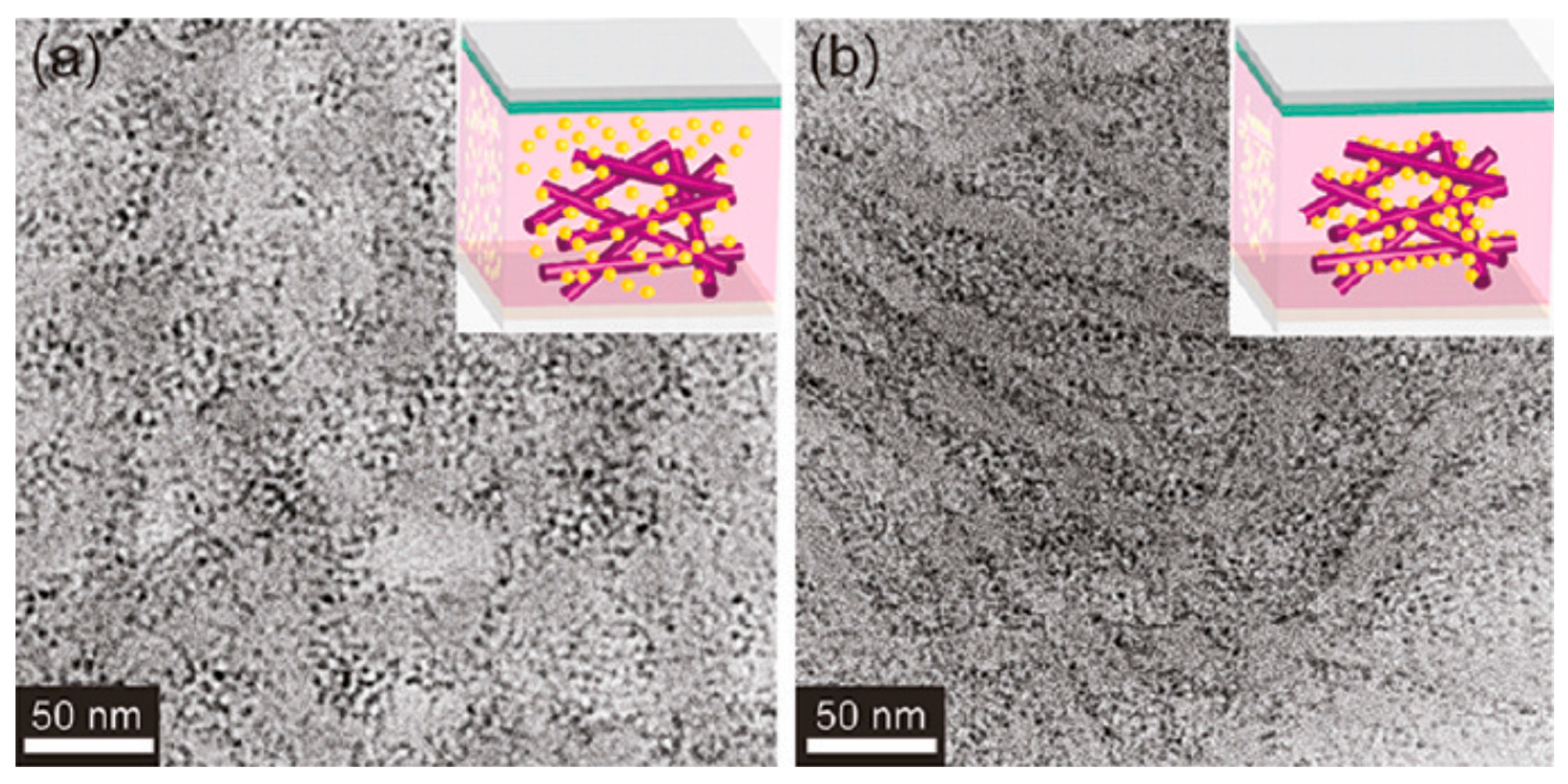
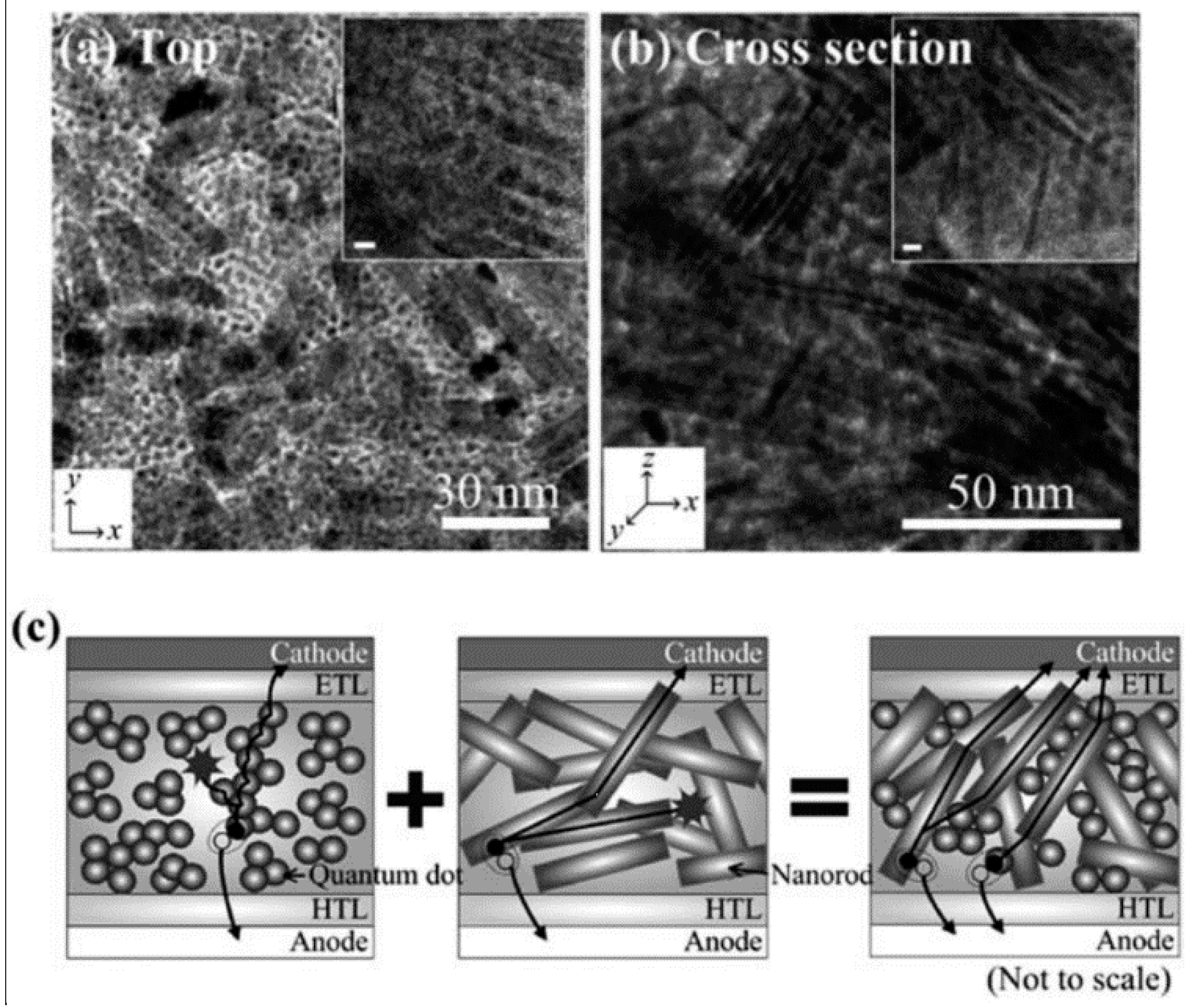
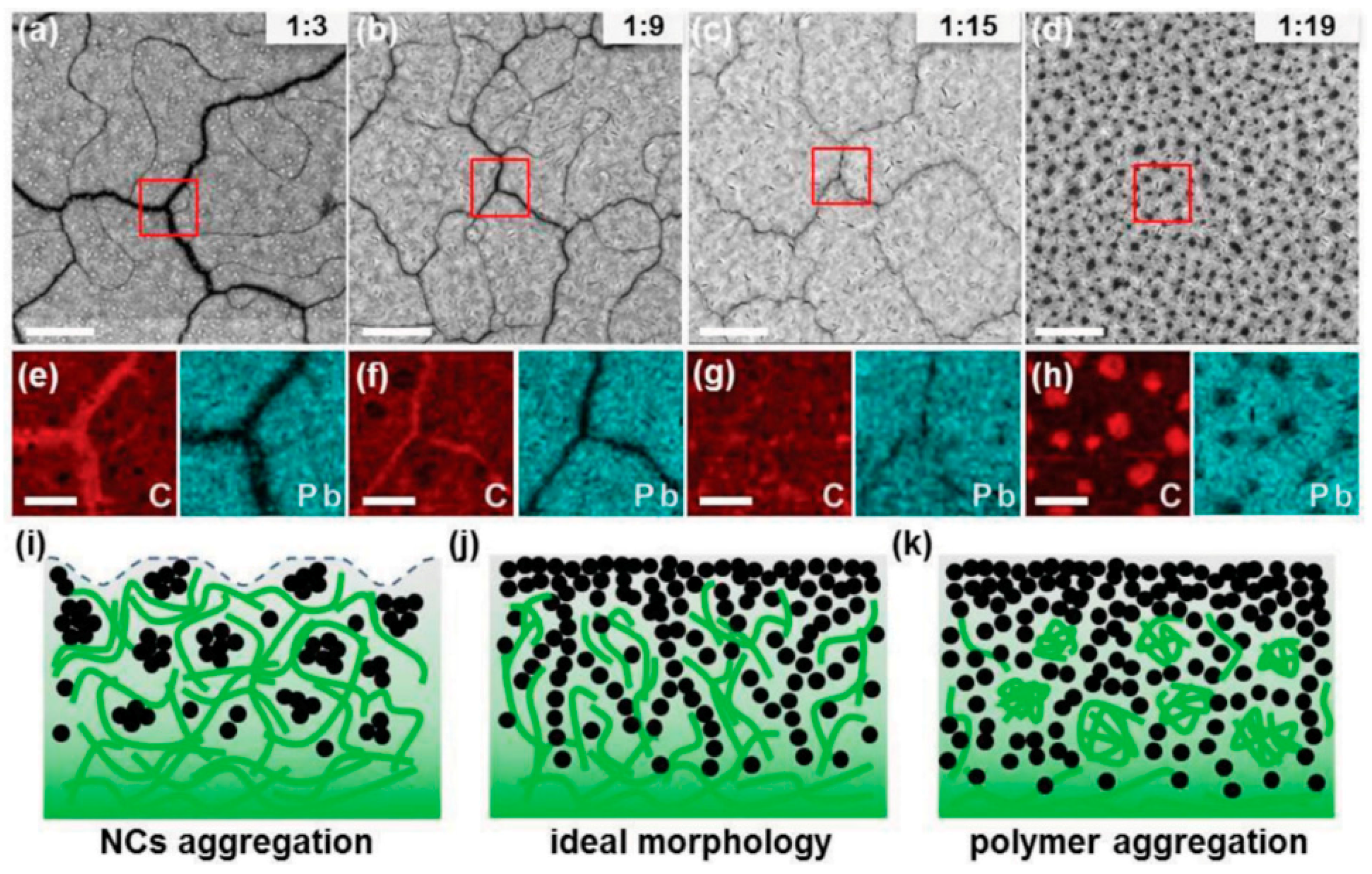
5.4. Achieving a Bicontinuous Percolation Network in Blends of QDs with Conjugated Polymers
6. Proposed and Realized Solutions to the Factors Limiting Performance in QD–Polymer Hybrid Solar Cells
6.1. Interfacial Engineering
6.2. Achieving a Bicontinuous Percolation Network in Blends of QDs with Conjugated Polymers
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Conibeer, G.; Green, M.; Corkish, R.; Cho, Y.; Cho, E.-C.; Jiang, C.-W.; Fangsuwannarak, T.; Pink, E.; Huang, Y.; Puzzer, T.; et al. Silicon nanostructures for third generation photovoltaic solar cells. Thin Solid Films 2006, 511–512, 654–662. [Google Scholar] [CrossRef]
- Bagnall, D.M.; Boreland, M. Photovoltaic technologies. Energy Policy 2008, 36, 4390–4396. [Google Scholar] [CrossRef]
- Dubey, A.; Saini, P.; Qiao, Q. Conjugated polymers-based blends, composites and copolymers for photovoltaics. In Fundamentals of Conjugated Polymer Blends, Copolymers and Composites; John Wiley & Sons, Inc.: New York, NY, USA, 2015; pp. 281–338. [Google Scholar]
- Snaith, H.J. Perovskites: The emergence of a new era for low-cost, high-efficiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Devižis, A.; de Jonghe-Risse, J.; Hany, R.; Nüesch, F.; Jenatsch, S.; Gulbinas, V.; Moser, J.-E. Dissociation of charge transfer states and carrier separation in bilayer organic solar cells: A time-resolved electroabsorption spectroscopy study. J. Am. Chem. Soc. 2015, 137, 8192–8198. [Google Scholar] [CrossRef] [PubMed]
- Bakulin, A.A.; Rao, A.; Pavelyev, V.G.; van Loosdrecht, P.H.M.; Pshenichnikov, M.S.; Niedzialek, D.; Cornil, J.; Beljonne, D.; Friend, R.H. The role of driving energy and delocalized states for charge separation in organic semiconductors. Science 2012, 335, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Peumans, P.; Uchida, S.; Forrest, S.R. Efficient bulk heterojunction photovoltaic cells using small-molecular-weight organic thin films. Nature 2003, 425, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Solar cell efficiency tables (version 45). Prog. Photovolt. Res. Appl. 2015, 23, 1–9. [Google Scholar] [CrossRef]
- Guerrero, A.; Heidari, H.; Ripolles, T.S.; Kovalenko, A.; Pfannmöller, M.; Bals, S.; Kauffmann, L.-D.; Bisquert, J.; Garcia-Belmonte, G. Shelf life degradation of bulk heterojunction solar cells: Intrinsic evolution of charge transfer complex. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Guerrero, A.; Boix, P.P.; Marchesi, L.F.; Ripolles-Sanchis, T.; Pereira, E.C.; Garcia-Belmonte, G. Oxygen doping-induced photogeneration loss in P3HT:PCBM solar cells. Sol. Energy Mater. Sol. Cells 2012, 100, 185–191. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; Macdiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 2014, 5, 5293. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Fafarman, A.T.; Oh, S.J.; Ko, D.-K.; Kim, D.K.; Diroll, B.T.; Muramoto, S.; Gillen, J.G.; Murray, C.B.; Kagan, C.R. Bandlike transport in strongly coupled and doped quantum dot solids: A route to high-performance thin-film electronics. Nano Lett. 2012, 12, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
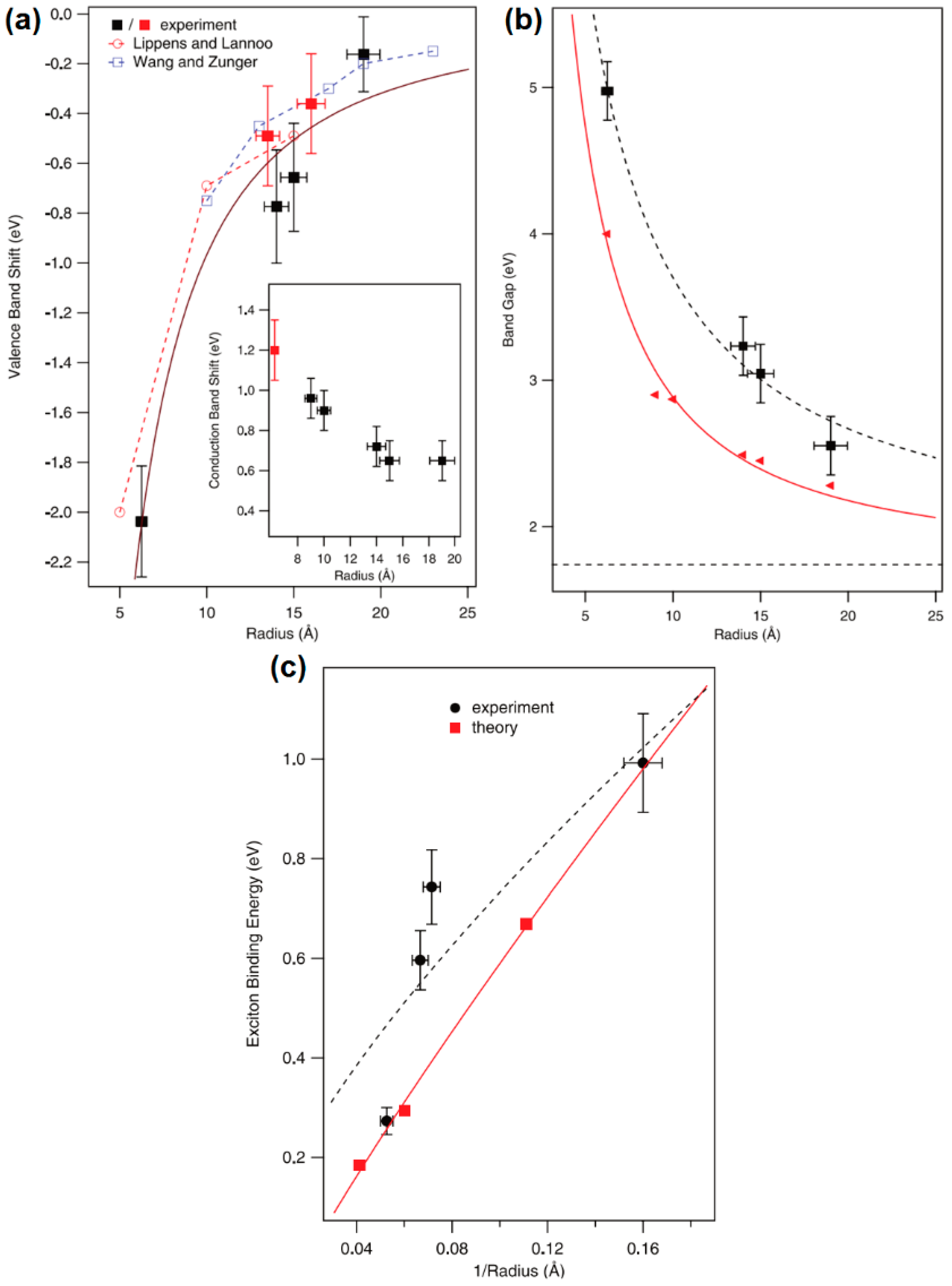
- Knupfer, M. Exciton binding energies in organic semiconductors. Appl. Phys. A 2003, 77, 623–626. [Google Scholar] [CrossRef]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Wise, F.W. Lead salt quantum dots: The limit of strong quantum confinement. Acc. Chem. Res. 2000, 33, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M.; Kamat, P.V. Quantum dot solar cells. Tuning photoresponse through size and shape control of CdSe−TiO2 architecture. J. Am. Chem. Soc. 2008, 130, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H.; Colbert, A.E.; Strein, E.; Janke, E.M.; Salvador, M.; Schlenker, C.W.; Ginger, D.S. Size-dependent charge transfer yields in conjugated polymer/quantum dot blends. J. Phys. Chem. C 2014, 118, 5710–5715. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, Y.; Wu, H.; Yan, L.; Wang, Z.G.; Zhao, J.; Yu, W.W.; Rogach, A.L. Pbse quantum dot films with enhanced electron mobility employed in hybrid polymer/nanocrystal solar cells. RSC Adv. 2016, 6, 17029–17035. [Google Scholar] [CrossRef]
- Tan, L.C.; Shi, Y.Q.; Chen, Y.W. Assembly of quantum dots in polymer solar cells driven by orientational switching of mesogens under electric field. Sol. Energy 2016, 129, 184–191. [Google Scholar] [CrossRef]
- Lu, H.P.; Joy, J.; Gaspar, R.L.; Bradforth, S.E.; Brutchey, R.L. Lodide-passivated colloidal pbs nanocrystals leading to highly efficient polymer:Nanocrystal hybrid solar cells. Chem. Mater. 2016, 28, 1897–1906. [Google Scholar] [CrossRef]
- Yuan, J.Y.; Gallagher, A.; Liu, Z.K.; Sun, Y.X.; Ma, W.L. High-efficiency polymer-PbS hybrid solar cells via molecular engineering. J. Mater. Chem. A 2015, 3, 2572–2579. [Google Scholar] [CrossRef]
- Tayakoli, M.M.; Aashuri, H.; Simchi, A.; Kalytchuk, S.; Fan, Z.Y. Quasi core/shell lead sulfide/graphene quantum dots for bulk heterojunction solar cells. J. Phys. Chem. C 2015, 119, 18886–18895. [Google Scholar] [CrossRef]
- Sun, Y.X.; Liu, Z.K.; Yuan, J.Y.; Chen, J.M.; Zhou, Y.; Huang, X.D.; Ma, W.L. Polymer selection toward efficient polymer/PbSe planar heterojunction hybrid solar cells. Org. Electron. 2015, 24, 263–271. [Google Scholar] [CrossRef]
- Chen, Z.L.; Liu, F.Y.; Zeng, Q.S.; Cheng, Z.K.; Du, X.H.; Jin, G.; Zhang, H.; Yang, B. Efficient aqueous-processed hybrid solar cells from a polymer with a wide bandgap. J. Mater. Chem. A 2015, 3, 10969–10975. [Google Scholar] [CrossRef]
- Kim, K.M.; Jeon, J.H.; Kim, Y.Y.; Lee, H.K.; Park, O.O.; Wang, D.H. Effects of ligand exchanged CdSe quantum dot interlayer for inverted organic solar cells. Org. Electron. 2015, 25, 44–49. [Google Scholar] [CrossRef]
- Yao, S.; Chen, Z.; Li, F.; Xu, B.; Song, J.; Yan, L.; Jin, G.; Wen, S.; Wang, C.; Yang, B.; et al. High-efficiency aqueous-solution-processed hybrid solar cells based on P3HT dots and CdTe nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 7146–7152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Y.; Yuan, J.; Wei, H.; Huang, X.; Han, L.; Wang, W.; Wang, H.; Ma, W. High-efficiency hybrid solar cells based on polymer/pbsxse1-x nanocrystals benefiting from vertical phase segregation. Adv. Mater. 2013, 25, 5772–5778. [Google Scholar] [CrossRef] [PubMed]
- Leatherdale, C.A.; Woo, W.K.; Mikulec, F.V.; Bawendi, M.G. On the absorption cross section of CdSe nanocrystal quantum dots. J. Phys. Chem. B 2002, 106, 7619–7622. [Google Scholar] [CrossRef]
- Guldi, D.M.; Prato, M. Excited-state properties of C60 fullerene derivatives. Acc. Chem. Res. 2000, 33, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Peet, J.; Hu, S.; Azoulay, J.; Bazan, G.; Dadmun, M. The role of fullerene mixing behavior in the performance of organic photovoltaics: Pcbm in low-bandgap polymers. Adv. Funct. Mater. 2014, 24, 140–150. [Google Scholar] [CrossRef]
- Strein, E.; Colbert, A.; Subramaniyan, S.; Nagaoka, H.; Schlenker, C.W.; Janke, E.; Jenekhe, S.A.; Ginger, D.S. Charge generation and energy transfer in hybrid polymer/infrared quantum dot solar cells. Energy Environ. Sci. 2013, 6, 769–775. [Google Scholar] [CrossRef]
- Pattantyus-Abraham, A.G.; Kramer, I.J.; Barkhouse, A.R.; Wang, X.; Konstantatos, G.; Debnath, R.; Levina, L.; Raabe, I.; Nazeeruddin, M.K.; Grätzel, M.; et al. Depleted-heterojunction colloidal quantum dot solar cells. ACS Nano 2010, 4, 3374–3380. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Qiu, F.; Lin, Z. Toward high-performance organic-inorganic hybrid solar cells: Bringing conjugated polymers and inorganic nanocrystals in close contact. J. Phys. Chem. Lett. 2013, 4, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Reiss, P.; Couderc, E.; De Girolamo, J.; Pron, A. Conjugated polymers/semiconductor nanocrystals hybrid materials-preparation, electrical transport properties and applications. Nanoscale 2011, 3, 446–489. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, Y.; Miranti, R.; Fron, E.; Khetubol, A.; Vandenplas, E.; Cheyns, D.; Borchert, H.; Parisi, J.; van der Auweraer, M. Charge separation dynamics at bulk heterojunctions between poly(3-hexylthiophene) and pbs quantum dots. J. Appl. Phys. 2015, 118, 055502. [Google Scholar] [CrossRef]
- Noone, K.M.; Strein, E.; Anderson, N.C.; Wu, P.-T.; Jenekhe, S.A.; Ginger, D.S. Broadband absorbing bulk heterojunction photovoltaics using low-bandgap solution-processed quantum dots. Nano Lett. 2010, 10, 2635–2639. [Google Scholar] [CrossRef] [PubMed]
- Mastria, R.; Rizzo, A.; Giansante, C.; Ballarini, D.; Dominici, L.; Inganaes, O.; Gigli, G. Role of polymer in hybrid polymer/PbS quantum dot solar cells. J. Phys. Chem. C 2015, 119, 14972–14979. [Google Scholar] [CrossRef]
- Barford, W. Excitons in conjugated polymers: A tale of two particles. J. Phys. Chem. A 2013, 117, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Meulenberg, R.W.; Lee, J.R.I.; Wolcott, A.; Zhang, J.Z.; Terminello, L.J.; van Buuren, T. Determination of the exciton binding energy in cdse quantum dots. ACS Nano 2009, 3, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Forster, T. 10th spiers memorial lecture. Transfer mechanisms of electronic excitation. Discuss. Faraday Soc. 1959, 27, 7–17. [Google Scholar] [CrossRef]
- Dexter, D.L. A theory of sensitized luminescence in solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Patra, A. Interactions of π-conjugated polymers with inorganic nanocrystals. J. Photochem. Photobiol. C 2014, 20, 51–70. [Google Scholar] [CrossRef]
- Couderc, E.; Greaney, M.J.; Brutchey, R.L.; Bradforth, S.E. Direct spectroscopic evidence of ultrafast electron transfer from a low band gap polymer to cdse quantum dots in hybrid photovoltaic thin films. J. Am. Chem. Soc. 2013, 135, 18418–18426. [Google Scholar] [CrossRef] [PubMed]
- Lutich, A.A.; Jiang, G.; Susha, A.S.; Rogach, A.L.; Stefani, F.D.; Feldmann, J. Energy transfer versus charge separation in type-ii hybrid organic−inorganic nanocomposites. Nano Lett. 2009, 9, 2636–2640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Z. Direct observation of the size dependence of dexter energy transfer from polymer to small pbs quantum dots. Appl. Phys. Lett. 2008, 93, 083106. [Google Scholar] [CrossRef]
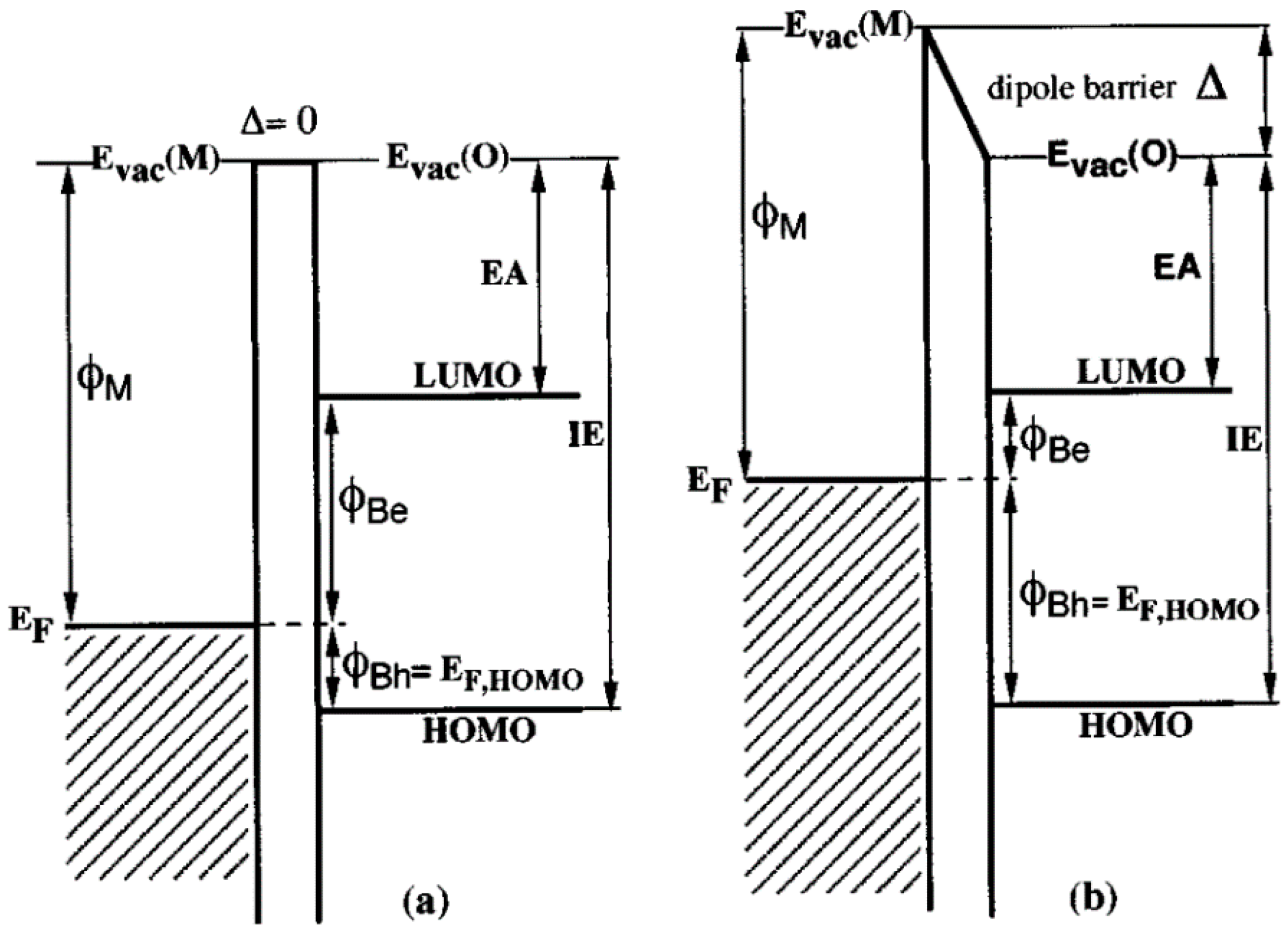
- Hill, I.G.; Rajagopal, A.; Kahn, A.; Hu, Y. Molecular level alignment at organic semiconductor-metal interfaces. Appl. Phys. Lett. 1998, 73, 662–664. [Google Scholar] [CrossRef]
- Seo, J.H.; Gutacker, A.; Sun, Y.; Wu, H.; Huang, F.; Cao, Y.; Scherf, U.; Heeger, A.J.; Bazan, G.C. Improved high-efficiency organic solar cells via incorporation of a conjugated polyelectrolyte interlayer. J. Am. Chem. Soc. 2011, 133, 8416–8419. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Shi, Y.; Qiu, W.; Wang, L.; Nan, Y.; Shi, M.; Li, H.; Chen, H. High efficiency hybrid solar cells using post-deposition ligand exchange by monothiols. Phys. Chem. Chem. Phys. 2012, 14, 12094–12098. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Lim, K.-G.; Shim, M.; Moon, H.C.; Park, J.; Jeon, G.; Shin, J.; Cho, K.; Lee, T.-W.; Kim, J.K. Air-stable inverted structure of hybrid solar cells using a cesium-doped ZnO electron transport layer prepared by a sol-gel process. J. Mater. Chem. A 2013, 1, 11802–11808. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and metallic polymers: The fourth generation of polymeric materials (nobel lecture). Angew. Chem. Int. Ed. 2001, 40, 2591–2611. [Google Scholar] [CrossRef]
- Xu, T.; Yu, L. How to design low bandgap polymers for highly efficient organic solar cells. Mater. Today 2014, 17, 11–15. [Google Scholar] [CrossRef]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid nanorod-polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef] [PubMed]
- Askar, A.M.; Shankar, K. Exciton binding energy in organic-inorganic tri-halide perovskites. J. Nanosci. Nanotechnol. 2016, 16, 5890–5901. [Google Scholar] [CrossRef] [PubMed]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341. [Google Scholar] [CrossRef]
- Kettle, J.; Horie, M.; Majewski, L.A.; Saunders, B.R.; Tuladhar, S.; Nelson, J.; Turner, M.L. Optimisation of pcpdtbt solar cells using polymer synthesis with suzuki coupling. Sol. Energy Mater. Sol. Cells 2011, 95, 2186–2193. [Google Scholar] [CrossRef]
- Saunders, B.R. Hybrid polymer/nanoparticle solar cells: Preparation, principles and challenges. J. Colloid Interface Sci. 2012, 369, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; McCullough, R.D. End group modification of regioregular polythiophene through postpolymerization functionalization. Macromolecules 2002, 35, 9882–9889. [Google Scholar] [CrossRef]
- Hines, M.A.; Scholes, G.D. Colloidal PbS nanocrystals with size-tunable near-infrared emission: Observation of post-synthesis self-narrowing of the particle size distribution. Adv. Mater. 2003, 15, 1844–1849. [Google Scholar] [CrossRef]
- Buhro, W.E.; Colvin, V.L. Semiconductor nanocrystals: Shape matters. Nat. Mater. 2003, 2, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.E.; Beard, M.C.; Norman, A.G.; Ahrenkiel, S.P.; Johnson, J.C.; Yu, P.; Mićić, O.I.; Ellingson, R.J.; Nozik, A.J. Pbte colloidal nanocrystals: Synthesis, characterization, and multiple exciton generation. J. Am. Chem. Soc. 2006, 128, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Nozik, A.J. Multiple exciton generation in semiconductor quantum dots. Chem. Phys. Lett. 2008, 457, 3–11. [Google Scholar] [CrossRef]
- Nozik, A.J.; Beard, M.C.; Luther, J.M.; Law, M.; Ellingson, R.J.; Johnson, J.C. Semiconductor quantum dots and quantum dot arrays and applications of multiple exciton generation to third-generation photovoltaic solar cells. Chem. Rev. 2010, 110, 6873–6890. [Google Scholar] [CrossRef] [PubMed]
- Beard, M.C.; Midgett, A.G.; Hanna, M.C.; Luther, J.M.; Hughes, B.K.; Nozik, A.J. Comparing multiple exciton generation in quantum dots to impact ionization in bulk semiconductors: Implications for enhancement of solar energy conversion. Nano Lett. 2010, 10, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Kambhampati, P. False multiple exciton recombination and multiple exciton generation signals in semiconductor quantum dots arise from surface charge trapping. J. Chem. Phys. 2011, 134, 094706. [Google Scholar] [CrossRef] [PubMed]
- Žídek, K.; Zheng, K.; Abdellah, M.; Lenngren, N.; Chábera, P.; Pullerits, T. Ultrafast dynamics of multiple exciton harvesting in the CdSe–ZnO system: Electron injection versus auger recombination. Nano Lett. 2012, 12, 6393–6399. [Google Scholar] [CrossRef] [PubMed]
- de Mello Donegá, C.; Liljeroth, P.; Vanmaekelbergh, D. Physicochemical evaluation of the hot-injection method, a synthesis route for monodisperse nanocrystals. Small 2005, 1, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Radychev, N.; Scheunemann, D.; Kruszynska, M.; Frevert, K.; Miranti, R.; Kolny-Olesiak, J.; Borchert, H.; Parisi, J. Investigation of the morphology and electrical characteristics of hybrid blends based on poly(3-hexylthiophene) and colloidal cuins2 nanocrystals of different shapes. Org. Electron. 2012, 13, 3154–3164. [Google Scholar] [CrossRef]
- Oosterhout, S.D.; Wienk, M.M.; van Bavel, S.S.; Thiedmann, R.; Koster, L.J.A.; Gilot, J.; Loos, J.; Schmidt, V.; Janssen, R.A.J. The effect of three-dimensional morphology on the efficiency of hybrid polymer solar cells. Nat. Mater. 2009, 8, 818–824. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Qiu, F.; Lin, Z. Conjugated rod-coil and rod-rod block copolymers for photovoltaic applications. J. Mater. Chem. 2011, 21, 17039–17048. [Google Scholar] [CrossRef]
- Sun, Y.; Chien, S.-C.; Yip, H.-L.; Chen, K.-S.; Zhang, Y.; Davies, J.A.; Chen, F.-C.; Lin, B.; Jen, A.K.Y. Improved thin film morphology and bulk-heterojunction solar cell performance through systematic tuning of the surface energy of conjugated polymers. J. Mater. Chem. 2012, 22, 5587–5595. [Google Scholar] [CrossRef]
- Peet, J.; Kim, J.Y.; Coates, N.E.; Ma, W.L.; Moses, D.; Heeger, A.J.; Bazan, G.C. Efficiency enhancement in low-bandgap polymer solar cells by processing with alkane dithiols. Nat. Mater. 2007, 6, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Hailegnaw, B.; Adam, G.; Yohannes, T. Effect of short chain iodoalkane solvent additives on photovoltaic performance of poly(3-hexylthiophene) and phenyl-c61-butyric acid methyl ester based bulk heterojunction solar cells. Thin Solid Films 2015, 589, 272–277. [Google Scholar] [CrossRef]
- Lee, J.K.; Ma, W.L.; Brabec, C.J.; Yuen, J.; Moon, J.S.; Kim, J.Y.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing additives for improved efficiency from bulk heterojunction solar cells. J. Am. Chem. Soc. 2008, 130, 3619–3623. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-M.; Xu, Z.; Hong, Z.; Yang, Y. Interface investigation and engineering—Achieving high performance polymer photovoltaic devices. J. Mater. Chem. 2010, 20, 2575–2598. [Google Scholar] [CrossRef]
- Das, S.; Alford, T.L. Optimization of the zinc oxide electron transport layer in P3HT:PC61BM based organic solar cells by annealing and yttrium doping. RSC Adv. 2015, 5, 45586–45591. [Google Scholar] [CrossRef]
- Kemerink, M.; Timpanaro, S.; de Kok, M.M.; Meulenkamp, E.A.; Touwslager, F.J. Three-dimensional inhomogeneities in pedot:Pss films. J. Phys. Chem. B 2004, 108, 18820–18825. [Google Scholar] [CrossRef]
- Ren, S.; Chang, L.-Y.; Lim, S.-K.; Zhao, J.; Smith, M.; Zhao, N.; Bulović, V.; Bawendi, M.; Gradečak, S. Inorganic–organic hybrid solar cell: Bridging quantum dots to conjugated polymer nanowires. Nano Lett. 2011, 11, 3998–4002. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Cho, M.J.; Lee, D.; Cartwright, A.N.; Prasad, P.N. Efficient heterojunction photovoltaic cell utilizing nanocomposites of lead sulfide nanocrystals and a low-bandgap polymer. Adv. Mater. 2011, 23, 3984–3988. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Luther, J.M.; Zheng, H.; Wu, Y.; Alivisatos, A.P. Photovoltaic devices employing ternary pbsxse1-x nanocrystals. Nano Lett. 2009, 9, 1699–1703. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-H.; García de Arquer, F.P.; Yoon, Y.J.; Lan, X.; Liu, M.; Voznyy, O.; Yang, Z.; Fan, F.; Ip, A.H.; Kanjanaboos, P.; et al. High-efficiency colloidal quantum dot photovoltaics via robust self-assembled monolayers. Nano Lett. 2015, 15, 7691–7696. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Dou, L.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.-C.; Gao, J.; Li, G.; et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4, 1446. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Wang, L.; Zhang, Y.; Ma, R.; Zuo, L.; Mai, J.; Lau, T.-K.; Du, S.; Lu, X.; Shi, M.; et al. Improving polymer/nanocrystal hybrid solar cell performance via tuning ligand orientation at cdse quantum dot surface. ACS Appl. Mater. Interfaces 2014, 6, 19154–19160. [Google Scholar] [CrossRef] [PubMed]
- Munro, A.M.; Ginger, D.S. Photoluminescence quenching of single cdse nanocrystals by ligand adsorption. Nano Lett. 2008, 8, 2585–2590. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nichols, V.M.; Zhou, D.; Lim, C.; Pau, G.S.H.; Bardeen, C.J.; Tang, M.L. Observation of multiple, identical binding sites in the exchange of carboxylic acid ligands with CdS nanocrystals. Nano Lett. 2014, 14, 3382–3387. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Reynolds, L.X.; MacLachlan, A.; Lutz, T.; Ashraf, R.S.; Zhang, W.; Nielsen, C.B.; McCulloch, I.; Rebois, D.G.; Kirchartz, T.; et al. Influence of crystallinity and energetics on charge separation in polymer–inorganic nanocomposite films for solar cells. Sci. Rep. 2013, 3, 1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandewal, K.; Himmelberger, S.; Salleo, A. Structural factors that affect the performance of organic bulk heterojunction solar cells. Macromolecules 2013, 46, 6379–6387. [Google Scholar] [CrossRef]
- Gao, F.; Li, Z.; Wang, J.; Rao, A.; Howard, I.A.; Abrusci, A.; Massip, S.; McNeill, C.R.; Greenham, N.C. Trap-induced losses in hybrid photovoltaics. ACS Nano 2014, 8, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Wagenpfahl, A.; Rauh, D.; Binder, M.; Deibel, C.; Dyakonov, V. S-shaped current-voltage characteristics of organic solar devices. Phys. Rev. B 2010, 82, 115306. [Google Scholar] [CrossRef]
- Liu, Y.; Gibbs, M.; Puthussery, J.; Gaik, S.; Ihly, R.; Hillhouse, H.W.; Law, M. Dependence of carrier mobility on nanocrystal size and ligand length in pbse nanocrystal solids. Nano Lett. 2010, 10, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhny, G.; Murray, R.W. Ligand effects on optical properties of cdse nanocrystals. J. Phys. Chem. B 2005, 109, 7012–7021. [Google Scholar] [CrossRef] [PubMed]
- Albero, J.; Clifford, J.N.; Palomares, E. Quantum dot based molecular solar cells. Coord. Chem. Rev. 2014, 263, 53–64. [Google Scholar] [CrossRef]
- Nam, M.; Lee, T.; Kim, S.; Kim, S.; Kim, S.W.; Lee, K.K. Two strategies to enhance efficiency of PbS quantum dot solar cells: Removing surface organic ligands and configuring a bilayer heterojunction with a new conjugated polymer. Org. Electron. 2014, 15, 391–398. [Google Scholar] [CrossRef]
- Moule, A.J.; Chang, L.; Thambidurai, C.; Vidu, R.; Stroeve, P. Hybrid solar cells: Basic principles and the role of ligands. J. Mater. Chem. 2012, 22, 2351–2368. [Google Scholar] [CrossRef]
- Celik, D.; Krueger, M.; Veit, C.; Schleiermacher, H.F.; Zimmermann, B.; Allard, S.; Dumsch, I.; Scherf, U.; Rauscher, F.; Niyamakom, P. Performance enhancement of cdse nanorod-polymer based hybrid solar cells utilizing a novel combination of post-synthetic nanoparticle surface treatments. Sol. Energy Mater. Sol. Cells 2012, 98, 433–440. [Google Scholar] [CrossRef]
- Jeltsch, K.F.; Schädel, M.; Bonekamp, J.-B.; Niyamakom, P.; Rauscher, F.; Lademann, H.W.A.; Dumsch, I.; Allard, S.; Scherf, U.; Meerholz, K. Efficiency enhanced hybrid solar cells using a blend of quantum dots and nanorods. Adv. Funct. Mater. 2012, 22, 397–404. [Google Scholar] [CrossRef]
- Colbert, A.E.; Wu, W.; Janke, E.M.; Ma, F.; Ginger, D.S. Effects of ligands on charge generation and recombination in hybrid polymer/quantum dot solar cells. J. Phys. Chem. C 2015, 119, 24733–24739. [Google Scholar] [CrossRef]
- Bloom, B.P.; Mendis, M.N.; Wierzbinski, E.; Waldeck, D.H. Eliminating fermi-level pinning in PbS quantum dots using an alumina interfacial layer. J. Mater. Chem. C 2016, 4, 704–712. [Google Scholar] [CrossRef]
- Vokhmintcev, K.V.; Samokhvalov, P.S.; Nabiev, I. Charge transfer and separation in photoexcited quantum dot-based systems. Nano Today 2016, 11, 189–211. [Google Scholar] [CrossRef]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The surface science of nanocrystals. Nat. Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-W.; Lin, W.-H.; Hsu, Y.-J.; Wei, K.-H. Conjugated polymer/nanocrystal nanocomposites for renewable energy applications in photovoltaics and photocatalysis. Small 2014, 10, 4427–4442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lin, Z. Crafting semiconductor organic−inorganic nanocomposites via placing conjugated polymers in intimate contact with nanocrystals for hybrid solar cells. Adv. Mater. 2012, 24, 4353–4368. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hu, J.; Liu, X.; Qiu, X.; Wei, Z. Stepwise self-assembly of P3HT/CdSe hybrid nanowires with enhanced photoconductivity. Macromol. Rapid Commun. 2009, 30, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Lai, C.-W.; Wu, I.C.; Pan, H.-R.; Chen, I.W.P.; Peng, Y.-K.; Liu, C.-L.; Chen, C.-H.; Chou, P.-T. Enhanced performance and air stability of 3.2% hybrid solar cells: How the functional polymer and cdte nanostructure boost the solar cell efficiency. Adv. Mater. 2011, 23, 5451–5455. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.; Kim, S.; Kim, S.; Kim, S.W.; Lee, K. Efficient hybrid solar cells using pbsxse1-x quantum dots and nanorods for broad-range photon absorption and well-assembled charge transfer networks. Nanoscale 2013, 5, 8202–8209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Stalder, R.; Xie, D.; Cao, W.; Zheng, Y.; Yang, Y.; Plaisant, M.; Holloway, P.H.; Schanze, K.S.; Reynolds, J.R.; et al. Enhancing the efficiency of solution-processed polymer:Colloidal nanocrystal hybrid photovoltaic cells using ethanedithiol treatment. ACS Nano 2013, 7, 4846–4854. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, H.; Du, X.; Cheng, X.; Chen, X.; Jiang, Y.; Yang, B. From planar-heterojunction to n-i structure: An efficient strategy to improve short-circuit current and power conversion efficiency of aqueous-solution-processed hybrid solar cells. Energy Environ. Sci. 2013, 6, 1597–1603. [Google Scholar] [CrossRef]
- Lan, X.; Voznyy, O.; Garcia de Arquer, F.P.; Liu, M.; Xu, J.; Proppe, A.H.; Walters, G.; Fan, F.; Tan, H.; Liu, M.; et al. 10.6%-certified colloidal quantum dot solar cells via solvent-polarity-engineered halide passivation. Nano Lett. 2016, 16, 4630–4634. [Google Scholar] [CrossRef] [PubMed]



| Abbreviation | Name | Structure |
|---|---|---|
| P3HT | Poly(3-hexylthiophene) |  |
| PCPDTBT | Poly[2,6-(4,4-bis-(2-ethyhexyl)-4H-cyclopenta[2,1-b;3,4-b′]-dithiophene)-alt-4,7-(2,1,3 benzothiadiazole)] |  |
| MEH:PPV | Poly[2-methoxy, 5-(2′-ethyl-hexyloxy)-p-phenylenevinylene)] |  |
| P3DT | Poly(3-decylthiophene-2,5-diyl) |  |
| MDMO-PPV | Poly[2-methoxy-5-(3′,7′-dimethyloctyloxy)-1,4-phenylenevinylene] |  |
| PDTPBT | Poly(2,6-(N-(1-octylnonyl)dithieno[3,2-b:20,30-d]pyrrole)-alt-4,7-(2,1,3-benzothiadiazole)) |  |
| PTB1 | Poly(4,8-bis (octyloxy) benzo (1,2-b:4,5-b′ dithiophene-2,6-diyl) (2-((dodecyloxy) carbonyl) thieno(3,4-b) thiophenediyl)) |  |
| PSBTBT | Poly[(4,4′-bis(2-ethylhexyl)-dithieno[3,2-b:2′,3′-d]silole)-2,6-diyl-alt-(2,1,3-benzothiadiazole)-4,7-diyl] |  |
| PPV | Poly(p-phenylene vinylene) |  |
| PDFD | Poly[9,9-bis(3′-((N,N-dimethyl)-N-ethylammonium)-propyl)-2,7-fluorene-alt-1,4-phenylene] dibromide |  |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisslinger, R.; Hua, W.; Shankar, K. Bulk Heterojunction Solar Cells Based on Blends of Conjugated Polymers with II–VI and IV–VI Inorganic Semiconductor Quantum Dots. Polymers 2017, 9, 35. https://doi.org/10.3390/polym9020035
Kisslinger R, Hua W, Shankar K. Bulk Heterojunction Solar Cells Based on Blends of Conjugated Polymers with II–VI and IV–VI Inorganic Semiconductor Quantum Dots. Polymers. 2017; 9(2):35. https://doi.org/10.3390/polym9020035
Chicago/Turabian StyleKisslinger, Ryan, Weidi Hua, and Karthik Shankar. 2017. "Bulk Heterojunction Solar Cells Based on Blends of Conjugated Polymers with II–VI and IV–VI Inorganic Semiconductor Quantum Dots" Polymers 9, no. 2: 35. https://doi.org/10.3390/polym9020035