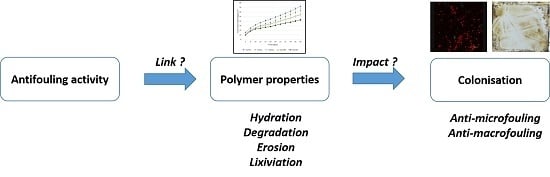
Influence of Biodegradable Polymer Properties on Antifouling Paints Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Copolymerization
2.2. Paints Preparation
2.3. Hydration
2.4. Degradation
2.5. Erosion
2.6. Lixiviation Tests
2.7. Field Immersion Test
2.7.1. Characteristics of Immersion Site
2.7.2. Microfouling Observations
2.7.3. Macrofouling Observations
2.8. Characterization Technique
2.8.1. Molecular Weight Measurements
2.8.2. Thermal Analysis
2.8.3. Nuclear Magnetic Resonance Spectroscopy
2.8.4. Karl Fisher Titration
2.8.5. Atomic Absorption Spectroscopy
2.8.6. Scanning Electronic Microscopy
2.8.7. Confocal Laser Scanning Microscopy
3. Results and Discussion
3.1. Characterization of Biodegradable Binders
3.2. Properties of Biodegradable Paints
3.2.1. Hydration
3.2.2. Degradation
3.2.3. Erosion
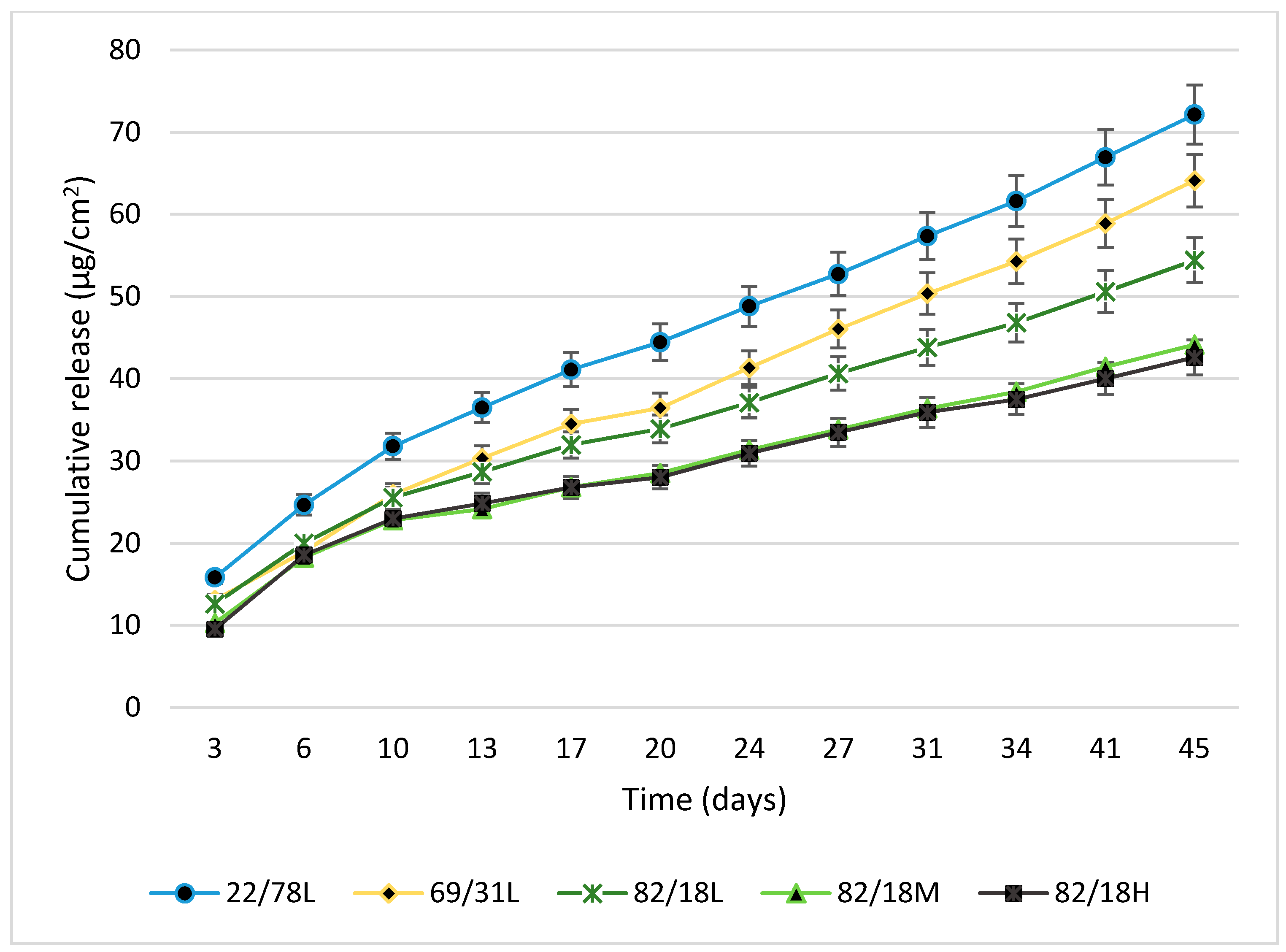
3.2.4. Biocide Release
3.3. Influence of Binders on Antifouling Properties
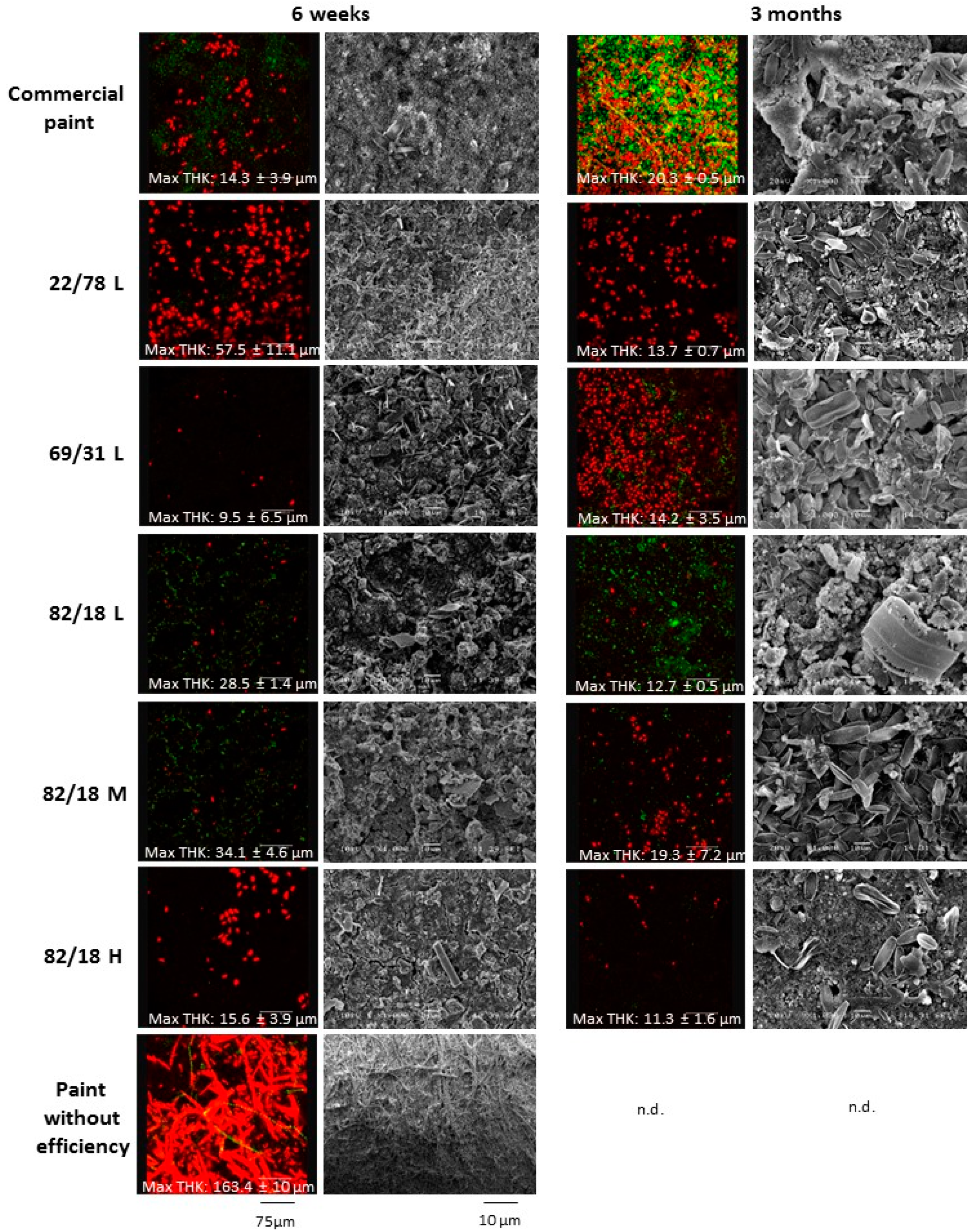
3.3.1. Paints Activity against Micro-Fouling
3.3.2. Paints Activity against Macro-Fouling
3.3.3. Influence of Polymer Characteristics on Colonization
3.3.4. Link between Micro- and Macrofouling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wahl, M. Marine epibiosis. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef]
- Qian, P.Y.; Lau, S.C.K.; Dahms, H.U.; Dobretsov, S.; Harder, T. Marine Biofilms as Mediator of Colonization by Marine Macroorganisms: Implications for Antifouling and Aquaculture. Mar. Biotechnol. 2007, 9, 339–410. [Google Scholar] [CrossRef] [PubMed]
- Gittens, J.E.; Smith, T.J.; Suleiman, R.; Akid, R. Current and emerging environmentally-friendly systems for fouling control in the marine environment. Biotechnol. Adv. 2013, 31, 1738–1753. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Bright, F.V.; Pagliaro, M. Ecofriendly Antifouling Marine Coatings. ACS Sustain. Chem. Eng. 2015, 3, 559–565. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Catarina, A.; Esteves, C.; de With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef]
- Bressy, C.; Hellio, C.; Nguyen, M.N.; Tanguy, B.; Maréchal, J.P.; Margaillan, A. Optimized silyl esteer diblock methacrylic copolymers: A new class of binders for chemically active antifouling coatings. Prog. Org. Coat. 2014, 77, 665–673. [Google Scholar] [CrossRef]
- Yang, W.J.; Neoh, K.G.; Kang, E.T.; Teo, S.L.M.; Rittschof, D. polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Pérez, M.; Garcia, M.; Blustein, G. Evaluation of low copper content antifouling paints containing natural phenolic compounds as bioactive additives. Mar. Environ. Res. 2015, 109, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wallström, E.; Jespersen, H.T.; Schaumburg, K. A new concept for antifouling paint for Yachts. Prog. Org. Coat. 2011, 72, 109–114. [Google Scholar] [CrossRef]
- Yi, J.; Huang, C.; Zhuang, H.; Gong, H.; Zhang, C.; Ren, R.; Ma, Y. Degradable polyurethane based on star-shaped polyester polyols (trimethylolpropane and ε-caprolactone) for marine antifouling. Prog. Org. Coat. 2015, 87, 161–170. [Google Scholar] [CrossRef]
- Marceaux, S.; Bressy, C.; Perrin, F.X.; Martin, C.; Margaillan, A. Development of polyorganosilazane-silicone marine coatings. Prog. Org. Coat. 2014, 77, 1919–1928. [Google Scholar] [CrossRef]
- Azemar, F.; Faÿ, F.; Réhel, K.; Linossier, I. Development of hybrid antifouling paint. Prog. Org. Coat. 2015, 87, 10–19. [Google Scholar] [CrossRef]
- Faÿ, F.; Renard, E.; Langlois, V.; Linossier, I.; Vallée-Réhel, K. Development of Poly(ε-caprolactone-co-l-lactide) and Poly(ε-caprolactone-co-δ-valerolactone) as New Degradable Binder used for Antifouling Paint. Eur. Polym. J. 2007, 43, 4800–4813. [Google Scholar] [CrossRef]
- Carteau, D.; Vallée-Réhel, K.; Linossier, I.; Quiniou, F.; Davy, R.; Compère, C.; Delbury, M.; Faÿ, F. Development of environmentally friendly antifouling paints using biodegradable polymer and non toxic substances. Prog. Org. Coat. 2014, 77, 485–493. [Google Scholar] [CrossRef]
- Faÿ, F.; Linossier, I.; Langlois, V.; Renard, E.; Vallée-Rehel, K. Degradation and controlled release behavior of e-caprolactone Copolymers in Biodegradable Antifouling Coatings. Biomacromolecules 2006, 7, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Faÿ, F.; Linossier, I.; Peron, J.J.; Langlois, V.; Vallee-Rehel, K. Antifouling activity of marine paints: Study of erosion. Prog. Org. Coat. 2007, 60, 194–206. [Google Scholar] [CrossRef]
- Zang, J.; Lin, C.; Wang, L.; Zheng, J.; Xu, F.; Sun, F. Study on the correlation of lab assay and field test for fouling-release coatings. Prog. Org. Coat. 2013, 76, 1430–1434. [Google Scholar] [CrossRef]
- Thouvenin, M.; Langlois, V.; Briandet, R.; Langlois, J.Y.; Guerin, P.; Peron, J.J.; Haras, D.; Vallée-Réhel, K. Study of Erodable Paint Properties Involved in Antifouling Activity. Biofouling 2003, 19, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K.; Weinell, C. Reaction rate estimation of controlled-release antifouling paint binders: Rosin-based systems. Prog. Org. Coat. 2005, 53, 256–275. [Google Scholar] [CrossRef]
- Stafslien, S.J.; Christianson, D.; Daniels, J.; VanderWal, L.; Chernykh, A.; Chisholm, B.J. Combinatorial materials research applied to the development of new surface coatings XVI: Foulinf-release properties of amphiphilic polysiloxane coatings. Biofouling 2015, 31, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.; Nicholas, C.; Daniels, J.; Stafslien, S.J.; Brewer, L.H.; Wendt, D.E.; Bright, F.V.; Detty, M. A comparison of non-biocidal xerogel and commercial coatings toward micro- and macrofouling organisms. Biofouling 2012, 28, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Cassé, F.; Swain, G.W. The development of microfouling on four commercial antifouling coatings under static and dynamic immersion. Int. Biodeterior. Biodegrad. 2006, 57, 179–185. [Google Scholar] [CrossRef]
- Jelic-Mrcelic, G.; Sliskovic, M.; Antolic, B. Biofouling communities on test panels coated with TBT and TBT-free copper based antifouling paints. Biofouling 2006, 22, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Molino, P.J.; Campbell, E.; Wetherbee, R. Development of the initial diatom microfouling layer on antifouling and fouling-release surfaces in temperate and tropical Australia. Biofouling 2009, 25, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Dobretsov, S.; Thomason, J.C. The development of marine biofilms on two commercial non-biocidal coatings: A comparison between silicone and fluoropolymer. Biofouling 2011, 27, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Zargiel, K.A.; Swain, G.W. Static vs. dynamic settlement and adhesion of diatoms to ship hull coating. Biofouling 2014, 30, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Briand, J.F.; Djeridi, I.; Jamet, D.; Coupé, S.; Bressy, C.; Molmeret, M.; Le Berre, B.; Rimet, F.; Bouchez, A.; Blache, Y. Pioneer marine biofilms on artificial surfaces including coatings immersed in two contrasting French Mediterranean coast sites. Biofouling 2012, 28, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lang, Y.; Sun, Q.; Liang, S.; Liu, Y.; Zhang, Z. Effect of anti-biofouling potential of multi-walled carbon nanotubes-filles polydimethylsiloxane composites on pioneer microbial colonization. Colloids Surf. B Biointerfaces 2016, 145, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, T.; Abed, R.M.M.; Dobretsov, S.; Kidd, B.; Finnie, A.A. Long-term microfouling on commercial biocidal fouling control coatings. Biofouling 2014, 30, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.G.; Scardino, W.; Zalizniak, L.; Shimeta, J. Colonisation and succession of marine biofilm-dwelling ciliate assemblages on biocidal antifouling and fouling-release coatings in temperate Australia. Biofouling 2015, 31, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Maki, J.S.; Rittschof, D.; Teo, S.L.M. Early marine bacterial biofilm on a copper-based antifouling paint. Int. Biodeterior. Biodegrad. 2013, 83, 71–76. [Google Scholar] [CrossRef]
- Thouvenin, M.; Peron, J.J.; Charreteur, C.; Guerin, P.; Langlois, J.Y.; Vallée-Réhel, K. A study of the biocide release from antifouling paints. Prog. Org. Coat. 2002, 44, 75–83. [Google Scholar] [CrossRef]
- Faÿ, F.; Carteau, D.; Linossier, I.; Vallée-Rehel, K. Evaluation of anti-microfouling activity of marine paints by microscopical techniques. Prog. Org. Coat. 2011, 72, 579–585. [Google Scholar] [CrossRef]
- Bressy, C.; Briand, J.F.; Compère, C.; Réhel, K. Efficacy testing of biocides and biocidal coatings. In Biofouling Methods; First Published; Dobretsov, S., Williams, D.N., Thomason, J.C., Eds.; John Wiley & Sons, Ltd.: London, UK, 2014; pp. 332–345. [Google Scholar]
- Heydorn, A.; Nielson, A.T.; Hentze, M.; Sternberg, C.; Givskov, M.; Ersboll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 2001, 146, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Loriot, M.; Linossier, I.; Vallée-Réhel, K.; Faÿ, F. Syntheses, characterization, and hydrolytic degradation of P(e-caprolactone-co-d-valerolactone) copolymers: Influence of molecular weight. J. Appl. Polym. Sci. 2016, 133, 43007. [Google Scholar] [CrossRef]
- Jamshidi, K.; Hyon, S.H.; Ikada, Y. Thermal characterization of polylactides. Polymer 1988, 29, 2229–2234. [Google Scholar] [CrossRef]
- Peponi, L.; Navarro-Baena, I.; Baez, J.E.; Kenny, J.M.; Marcos-Fernandez, A. Effect of the molecular weight on the crystallinity of PCL-b-PLLA di-block copolymers. Polymer 2012, 53, 4561–4568. [Google Scholar] [CrossRef]
- Lin, W.J. Comparison of thermal characteristics and degradation properties of e-caprolactone copolymers. J. Biomed. Mater. Res. 1999, 47, 420–423. [Google Scholar] [CrossRef]
- Odent, J.; Raquez, J.M.; Duquesne, E.; Dubois, P. Random aliphatic copolyesters as new biodegradable impact modifiers for polylactide materials. Eur. Polym. J. 2012, 48, 331–340. [Google Scholar] [CrossRef]
- Fernandez, J.; Eyxeberria, A.; Sarasua, J.R. In vitro degradation studies and mechanical behavior of poly(ε-captolactone-co-δ-valerolactone) and poly(ε-caprolactone-co-l-lactide) with random and semi-alternating chain microstructures. Eur. Polym. J. 2015, 71, 585–595. [Google Scholar] [CrossRef]
- Almeida, E.; Diamantino, T.C.; de Sousa, O. Marine paints: The particular case of antifouling paints. Prog. Org. Coat. 2007, 59, 2–20. [Google Scholar] [CrossRef]
- Bressy, C.; Margaillan, A.; Faÿ, F.; Linossier, I.; Réhel, K. Tin-free polishing marine antifouling coatings. In Advances in Marine Antifouling Coatings and Technologies; Hellio, C., Yebra, D., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 445–491. [Google Scholar]
- Bressy, C.; Margaillan, A. Erosion study of poly(trialkylsilyl methacrylate)-based antifouling coatings. Prog. Org. Coat. 2009, 66, 400–405. [Google Scholar] [CrossRef]
- Sackett, C.K.; Narasimhan, B. Mathematical modeling of polymer erosion: Consequences for drug delivery. Int. J. Pharm. 2011, 418, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Ponjavic, P.; Nikolic, M.S.; Nikodinovic-Runic, J.; Jeremic, S.; Stevanovic, S.; Djonlagic, J. Degradation behavior of PCL/PEO/PCL and PCL/PEO block copolymers under controlled hydrolytic, enzymatic and composting conditions. Polym. Test. 2017, 57, 66–77. [Google Scholar] [CrossRef]
- Fernandez, J.; Larranaga, A.; Etxeberria, A.; Sarasua, J.R. Effects of chain microstructures and derived crystallization capability on hydrolytic degradation of poly(l-lactide/ε-caprolactone) copolymers. Polym. Degrad. Stab. 2013, 98, 481–489. [Google Scholar] [CrossRef]
- Sakkas, V.A.; Shibata, K.; Yamaguchi, Y.; Sugasawa, S.; Albanis, T. Aqueous phototransformation of zinc pyrithione: Degradation kinetics and by product identification by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2007, 1144, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrom, M.; Sjögren, M.; Jonsson, P.R.; Gôransson, U.; Lindh, L.; Arnebrant, T.; Pinori, E.; Elwing, H.; Berglin, M. Affinity states of biocides determine bioavailability and release rates in marine paints. Biofouling 2015, 31, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Pinori, E.; Berglin, M.; Brive, L.M.; Hulander, M.; Dahlström, M.; Elwing, H. Multi-seasonal barnacle (Balanus improvises) protection achieved by trace amounts of a macrocylic lactone (ivermectin) included in rosin-based coatings. Biofouling 2011, 27, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology-past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Klein, G.; Pierre, G.; Bellon-Fontaine, M.N.; Zhao, J.M.; Breret, M.; Maugard, T.; Graber, M. Marine diatom Navicula jeffreyi: From biochemical composition and physico-chemical surface properties to understanding the first step of benthic biofilm formation. J. Adhes. Sci. Technol. 2014, 28, 1739–1753. [Google Scholar] [CrossRef]
- Mieszkin, S.; Martin-Tanchereau, P.; Callow, M.E.; Callow, J.A. Effect of bacterial biofilms formed on fouling-release coatings from natural seawater and Cobetia marina, on the adhesion of two marine algae. Biofouling 2012, 28, 953–968. [Google Scholar] [CrossRef] [PubMed]
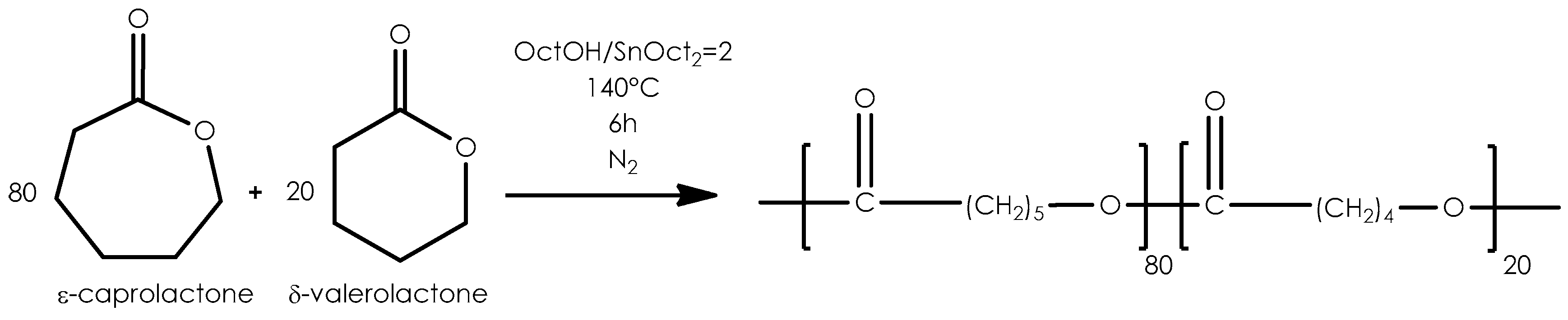


| Composition | Paint without biocide | P(CL-VL) paints |
|---|---|---|
| Polyacrylic | 21 | - |
| P(CL-VL) | - | 21 |
| Xylene | 32.4 | 32.4 |
| Zinc pyritione | - | 6 |
| Copper thiocyanate | - | 23 |
| Additives 1 | 46.6 | 17.6 |
| Surface covered by fouling (%) | Rank for intensity factor (I) | Type of fouling | Rank for severity factor (G) |
|---|---|---|---|
| No fouling | 0 | Biofilm | 1 |
| Up to 10% | 1 | Algae | 3 |
| From 10% to 20% | 2 | Non-encrousting species | 4 |
| From 20% to 40% | 3 | Encrouting species | 6 |
| From 40% to 60% | 4 | ||
| From 60% to 100% | 5 |
| Polymer | (CL/VL) 1 | Mn 2 (g·mol−1) | PDI | Yield (%) | ∆Hm 3 (J·g−1) | Tm 3 (°C) |
|---|---|---|---|---|---|---|
| 22/78L | 22/78 | 10,300 | 1.7 | 95 | 44 | 30 |
| 69/31L | 69/31 | 13,200 | 1.6 | 62 | 36 | 31 |
| 82/18L | 82/18 | 12,000 | 1.7 | 95 | 60 | 33 |
| 82/18M | 82/18 | 25,000 | 1.7 | 90 | 71 | 36 |
| 82/18H | 82/18 | 40,000 | 1.5 | 89 | 81 | 38 |
| Copolymer | Hydration (%) 1 | Degradation (%) 2 | Erosion 3 | Zinc pyrithione release (μg/cm2/day) 4 | ||
|---|---|---|---|---|---|---|
| 14 days | 8 months | 8 months | 8 months | to 10 days | from 10 to 45 days | |
| 22/78L | 7.8 ± 0.5 | 9.7 ± 0.6 | 26 | + | 8 | 4 |
| 69/31L | 20.6 ± 0.4 | 21.1 ± 1.0 | 12 | ++ | 6 | 4 |
| 82/18L | 9 ± 0.5 | 16.4 ± 0.5 | <1 | ++ | 6 | 3 |
| 82/18M | 4.9 ± 0.3 | 12.9 ± 1.0 | <1 | + | 6 | 2 |
| 82/18H | 4.6 ± 0.2 | 14.2 ± 0.6 | <1 | − | 7 | 2 |
| Paint ref. | 22/78L | 69/31L | 82/18L | 82/18M | 82/18H | Negative paint | ||
|---|---|---|---|---|---|---|---|---|
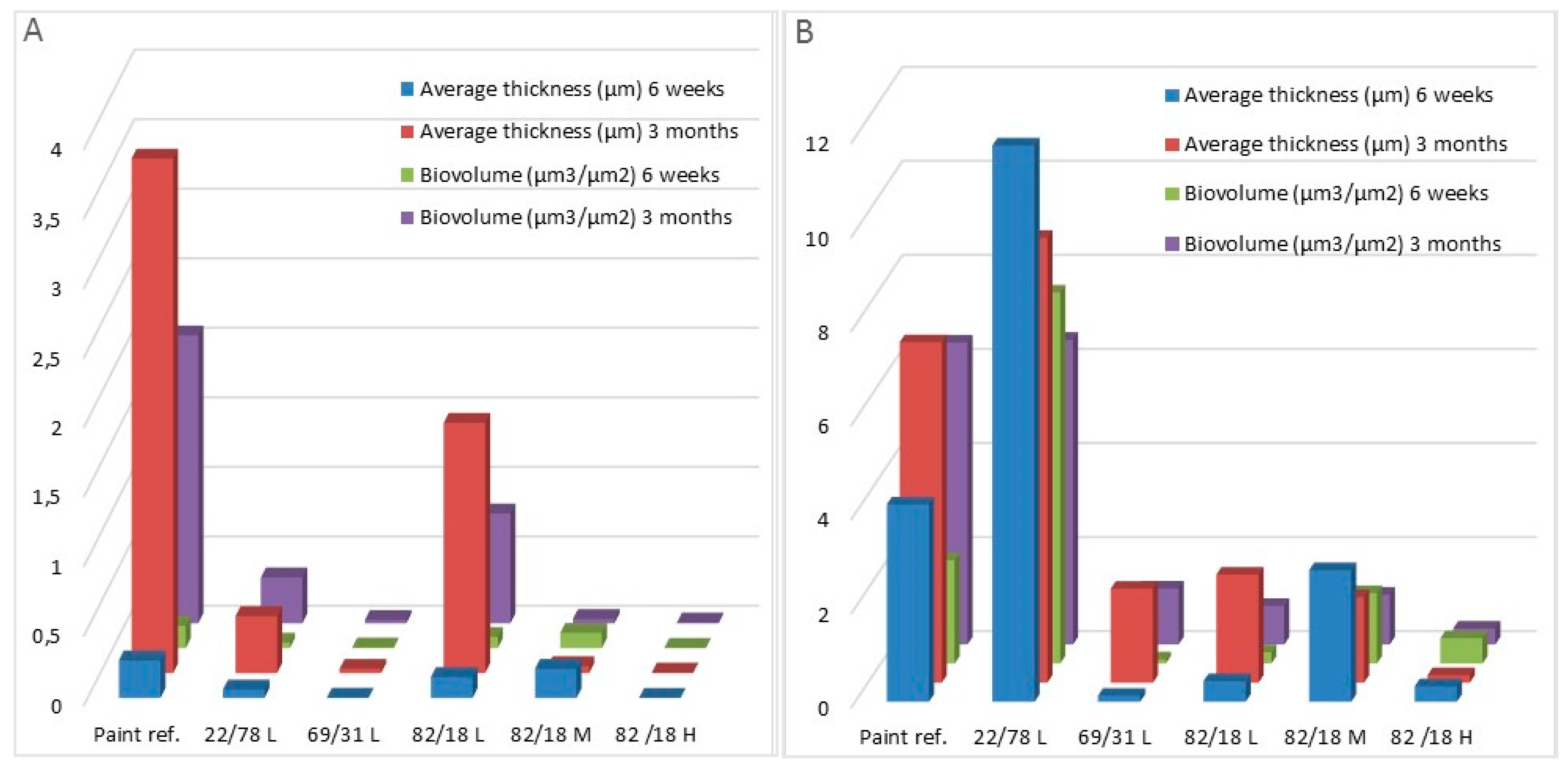
| Bacteria Biomass (μm3/μm2) | 6 weeks | 0.160 ± 0.030 | 0.040 ± 0.020 | 0.004± 0.004 | 0.080 ± 0.080 | 0.110 ± 0.015 | 0.002 ± 0.002 | 0.008 ± 0.008 |
| 3 months | 2.070 ± 0.230 | 0.330 ± 0.110 | 0.230 ± 0.020 | 0.790 ± 0.190 | 0.310 ± 0.015 | 0.005 ± 0.004 | n.d. | |
| Bacteria average Thickness (μm3/μm2) | 6 weeks | 0.270 ± 0.090 | 0.060 ± 0.040 | 0.0002 ± 0.002 | 0.150 ± 0.180 | 0.210 ± 0.033 | 0.001 ± 0.001 | 0.010 ± 0.010 |
| 3 months | 3.700 ± 0.360 | 0.410 ± 0.140 | 0.030 ± 0.040 | 1.790 ± 1.210 | 0.050 ± 0.020 | 0.003 ± 0.002 | n.d. | |
| Diatom Biomass (μm3/μm2) | 6 weeks | 2.200 ± 0.200 | 7.890 ± 2.920 | 0.092 ± 0.080 | 0.240 ± 0.090 | 1.500 ± 0.200 | 0.540 ± 0.260 | 35.400 ± 1.600 |
| 3 months | 6.420 ± 2.350 | 6.480 ± 0.790 | 1.190 ± 0.270 | 0.820 ± 0.200 | 1.060 ± 0.150 | 0.340 ± 0.400 | n.d. | |
| Diatom average Thickness (μm3/μm2) | 6 weeks | 4.200 ± 1.200 | 11.830 ± 4.680 | 0.130 ± 0.130 | 0.440 ± 0.250 | 2.800 ± 0.500 | 0.330 ± 0.180 | 86.700 ± 13.500 |
| 3 months | 7.240 ± 2.700 | 9.460 ± 0.440 | 2.000 ± 0.010 | 2.300 ± 1.260 | 1.820 ± 0.850 | 0.160 ± 0.010 | n.d. | |
| Maximum Thickness (μm) | 6 weeks | 14.300 ± 3.900 | 57.490 ± 10.800 | 9.490 ± 6.540 | 28.500 ± 1.010 | 34.100± 4.600 | 15.630 ± 3.900 | 163.4 ± 1.000 |
| 3 months | 20.350 ± 0.500 | 13.740 ± 0.730 | 14.170 ± 3.450 | 12.700 ± 0.500 | 19.330 ± 7.190 | 11.330 ± 1.620 | n.d. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loriot, M.; Linossier, I.; Vallée-Réhel, K.; Faÿ, F. Influence of Biodegradable Polymer Properties on Antifouling Paints Activity. Polymers 2017, 9, 36. https://doi.org/10.3390/polym9020036
Loriot M, Linossier I, Vallée-Réhel K, Faÿ F. Influence of Biodegradable Polymer Properties on Antifouling Paints Activity. Polymers. 2017; 9(2):36. https://doi.org/10.3390/polym9020036
Chicago/Turabian StyleLoriot, Marion, Isabelle Linossier, Karine Vallée-Réhel, and Fabienne Faÿ. 2017. "Influence of Biodegradable Polymer Properties on Antifouling Paints Activity" Polymers 9, no. 2: 36. https://doi.org/10.3390/polym9020036