RAFT Polymerization of Styrene and Maleimide in the Presence of Fluoroalcohol: Hydrogen Bonding Effects with Classical Alternating Copolymerization as Reference
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Typical Procedures for RAFT Copolymerization of St and N-PMI in Various Solvent Systems
2.3. Characterization
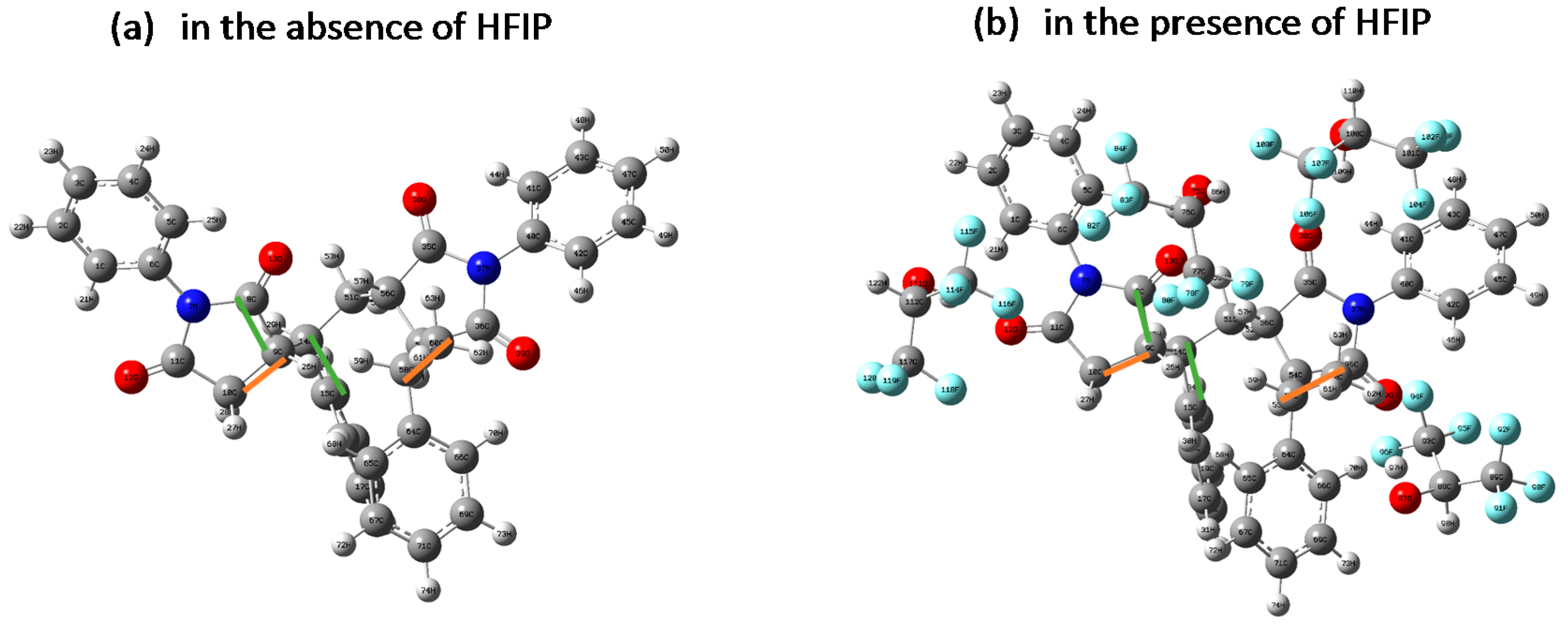
2.4. Computational Details
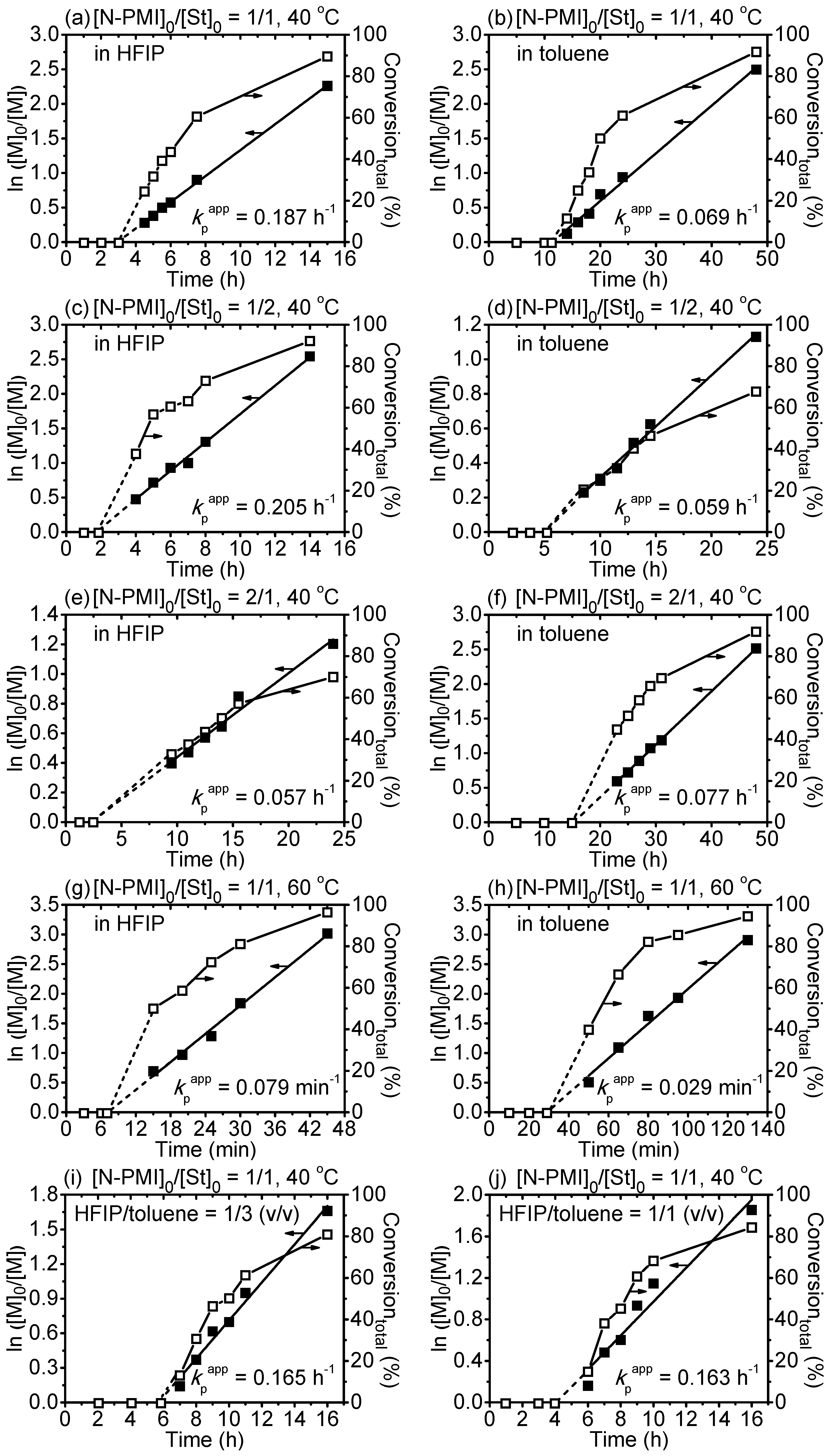
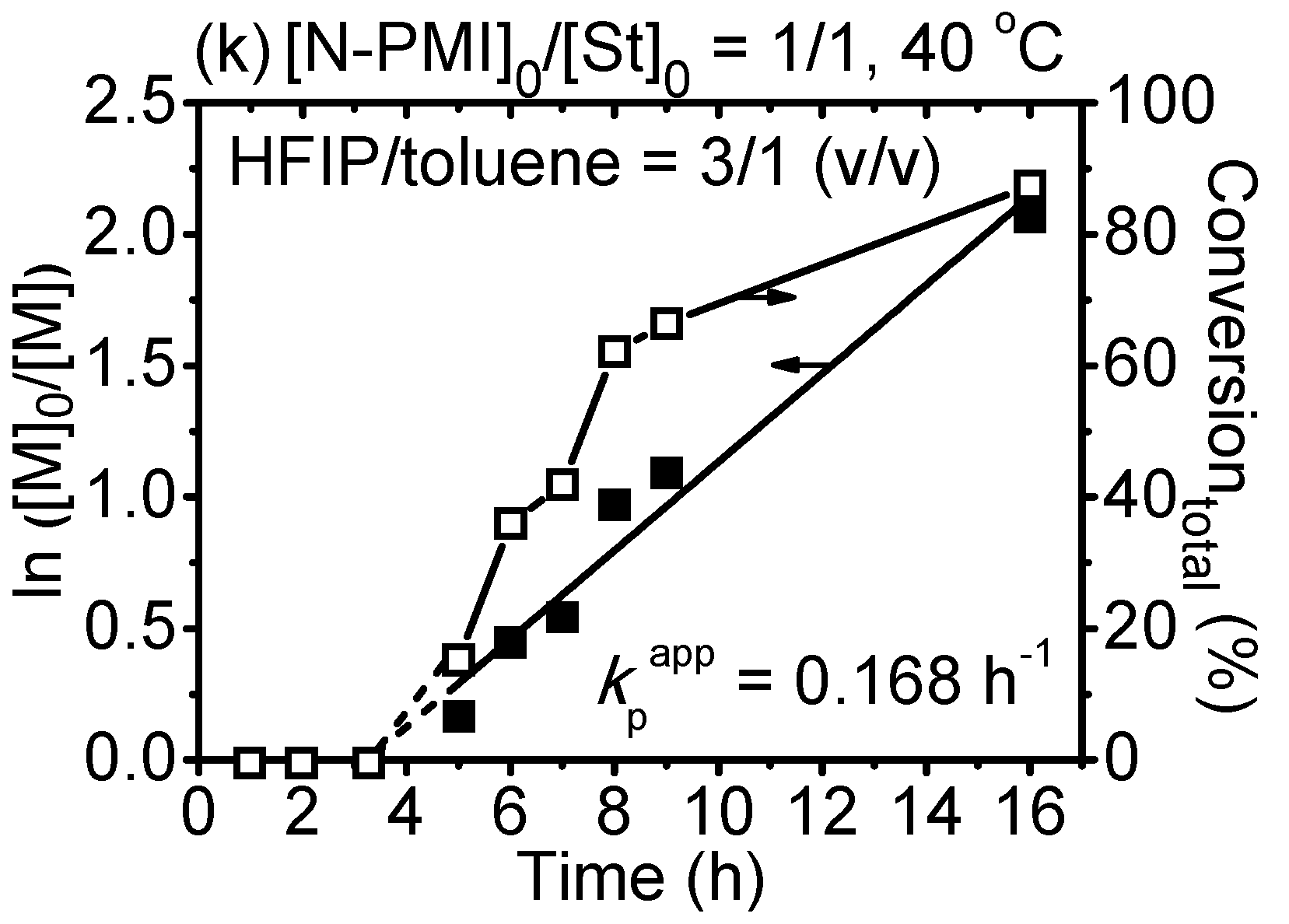
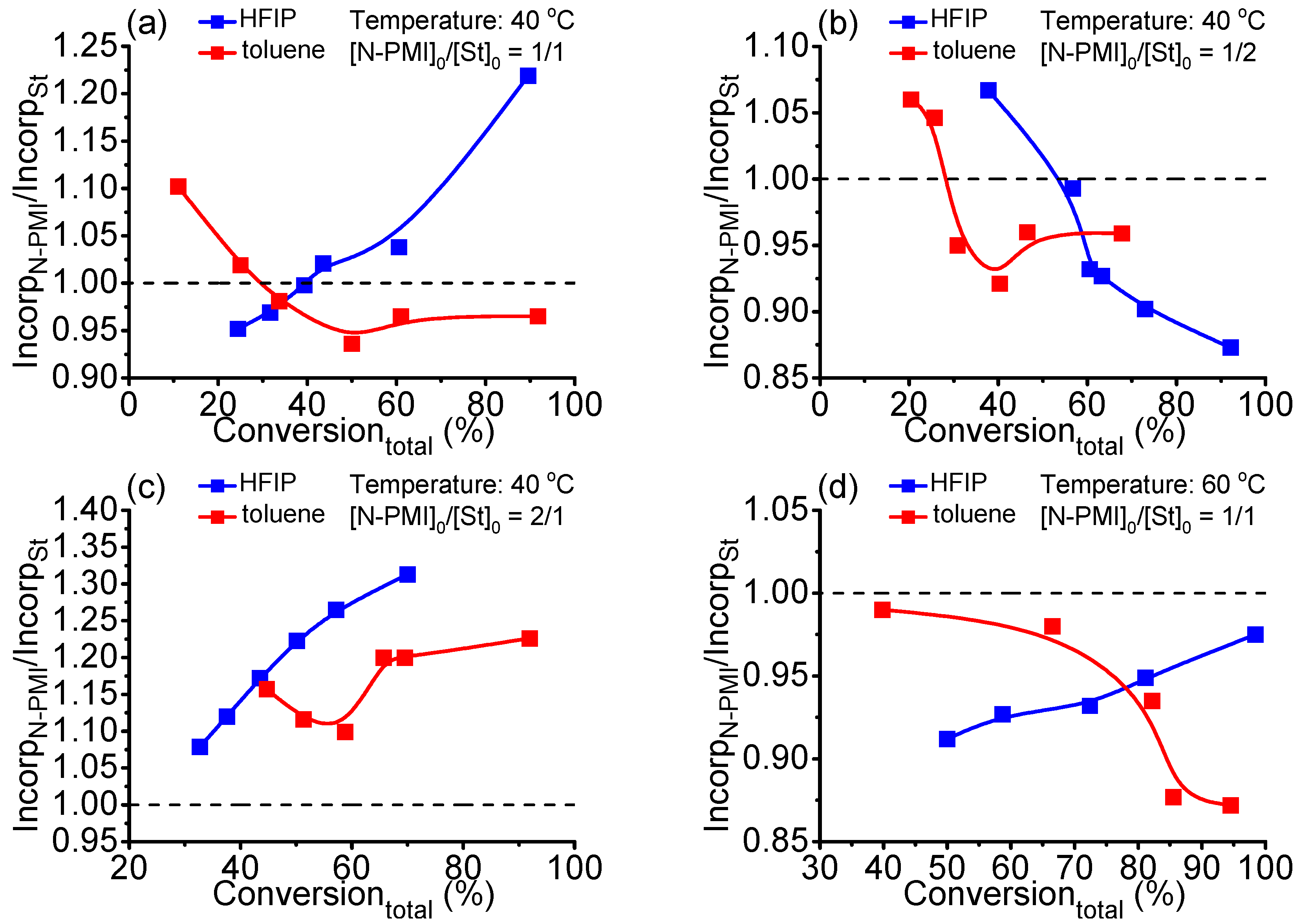
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamigaito, M.; Satoh, K. Stereoregulation in living radical polymerization. Macromolecules 2008, 41, 269–276. [Google Scholar] [CrossRef]
- Miura, Y.; Satoh, T.; Narumi, A.; Nishizawa, O.; Okamoto, Y.; Kakuchi, T. Atom transfer radical polymerization of methyl methacrylate in fluoroalcohol: Simultaneous control of molecular weight and tacticity. Macromolecules 2005, 38, 1041–1043. [Google Scholar] [CrossRef]
- Kamigaito, M.; Satoh, K. Stereospecific living radical polymerization for simultaneous control of molecular weight and tacticity. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6147–6158. [Google Scholar] [CrossRef]
- Miura, Y.; Satoh, T.; Narumi, A.; Nishizawa, O.; Okamoto, Y.; Kakuchi, T. Synthesis of well-defined syndiotactic poly(methyl methacrylate) with low-temperature atom transfer radical polymerization in fluoroalcohol. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1436–1446. [Google Scholar] [CrossRef]
- Satoh, K.; Matsuda, M.; Nagai, K.; Kamigaito, M. AAB-sequence living radical chain copolymerization of naturally occurring limonene with maleimide: An end-to-end sequence-regulated copolymer. J. Am. Chem. Soc. 2010, 132, 10003–10005. [Google Scholar] [CrossRef] [PubMed]
- Terayama, Y.; Kikuchi, M.; Kobayashi, M.; Takahara, A. Well-defined poly(sulfobetaine) brushes prepared by surface-initiated ATRP using a fluoroalcohol and ionic liquids as the solvents. Macromolecules 2011, 44, 104–111. [Google Scholar] [CrossRef]
- Yamada, K.; Nakano, T.; Okamoto, Y. Free-radical copolymerization of vinyl esters using fluoroalcohols as solvents: The solvent effect on the monomer reactivity ratio. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 220–228. [Google Scholar] [CrossRef]
- Wu, H.; Wan, Y.; Wang, W.; Wang, Y.; Zhou, N.; Zhang, W.; Li, X.; Zhang, Z.; Zhu, X. Hydrogen bonding promoting the controlled radical polymerization of 2-vinyl pyridine: Supramonomer for better control. Polym. Chem. 2015, 6, 2620–2625. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Cheng, Z.; Zhu, J.; Zhou, N.; Zhu, X. Favorable hydrogen bonding in room-temperature Cu(0)-mediated controlled radical polymerization of 4-vinylpyridine. Polym. Chem. 2012, 3, 2731–2734. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, H.; Zhang, L.; Zhou, Y.; Zhang, W.; Pan, X.; Zhang, Z.; Zhu, X. RAFT polymerization of N-vinylpyrrolidone mediated by cyanoprop-2-yl-1-dithionaphthalate in the presence of a fluoroalcohol: The possibility of altering monomer properties by hydrogen bonding? Polym. Chem. 2016, 7, 2015–2021. [Google Scholar] [CrossRef]
- Yang, P.; Mykhaylyk, O.O.; Jones, E.R.; Armes, S.P. RAFT dispersion alternating copolymerization of styrene with N-phenylmaleimide: Morphology control and application as an aqueous foam stabilizer. Macromolecules 2016, 49, 6731–6742. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Amani, J. Triphenylphosphine oxide supported on non-cross-linked maleimide–styrene copolymer: Application as a novel Hendrickson reagent. Tetrahedron Lett. 2008, 49, 2204–2207. [Google Scholar] [CrossRef]
- Zeng, C.; Seino, H.; Ren, J.; Hatanaka, K.; Yoshie, N. Self-healing bio-based furan polymers cross-linked with various bis-maleimides. Polymer 2013, 54, 5351–5357. [Google Scholar] [CrossRef]
- Ke, L.; Hu, D.; Lu, Y.; Feng, S.; Xie, Y.; Xu, W. Copolymerization of maleimide-based benzoxazine with styrene and the curing kinetics of the resultant copolymer. Polym. Degrad. Stab. 2013, 97, 132–138. [Google Scholar] [CrossRef]
- Pfeifer, S.; Lutz, J.F. A facile procedure for controlling monomer sequence distribution in radical chain polymerizations. J. Am. Chem. Soc. 2007, 129, 9542–9543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, W.; Xia, H.; Zhu, J.; Zhang, W.; Zhu, X. Single-electron transfer living radical polymerization (SET-LRP) of methyl methacrylate (MMA) with a typical RAFT agent as an Initiator. Macromolecules 2009, 42, 7360–7366. [Google Scholar] [CrossRef]
- Ebrahimi, H.P.; Hadi, J.S.; Abdulnabi, Z.A.; Bolandnazar, Z. Spectroscopic, thermal analysis and DFT computational studies of salen-type Schiff base complexes. Mol. Biomol. Spectrosc. 2014, 117, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Dennington, R.D.; Keith, T.A.; Millam, J.M. GaussView 5.0. 9; Gaussian Inc.: Wallingford, CT, USA, 2008. [Google Scholar]
- Kelen, T.; Tüdõs, F. Analysis of the linear methods for determining copolymerization reactivity ratios. I. A new improved linear graphic method. J. Macromol. Sci. Chem. 1975, 9, 1–27. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Kelen, T.; Tüdõs, F. Analysis of the linear methods for determining copolymerization reactivity ratios. II. A critical reexamination of cationic monomer reactivity ratios. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 2277–2289. [Google Scholar] [CrossRef]
- Mathammal, R.; Sangeetha, K.; Sangeetha, M.; Mekala, R.; Gadheeja, S. Molecular structure, vibrational, UV, NMR, HOMO-LUMO, MEP, NLO, NBO analysis of 3,5 di tert butyl 4 hydroxy benzoic acid. J. Mol. Struct. 2016, 1120, 1–14. [Google Scholar] [CrossRef]
- Polat, T.; Bulut, F.; Arican, I.; Kandemirli, F.; Yildirim, G. Vibrational assignments, spectroscopic investigation (FT-IR and FT-Raman), NBO, MEP, HOMO.LUMO analysis and intermolecular hydrogen bonding interactions of 7-fluoroisatin, 7-bromoisatin and 1-methylisatin—A comparative study. J. Mol. Struct. 2015, 1101, 189–211. [Google Scholar] [CrossRef]
- Fukui, K. Role of frontier orbitals in chemical reactions. Science 1982, 218, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Satoh, K.; Kamigaito, M. Stereospecific living radical polymerization: Dual control of chain length and tacticity for precision polymer synthesis. Chem. Rev. 2009, 109, 5120–5156. [Google Scholar] [CrossRef] [PubMed]
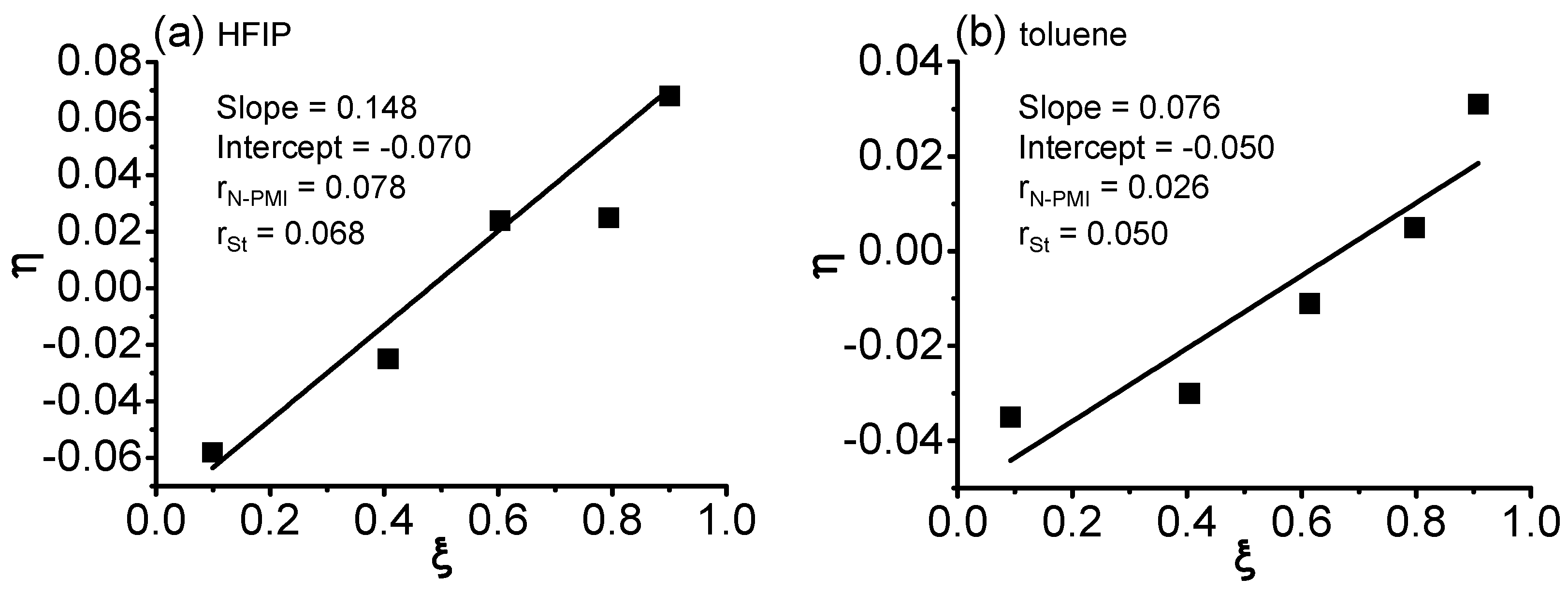

| Solvent | Time (h) | Convtotal (%) | Mn (kDa) | Ð | IncorpN-PMI/IncorpSt |
|---|---|---|---|---|---|
| 1H,1H,5H-Octafluoropentan-1-ol | 4.0 | 34.8 | 16.7 | 1.17 | 0.931 |
| 1H,1H,5H-Octafluoropentan-1-ol | 8.0 | 96.1 | 26.7 | 1.67 | 1.070 |
| 2,2,3,3-Tetrafluoro-1-propanol | 7.0 | 32.7 | 12.1 | 1.62 | 0.923 |
| 2,2,3,3-Tetrafluoro-1-propanol | 11.5 | 69.2 | 30.4 | 1.62 | 1.034 |
| Anisole | 11.5 | 30.9 | 11.8 | 1.67 | 1.087 |
| Anisole | 22.0 | 63.7 | 25.4 | 1.42 | 0.963 |
| 2,2,2-Trifluoroethanol | 5.0 | 47.4 | 18.5 | 1.21 | 0.909 |
| 2,2,2-Trifluoroethanol | 11.5 | 81.4 | 27.8 | 1.35 | 1.023 |
| Isopropanol | 8.0 | 16.9 | 5.4 | 1.42 | 0.894 |
| Isopropanol | 36.0 | 81.9 | 17.0 | 2.90 | 0.949 |
| HFIP b | 1.5 | 65.2 | 25.7 | 1.25 | 0.848 |
| HFIP b | 3.0 | 82.0 | 35.8 | 1.43 | 1.380 |
| Toluene b | 3.0 | 59.3 | 23.3 | 1.30 | 0.771 |
| Toluene b | 6.0 | 78.1 | 38.9 | 1.21 | 0.716 |
| Solvent | Time (h) | Temp. (°C) | [N-PMI]0/[St]0 | Mn (kDa) | Ð | IncorpN-PMI/IncorpSt | Tg (°C) |
|---|---|---|---|---|---|---|---|
| HFIP | 5.5 | 40 | 1:1 | 11.5 | 1.38 | 1.087:1 | 204.0 |
| Anisole | 11.5 | 40 | 1:1 | 11.8 | 1.67 | 0.998:1 | 211.3 |
| HFIP | 9.0 | 40 | 1:1 | 21.4 | 1.38 | 1.167:1 | 208.2 |
| Toluene | 24.0 | 40 | 1:1 | 21.3 | 1.48 | 0.965:1 | 219.8 |
| HFIP | 4.0 | 40 | 1:2 | 12.1 | 1.21 | 1.067:1 | 187.9 |
| Toluene | 10.0 | 40 | 1:2 | 12.1 | 1.38 | 1.046:1 | 202.5 |
| HFIP | 24.0 | 40 | 2:1 | 22.7 | 1.53 | 1.313:1 | 183.9 |
| Toluene | 29.0 | 40 | 2:1 | 22.6 | 1.39 | 1.200:1 | 213.7 |
| HFIP | 0.75 | 60 | 1:1 | 26.9 | 1.29 | 0.975:1 | 211.3 |
| Toluene | 1.33 | 60 | 1:1 | 27.4 | 1.27 | 0.935:1 | 212.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, F.; Liu, Q.; Zhang, Z.; Zhu, X. RAFT Polymerization of Styrene and Maleimide in the Presence of Fluoroalcohol: Hydrogen Bonding Effects with Classical Alternating Copolymerization as Reference. Polymers 2017, 9, 89. https://doi.org/10.3390/polym9030089
Yao F, Liu Q, Zhang Z, Zhu X. RAFT Polymerization of Styrene and Maleimide in the Presence of Fluoroalcohol: Hydrogen Bonding Effects with Classical Alternating Copolymerization as Reference. Polymers. 2017; 9(3):89. https://doi.org/10.3390/polym9030089
Chicago/Turabian StyleYao, Fangjun, Qingqing Liu, Zhengbiao Zhang, and Xiulin Zhu. 2017. "RAFT Polymerization of Styrene and Maleimide in the Presence of Fluoroalcohol: Hydrogen Bonding Effects with Classical Alternating Copolymerization as Reference" Polymers 9, no. 3: 89. https://doi.org/10.3390/polym9030089





