Preparation of RF-(VM-SiO2)n-RF/AM-Cellu Nanocomposites, and Use Thereof for the Modification of Glass and Filter Paper Surfaces: Creation of a Glass Thermoresponsive Switching Behavior and an Efficient Separation Paper Membrane
Abstract
:1. Introduction
2. Experimental Section
2.1. Measurements
2.2. Materials
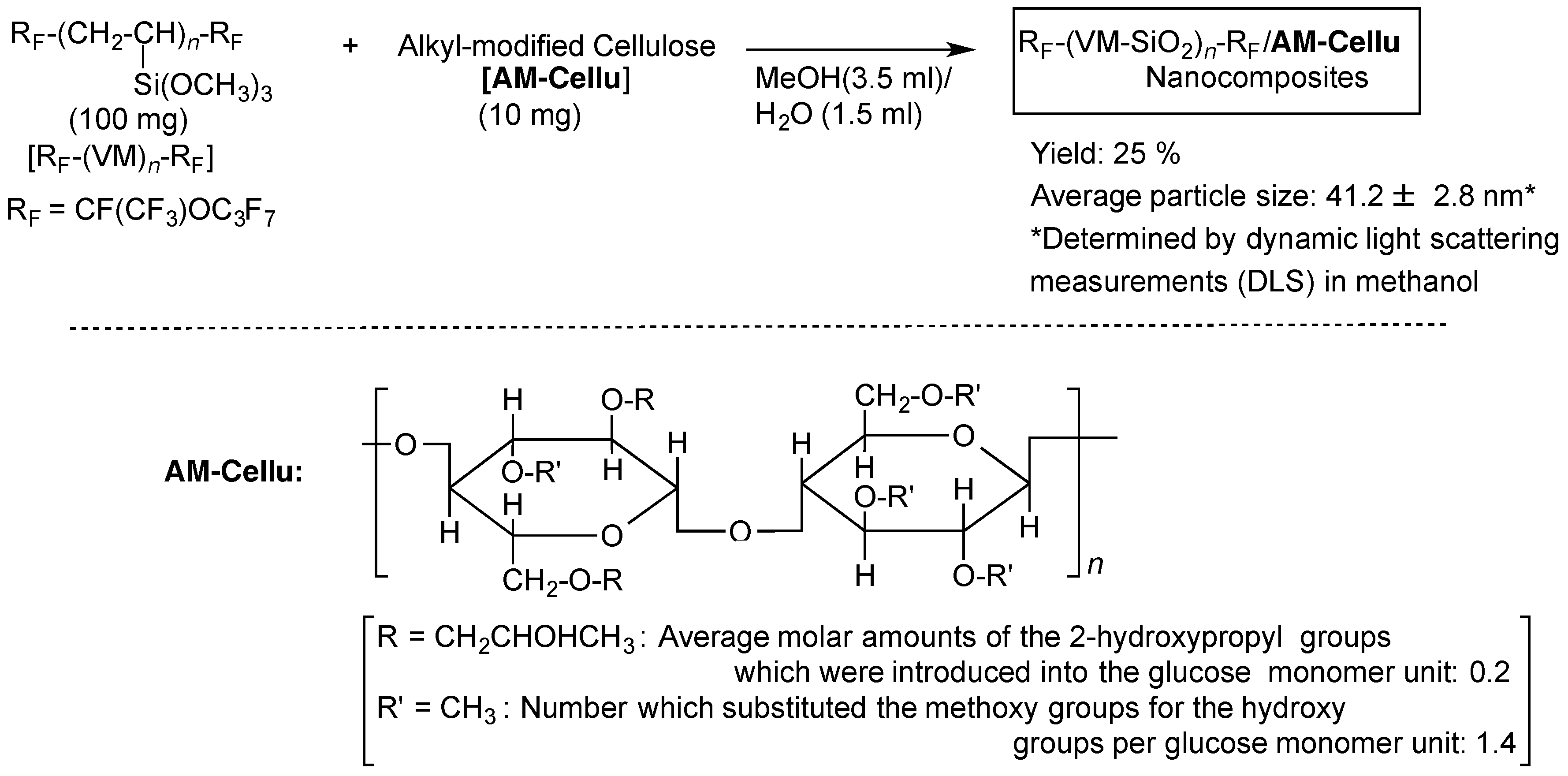
2.3. Preparation of Fluoroalkylated Vinyltrimethoxysilane Oligomeric Silica/AM-Cellu Nanocomposites [RF-(VM-SiO2)n-RF/AM-Cellu]
2.4. Surface Modification of Glass Treated with the RF-(VM-SiO2)n-RF/AM-Cellu Nanocomposites
2.5. Preparation of the Surfactant-Stabilized Water in Oil (1,2-Dichloroethane) Emulsion
3. Results and Discussion
3.1. Preparation of the RF-(VM-SiO2)n-RF/AM-Cellu Nanocomposites
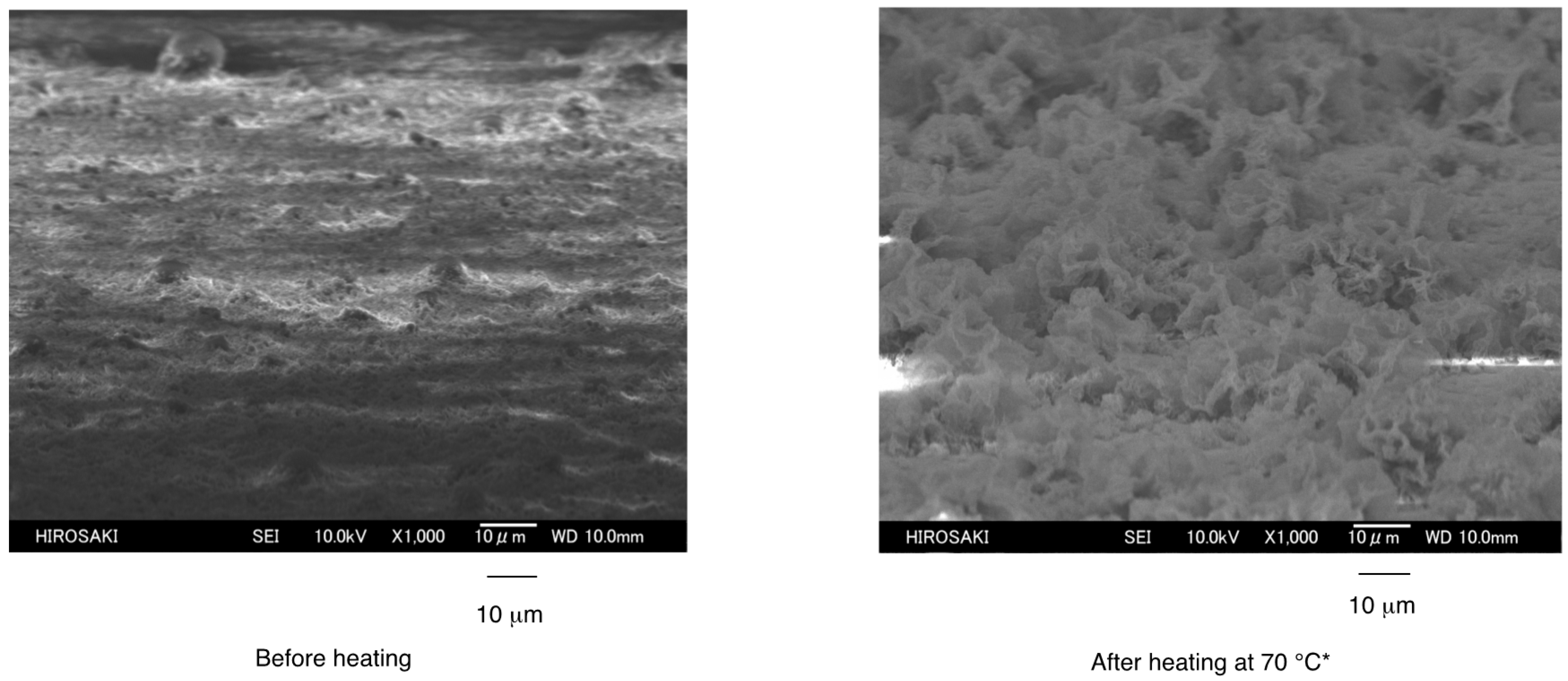
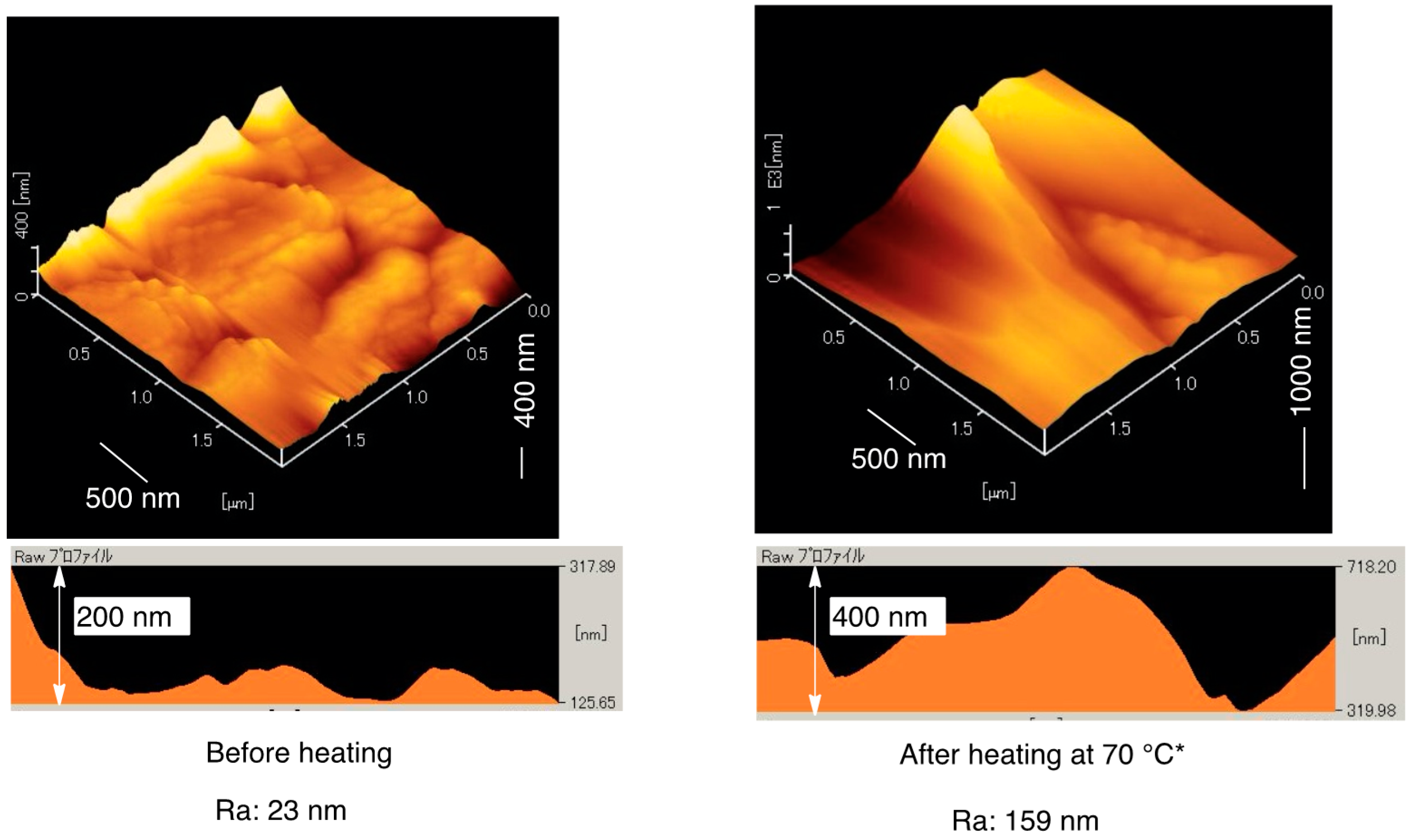
3.2. Surface Modification of Glass by Using the RF-(VM-SiO2)n-RF/AM-Cellu Nanocomposites
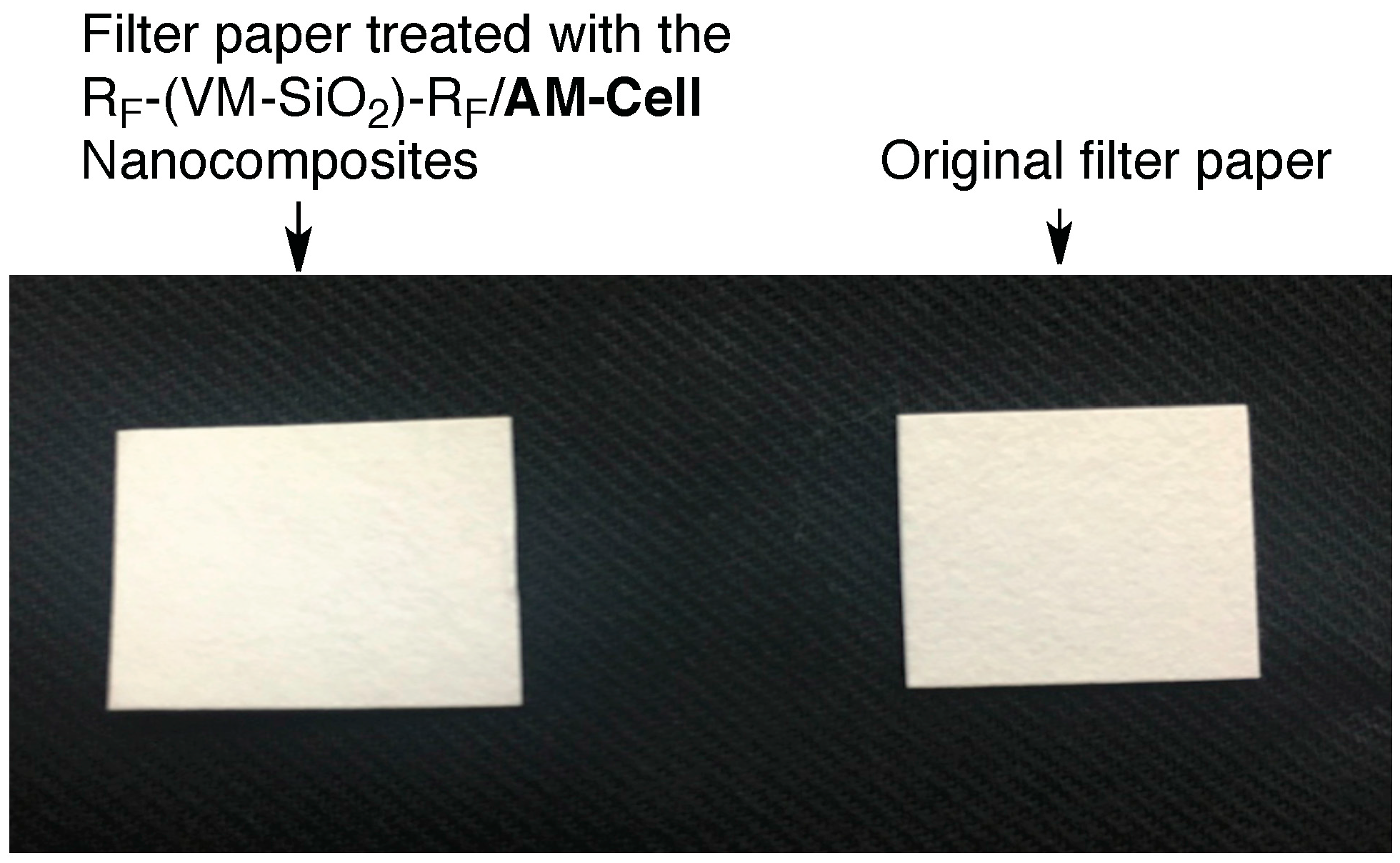
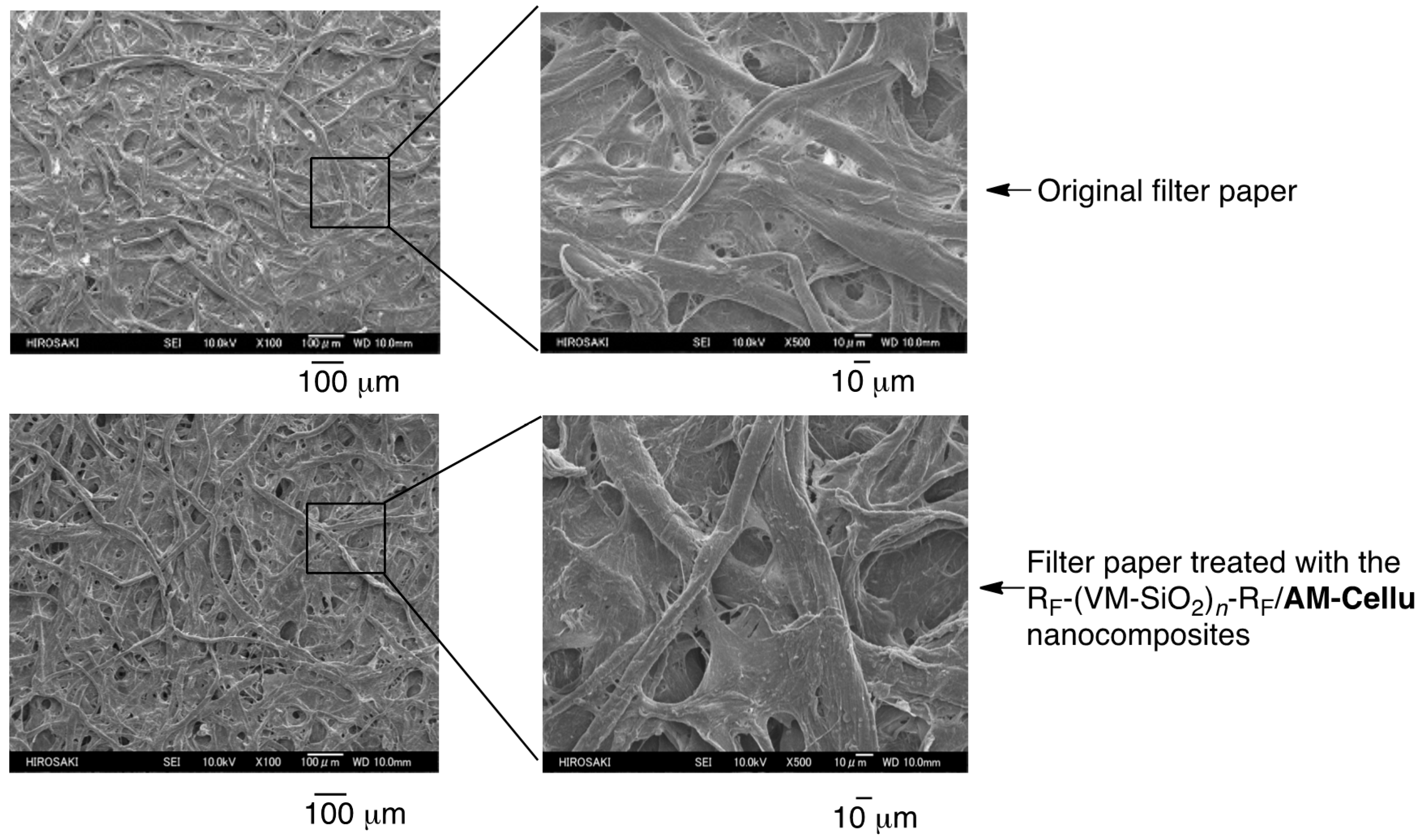
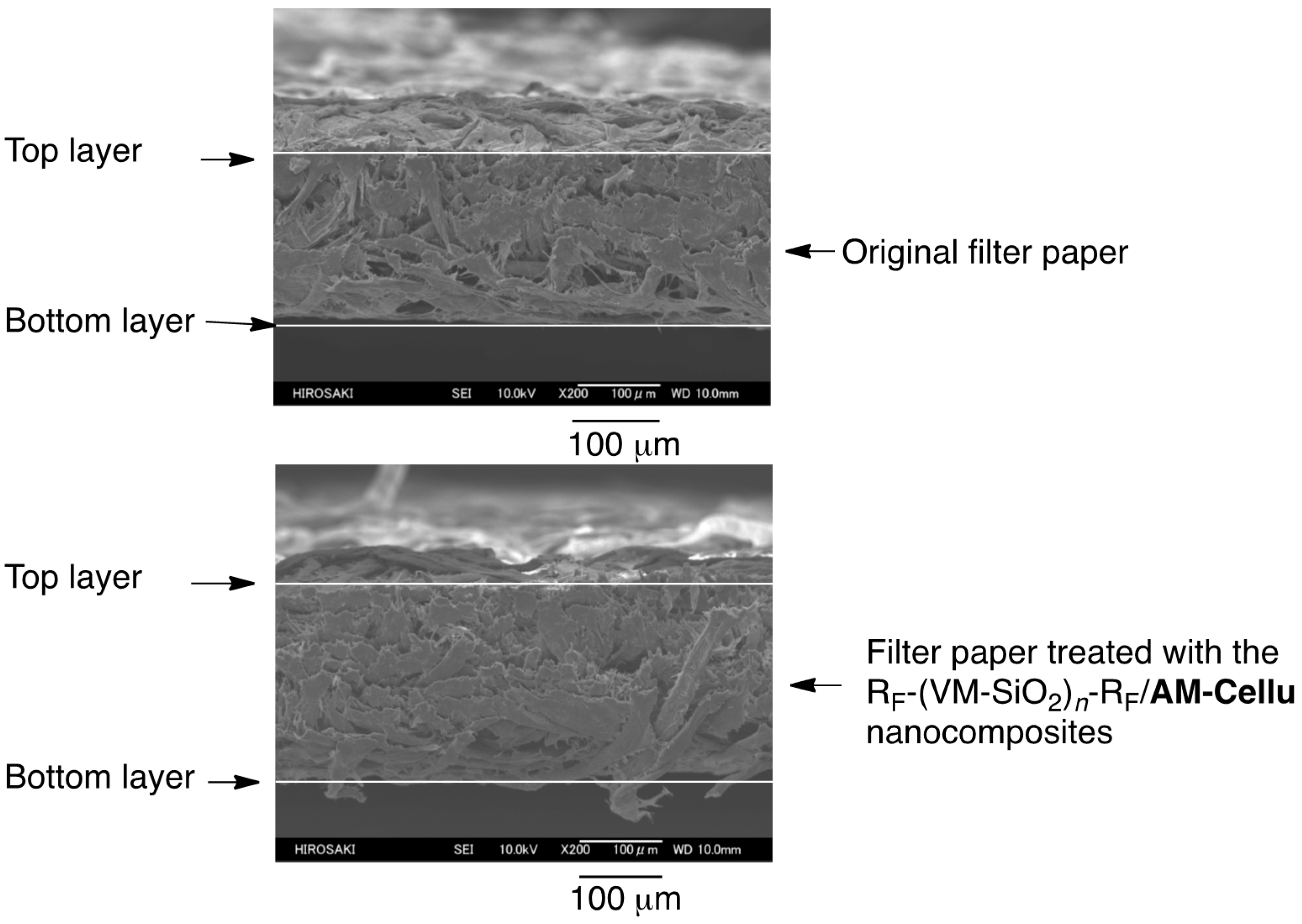
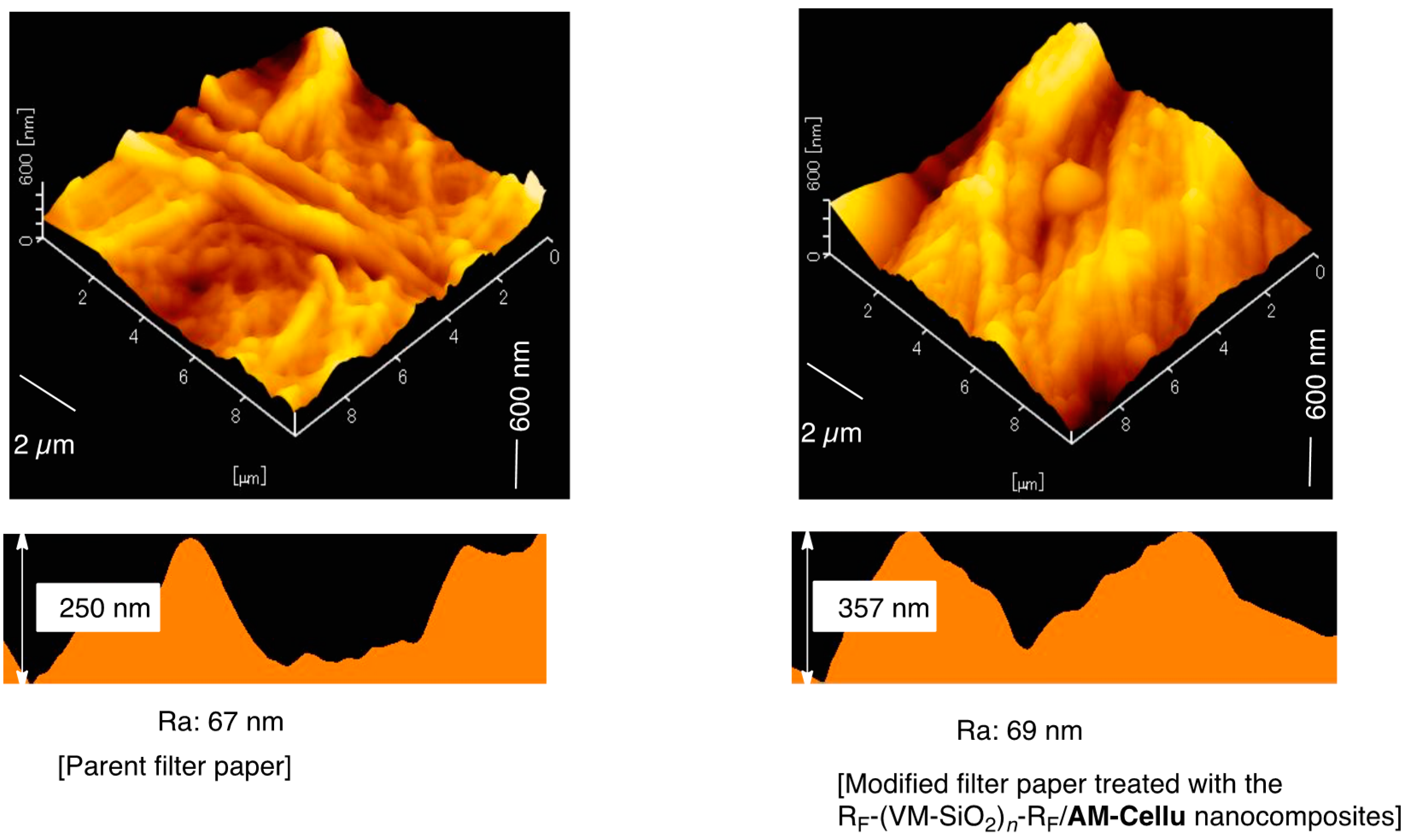
3.3. Surface Modification of Filter Paper by Using the RF-(VM-SiO2)n-RF/AM-Cellu Nanocomposites
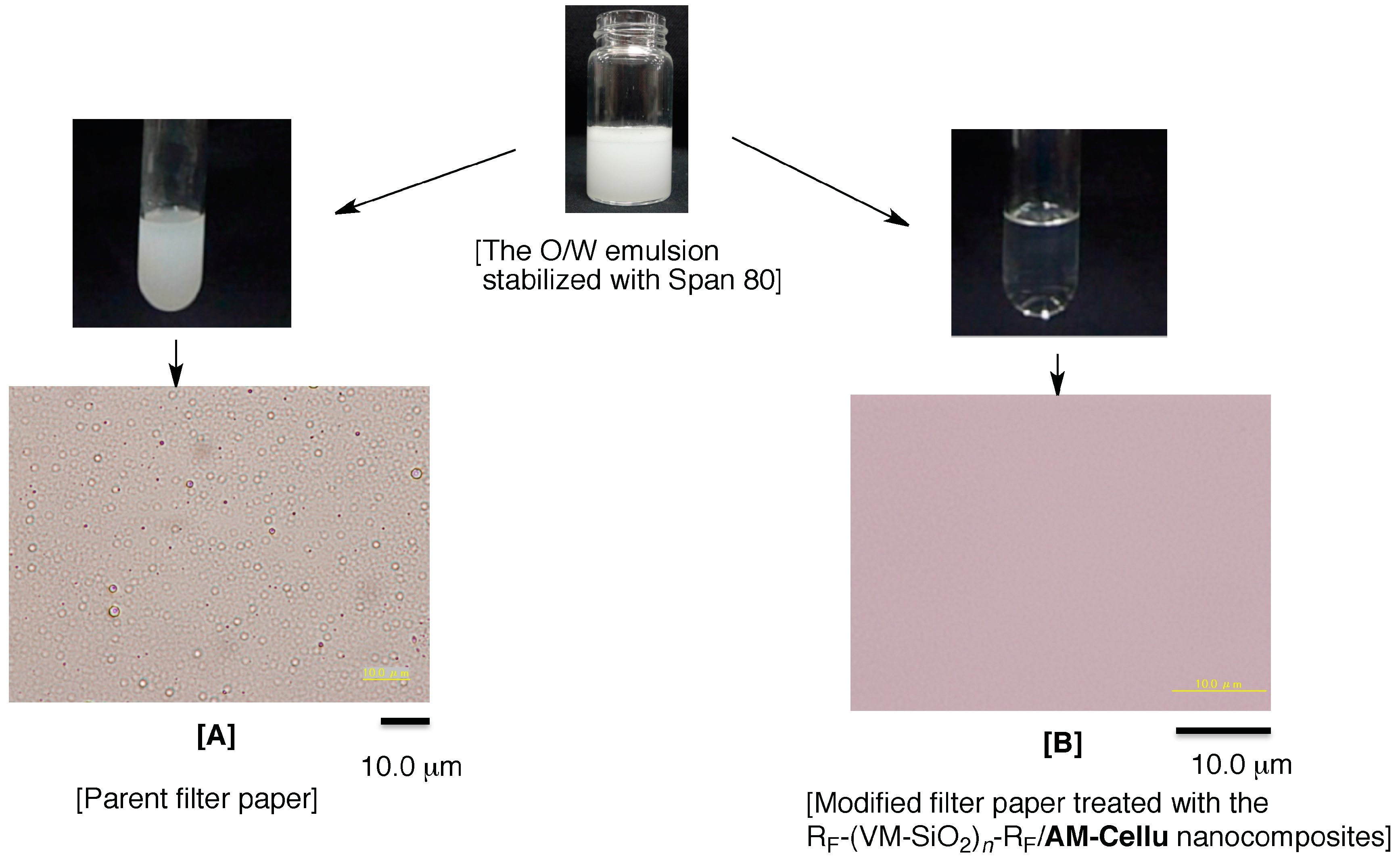
3.4. Separation of W/O Emulsion by Using the Modified Filter Paper Treated with the RF-(VM-SiO2)n-RF/AM-Cellu Nanocomposites as the Separation Membrane
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A.A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic coatings on cellulose-based materials: fabrication, properties, and applications. Adv. Mater. Interfaces 2014, 1, 1300026. [Google Scholar] [CrossRef]
- Li, S.; Wei, Y.; Huang, J. Facile fabrication of superhydrophobic cellulose materials by a nanocoating approach. Chem. Lett. 2010, 39, 20–21. [Google Scholar] [CrossRef]
- Arbatan, T.; Zhang, L.; Fang, X.-Y.; Shen, W. Cellulose nanofibers as binder for fabrication of superhydrophobic paper. Chem. Eng. J. 2012, 210, 74–79. [Google Scholar] [CrossRef]
- Yang, H.; Deng, Y. Preparation and physical properties of superhydrophobic papers. J. Colloid Interface Sci. 2008, 325, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wan, H.; Ye, Y.; Zhou, H.; Chen, J. One-step process to fabrication of transparent superhydrophobic SiO2 paper. Appl. Surface Sci. 2012, 261, 470–472. [Google Scholar] [CrossRef]
- Du, C.; Wang, J.; Chen, Z.; Chen, D. Durable superhydrophobic and superoleophilic filter paper for oil–water separation prepared by a colloidal deposition method. Appl. Surface Sci. 2014, 313, 304–310. [Google Scholar] [CrossRef]
- Li, S.; Xie, H.; Zhang, S.; Wang, X. Facile transformation of hydrophilic cellulose into superhydrophobic cellulose. Chem. Commun. 2007, 46, 4857–4859. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Wang, X. Fabrication of Superhydrophobic Cellulose-Based Materials through a Solution-Immersion Process. Langmuir 2008, 24, 5585–5590. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, D.; Lindqvist, J.; Ostmark, E.; Hult, A.; Malmstrom, E. Superhydrophobic bio-fibre surfaces via tailored grafting architecture. Chem. Commun. 2006, 34, 3594–3596. [Google Scholar] [CrossRef] [PubMed]
- Calmark, A.; Malmstrom, E. Atom transfer radical polymerization from cellulose fibers at ambient temperature. J. Am. Chem. Soc. 2002, 124, 900–901. [Google Scholar] [CrossRef]
- Wang, T.; Hu, X.; Dong, S. A general route to transform normal hydrophilic cloths into superhydrophobic surfaces. Chem. Commun. 2007, 18, 1849–1851. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H. Preparation and applications of novel fluoroalkyl end-capped oligomeric nanocomposites. Polym. Chem. 2012, 3, 46–65. [Google Scholar] [CrossRef]
- Goto, Y.; Takashima, H.; Takishita, K.; Sawada, H. Creation of coating surfaces possessing superhydrophobic and superoleophobic characteristics with fluoroalkyl end-capped vinyltrimethoxysilane oligomeric nanocomposites having biphenylene segments. J. Colloid Interface Sci. 2011, 362, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, Y.; Saito, T.; Yamada, M.; Sugita, M.; Sawada, H. Preparation and surface property of fluoroalkyl end-capped vinyltrimethoxysilane oligomer/talc composite-encapsulated organic compounds: Application for the separation of oil and water. ACS Appl. Mater. Interfaces 2015, 7, 13782–13793. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Tsushima, Y.; Sawada, H. Facile creation of superoleophobic and superhydrophilic surface by using fluoroalkyl end-capped vinyltrimethoxysilane oligomer/calcium silicide nanocomposites—Development of these nanocomposites to environmental cyclical type-fluorine recycle through formation of calcium fluoride. Colloid Polym. Sci. 2015, 293, 65–73. [Google Scholar]
- Ratcha, A.; Saito, T.; Takahashi, R.; Kongparakul, S.; Sawada, H. Preparation and thermal stability of fluoroalkyl end-capped vinyltrimethoxysilane oligomeric silica/poly(acrylonitrile-co-butadiene) nanocomposites—Application to the separation of oil and water. Colloid Polym. Sci. 2016, 294, 1529–1539. [Google Scholar] [CrossRef]
- Suzuki, J.; Takegahara, Y.; Oikawa, Y.; Chiba, M.; Yamada, S.; Sugiya, M.; Sawada, H. Preparation of fluoroalkyl end-capped vinyltrimethoxysilane oligomeric silica/poly(tetrafluoroethylene) nanocomposites possessing a superoleophilic/superhydrophobic characteristic: application to the separation of oil and water. J. Sol-Gel Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Sawada, H.; Nakayama, M. Synthesis of fluorine-containing organosilicon oligomers. J. Chem. Soc. Chem. Commun. 1991, 10, 677–678. [Google Scholar] [CrossRef]
- Sawada, H.; Suzuki, T.; Takashima, H.; Takishita, K. Preparation and properties of fluoroalkyl end-capped vinyltrimethoxysilane oligomeric nanoparticles—A new approach to facile creation of a completely superhydrophobic coating surface with these nanoparticles. Colloid Polym. Sci. 2008, 286, 1569–1574. [Google Scholar] [CrossRef]
- Sawada, H.; Ikematsu, Y.; Kawase, T.; Hayakawa, Y. Synthesis and Surface Properties of Novel Fluoroalkylated Flip-Flop-Type Silane Coupling Agents. Langmuir 1996, 12, 3529–3530. [Google Scholar] [CrossRef]
- Hirokawa, Y.; Tanaka, T. Nonionic N-isopropylacrylamide gel was found to undergo a discontinuous phase transition by changing a solvent composition or temperature. The observation that polymergel with and without charge can undergo a first order volume phase transition is an evidence for the universality of the phase transition of polymergels. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar]
- Si, Y.; Guo, Z. Superwetting Materials of Oil–Water Emulsion Separation. Chem. Lett. 2015, 44, 874–883. [Google Scholar] [CrossRef]
- Liu, K.; Tian, Y.; Jiang, L. Bio-inspired superoleophobic and smart materials: Design, fabrication, and application. Prog. Mater. Sci. 2013, 58, 503–564. [Google Scholar] [CrossRef]
- Celia, E.; Darmanin, T.; de Givenchy, E.T.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, M.; Lu, Q. Filter paper with selective absorption and separation of liquids that differ in surface tension. ACS Appl. Mater. Interfaces 2010, 2, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Wang, S.; Shi, Y.; Li, J. Fabrication of coral-like superhydrophobic coating on filter paper for water–oil separation. Appl. Surface Sci. 2012, 261, 764–769. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, S.; Shi, Y.; Li, J. Fabrication of superhydrophobic cotton textiles for water–oil separation based on drop-coating route. Carbohydr. Polym. 2013, 97, 59–64. [Google Scholar] [CrossRef] [PubMed]
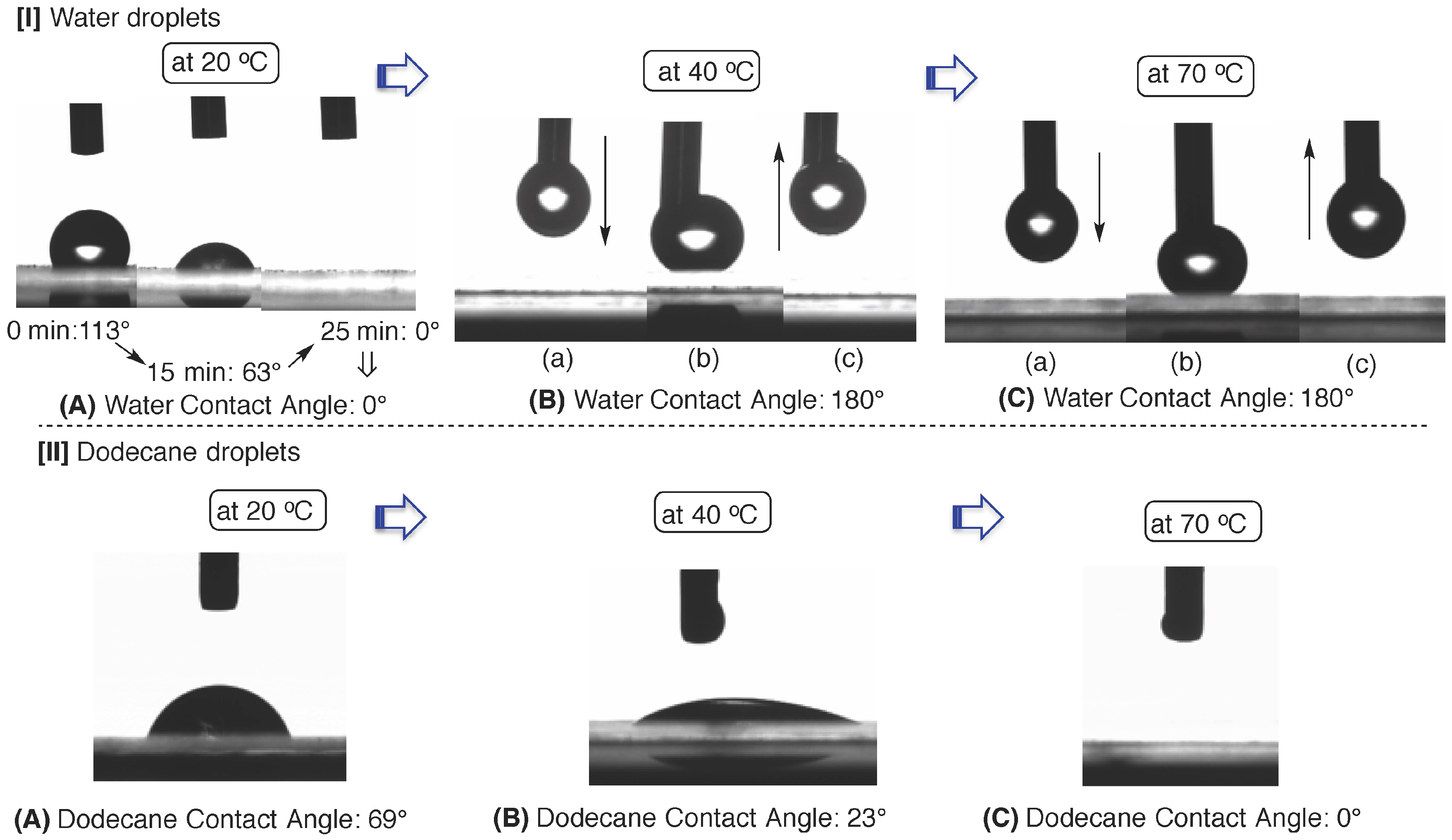
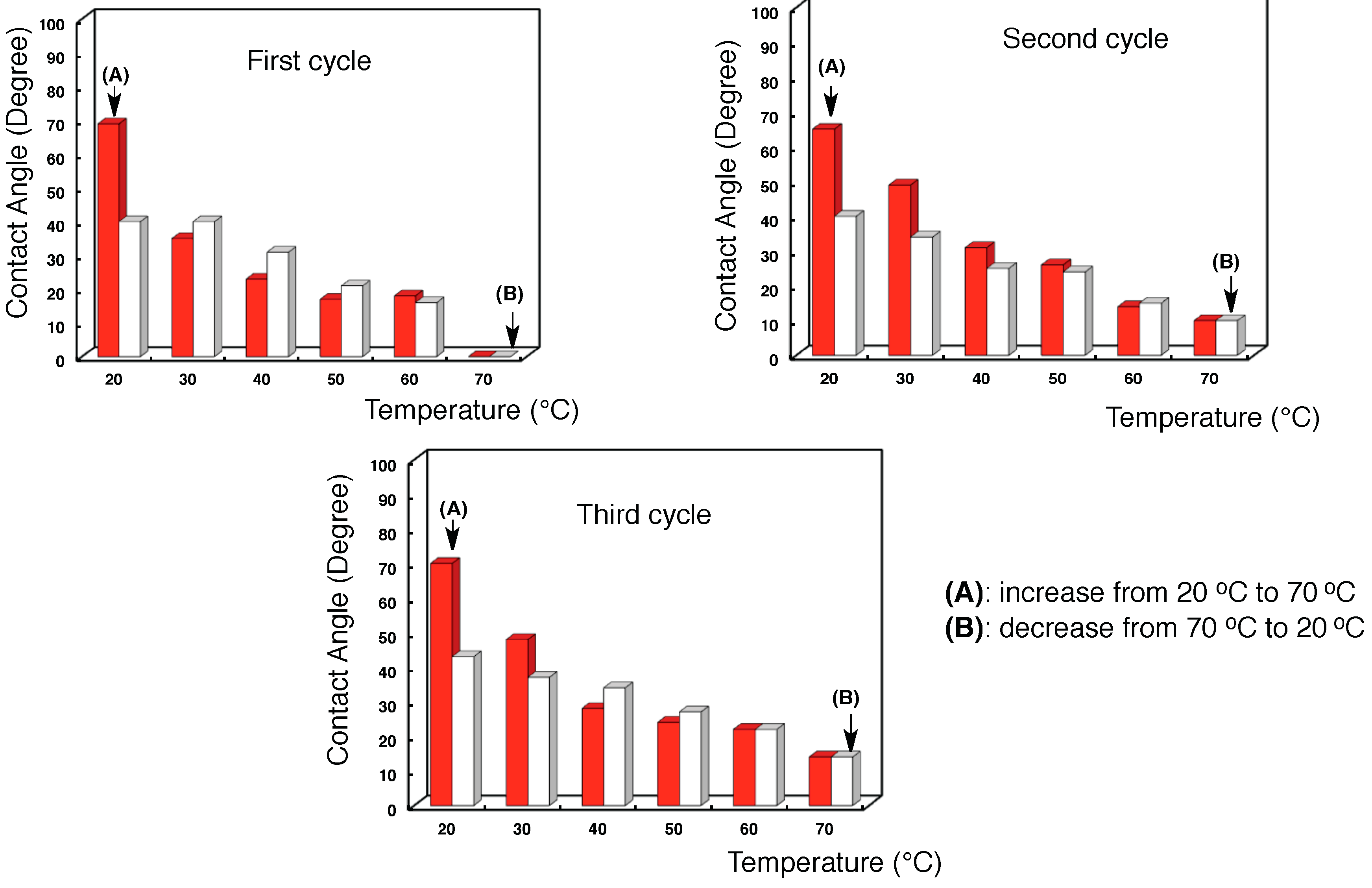
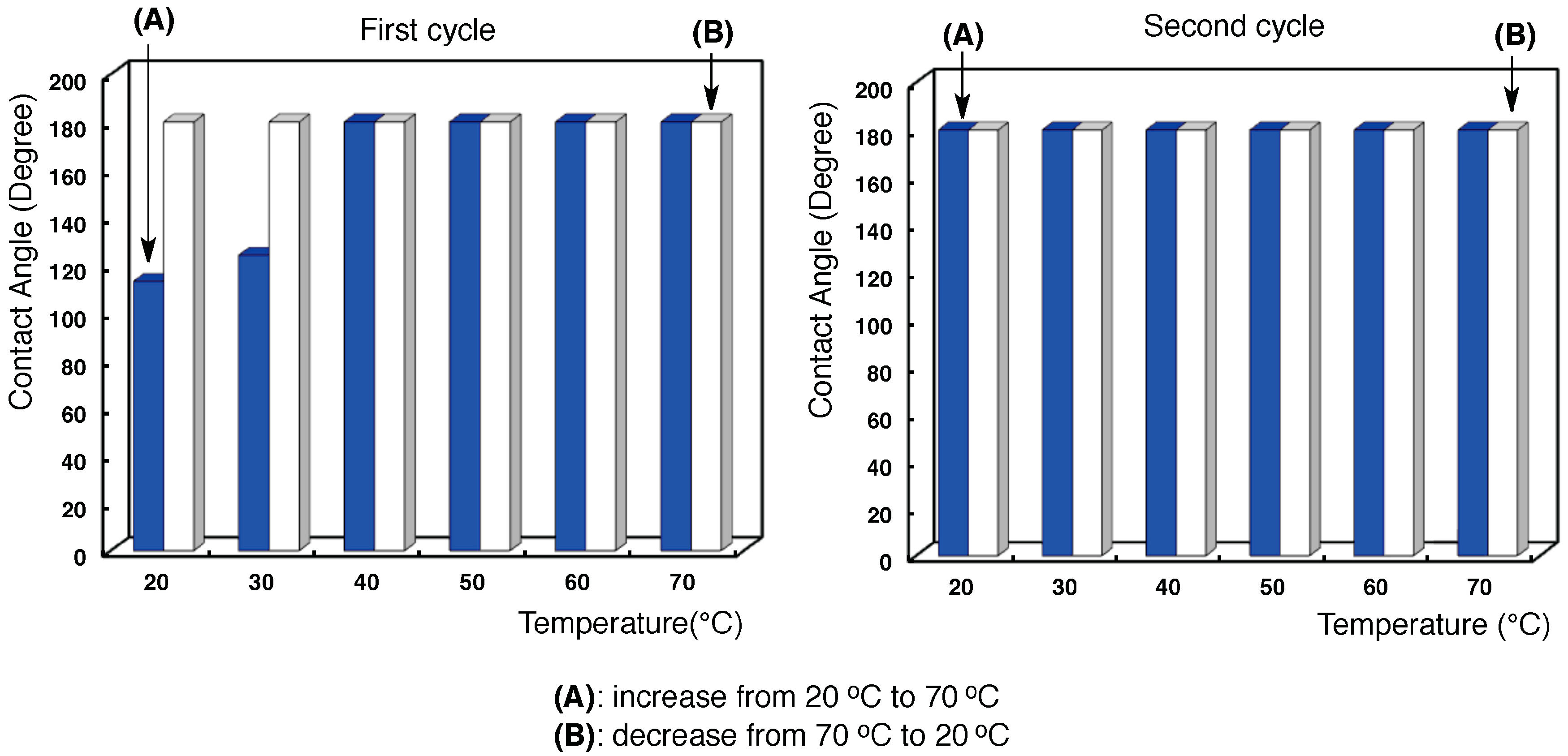
| Temperature (°C) | Contact angel (°) | |||||||
|---|---|---|---|---|---|---|---|---|
| Dodecane a | Water | |||||||
| Time (min) | ||||||||
| 0 | 5 | 10 | 15 | 20 | 25 | 30 | ||
| 20 | 69 | 113 | 95 | 88 | 63 | 38 | 0 | 0 |
| 30 | 35 | 124 | 96 | 86 | 75 | 51 | 17 | 0 |
| 40 | 23 | 180 | - b | - b | - b | - b | - b | - b |
| 50 | 17 | 180 | - c | - c | - c | - c | - c | - c |
| 60 | 18 | 180 | - c | - c | - c | - c | - c | - c |
| 70 | 0 | 180 | - c | - c | - c | - c | - c | - c |
| Temperature | 25 °C | 70 °C |
|---|---|---|
| Dodecane contact angle: | 0° | 0° |
| Water contact angle: | 180° | 180° |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, H.; Suto, Y.; Saito, T.; Oikawa, Y.; Yamashita, K.; Yamada, S.; Sugiya, M.; Suzuki, J.-i. Preparation of RF-(VM-SiO2)n-RF/AM-Cellu Nanocomposites, and Use Thereof for the Modification of Glass and Filter Paper Surfaces: Creation of a Glass Thermoresponsive Switching Behavior and an Efficient Separation Paper Membrane. Polymers 2017, 9, 92. https://doi.org/10.3390/polym9030092
Sawada H, Suto Y, Saito T, Oikawa Y, Yamashita K, Yamada S, Sugiya M, Suzuki J-i. Preparation of RF-(VM-SiO2)n-RF/AM-Cellu Nanocomposites, and Use Thereof for the Modification of Glass and Filter Paper Surfaces: Creation of a Glass Thermoresponsive Switching Behavior and an Efficient Separation Paper Membrane. Polymers. 2017; 9(3):92. https://doi.org/10.3390/polym9030092
Chicago/Turabian StyleSawada, Hideo, Yuki Suto, Tomoya Saito, Yuri Oikawa, Katsumi Yamashita, Satoshi Yamada, Masashi Sugiya, and Jun-ichi Suzuki. 2017. "Preparation of RF-(VM-SiO2)n-RF/AM-Cellu Nanocomposites, and Use Thereof for the Modification of Glass and Filter Paper Surfaces: Creation of a Glass Thermoresponsive Switching Behavior and an Efficient Separation Paper Membrane" Polymers 9, no. 3: 92. https://doi.org/10.3390/polym9030092
APA StyleSawada, H., Suto, Y., Saito, T., Oikawa, Y., Yamashita, K., Yamada, S., Sugiya, M., & Suzuki, J.-i. (2017). Preparation of RF-(VM-SiO2)n-RF/AM-Cellu Nanocomposites, and Use Thereof for the Modification of Glass and Filter Paper Surfaces: Creation of a Glass Thermoresponsive Switching Behavior and an Efficient Separation Paper Membrane. Polymers, 9(3), 92. https://doi.org/10.3390/polym9030092





