Simple Synthesis of Hydroxyl and Ethylene Functionalized Aromatic Polyamides as Sizing Agents to Improve Adhesion Properties of Aramid Fiber/Vinyl Epoxy Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
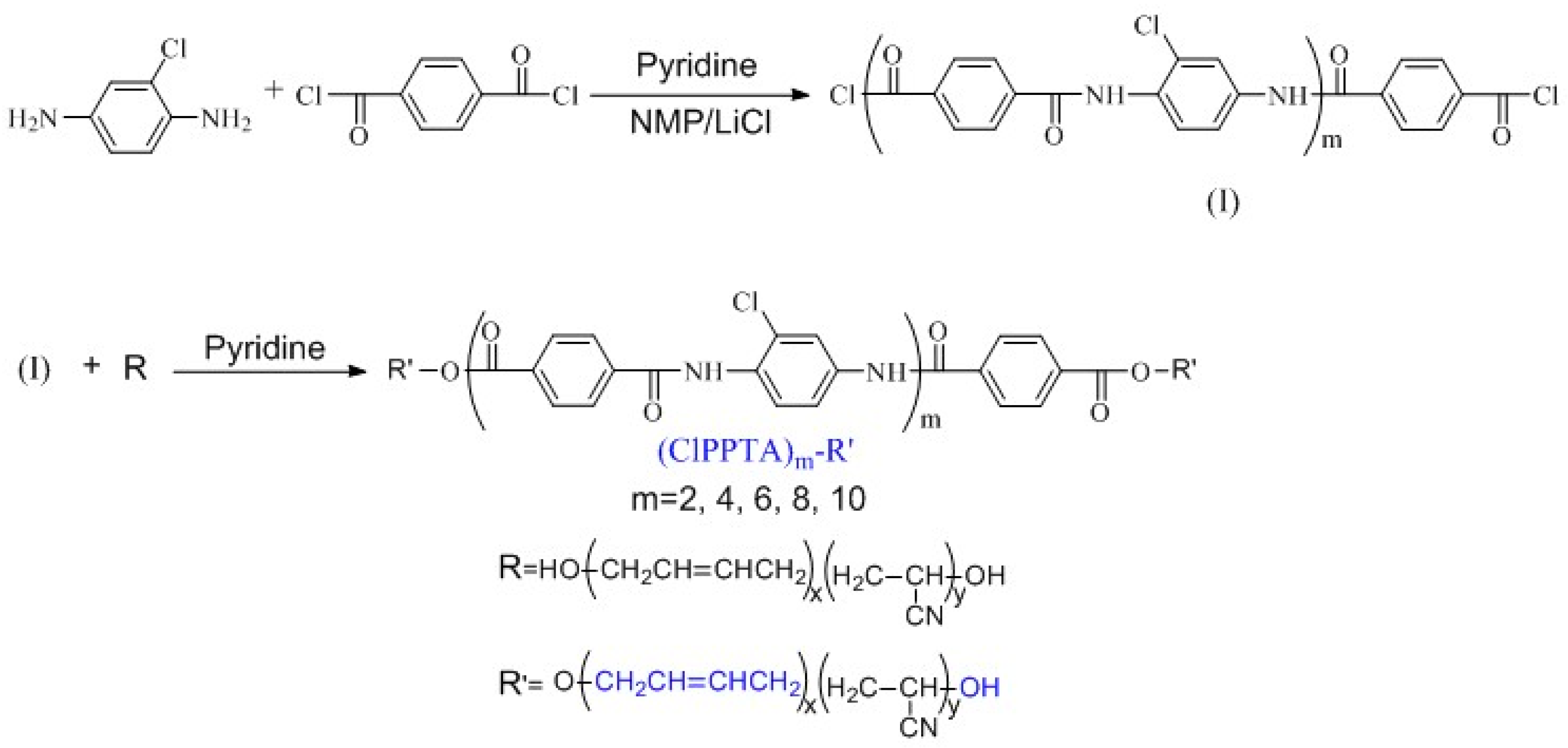
2.2. General Polymerization Procedure
2.3. Films Fabrication
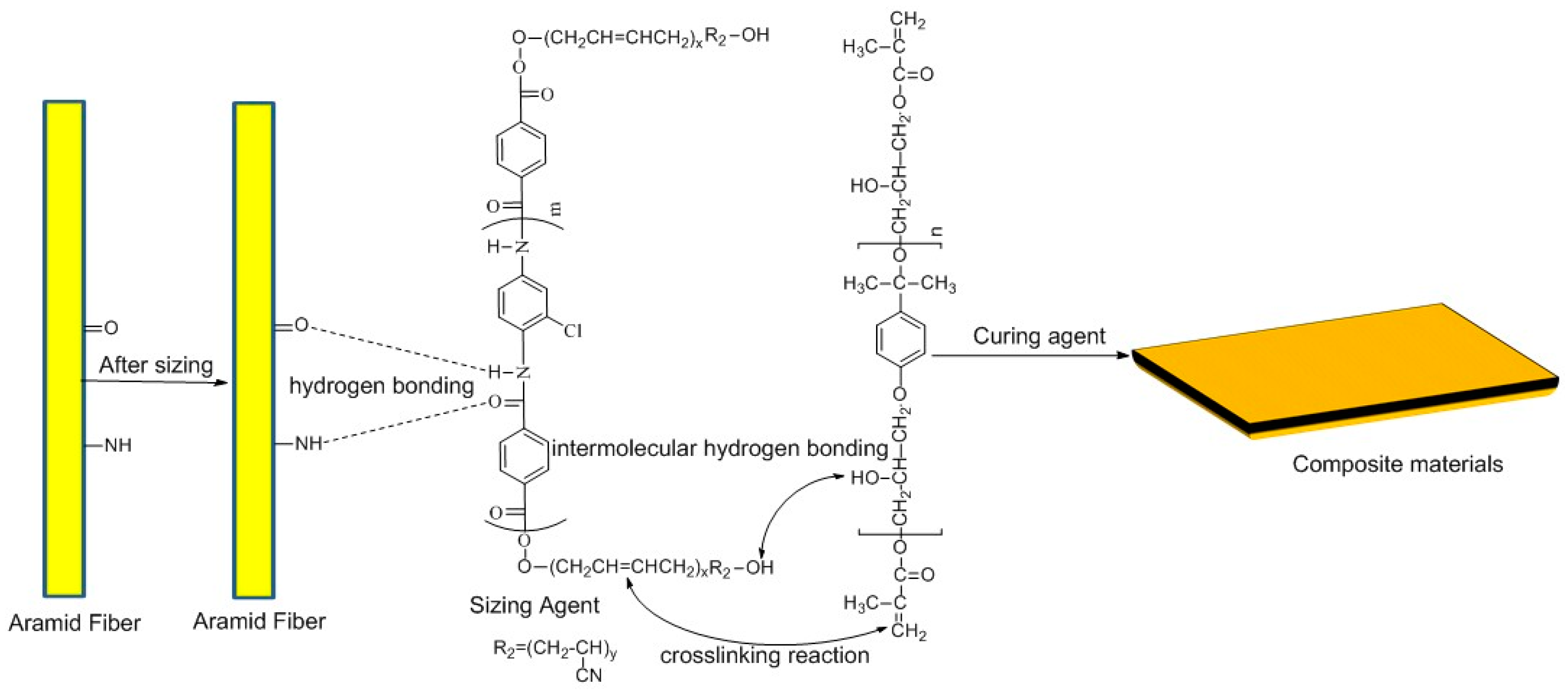
2.4. Sizing of Fibers Preparation
2.5. Characterization
3. Results and Discussion
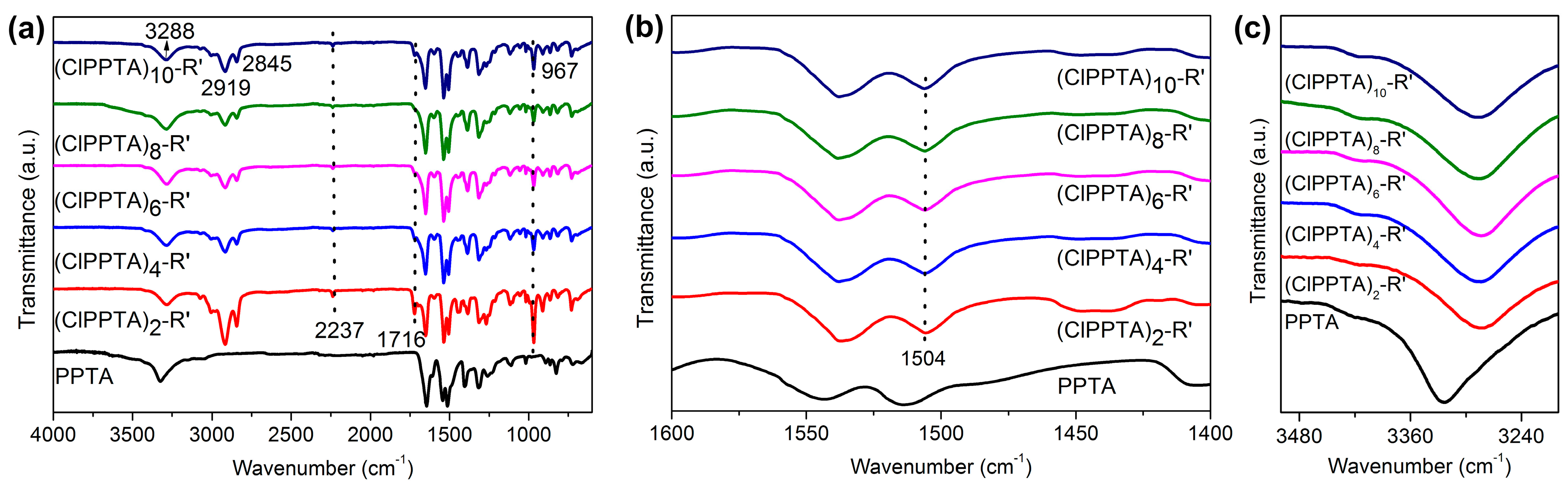
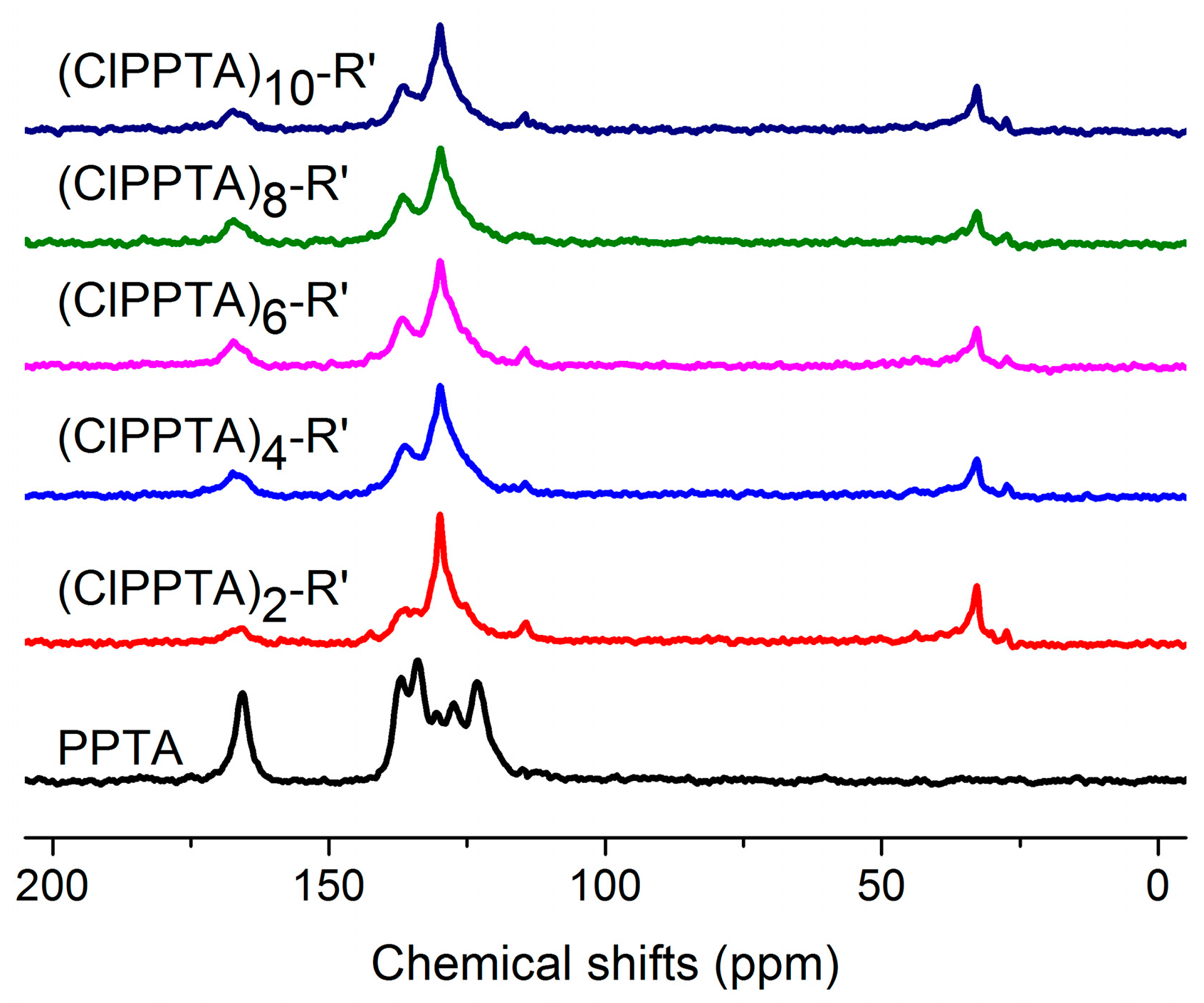
3.1. Molecular Structure
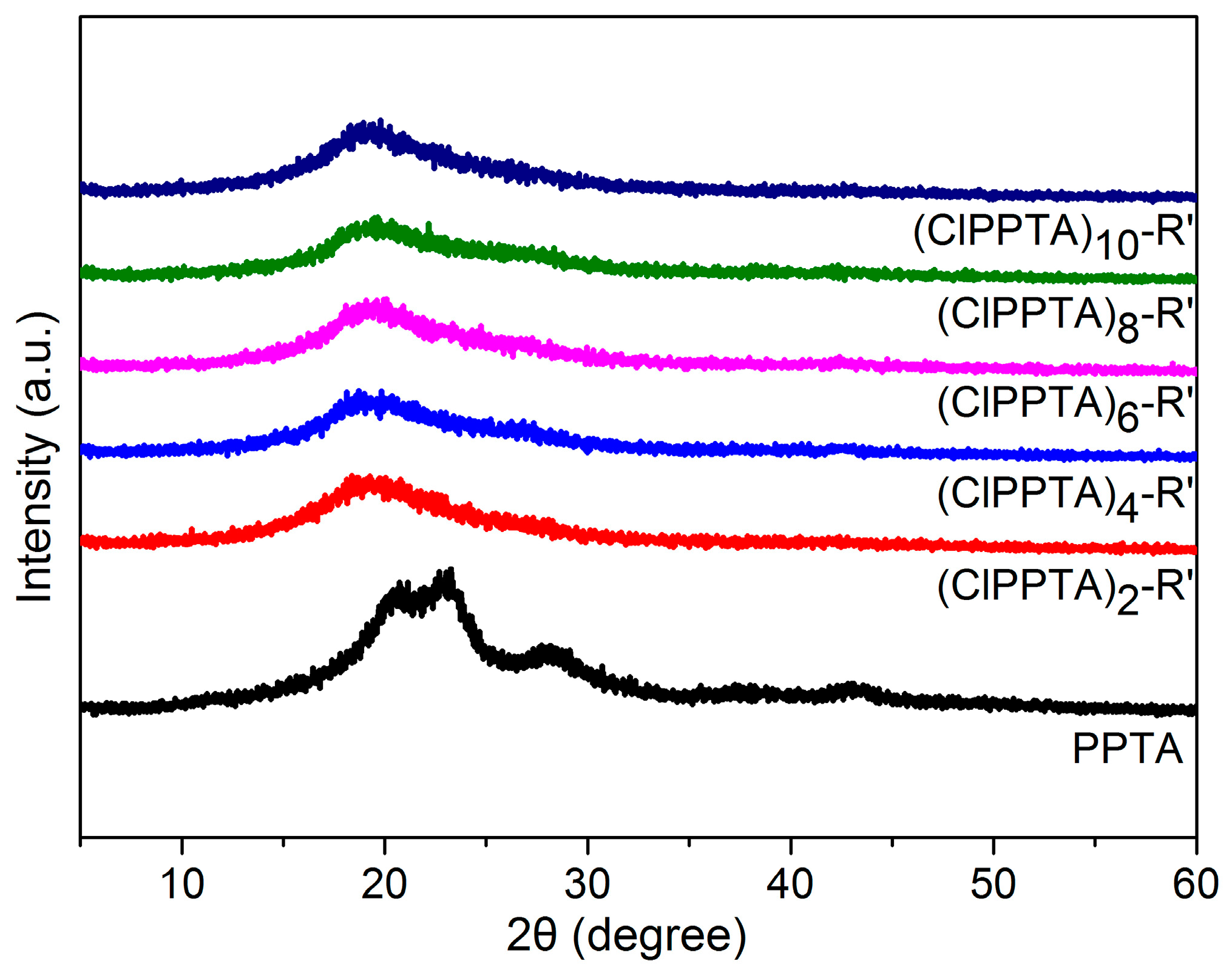
3.2. Crystalline Structures
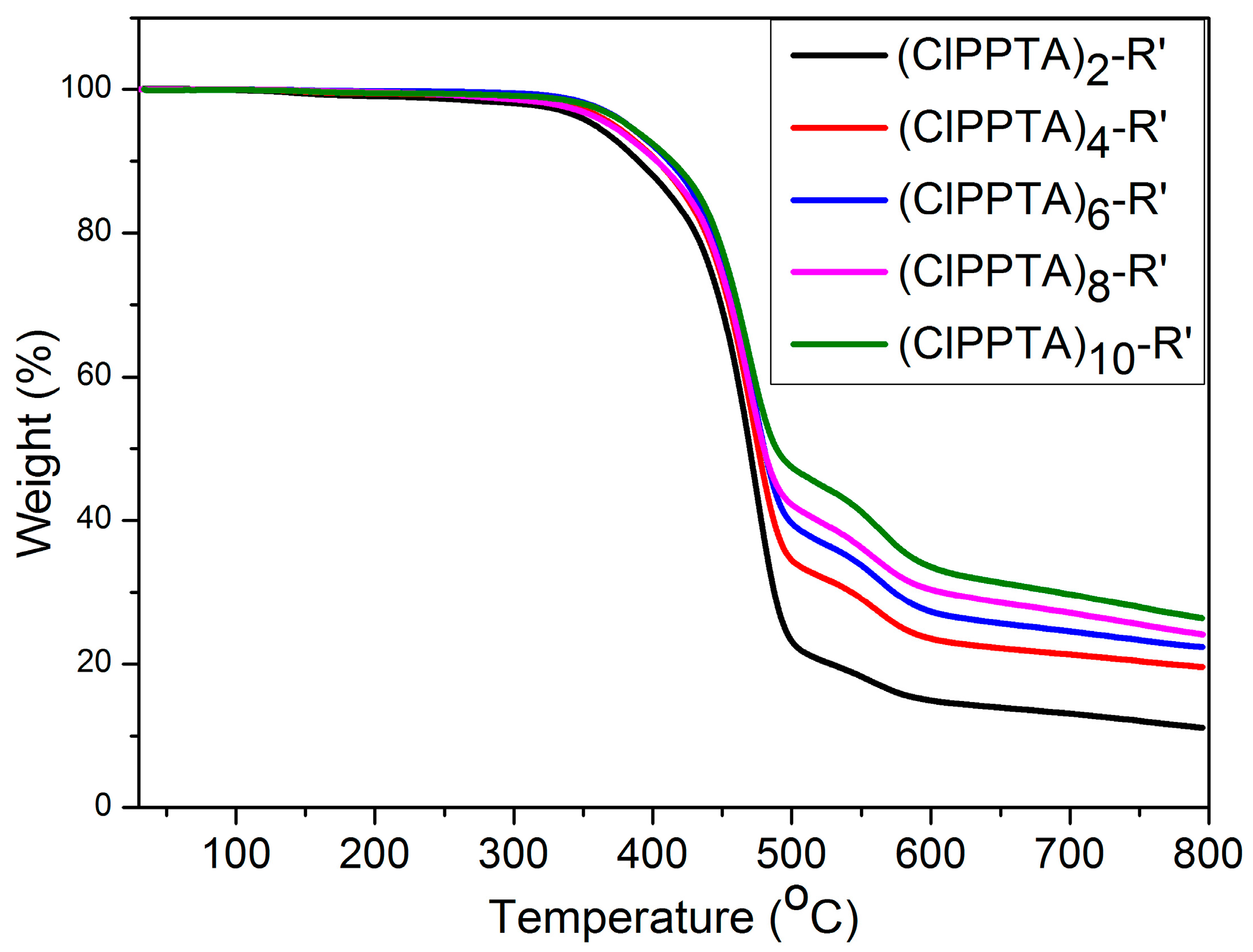
3.3. Thermal Stability
3.4. Solubility
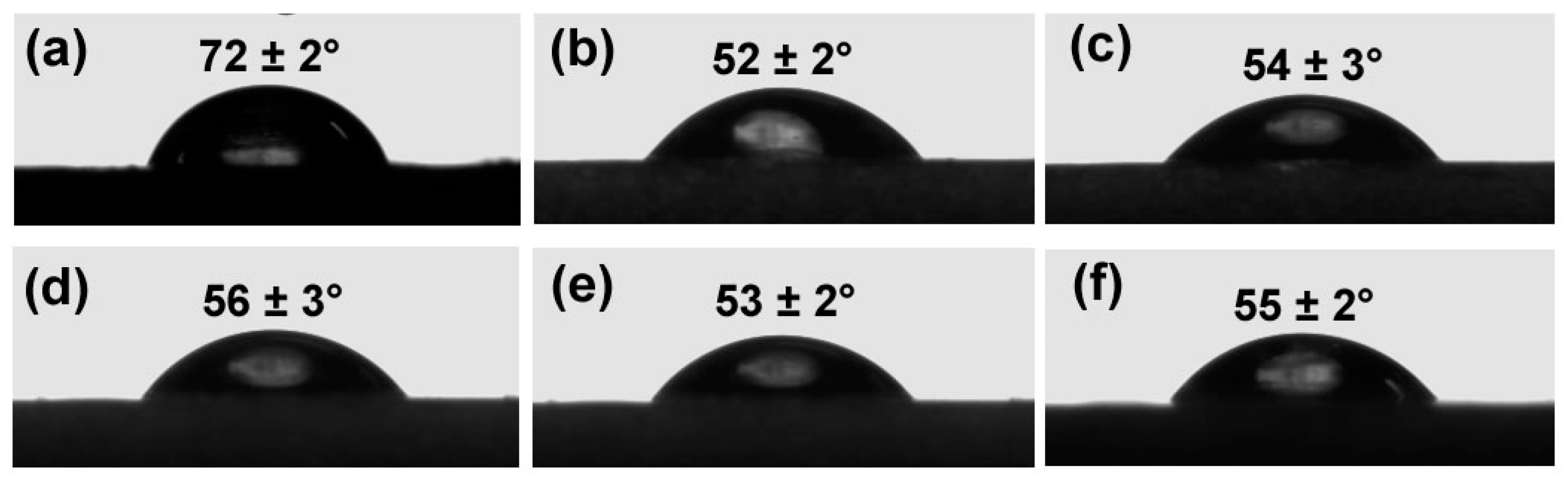
3.5. Wettability
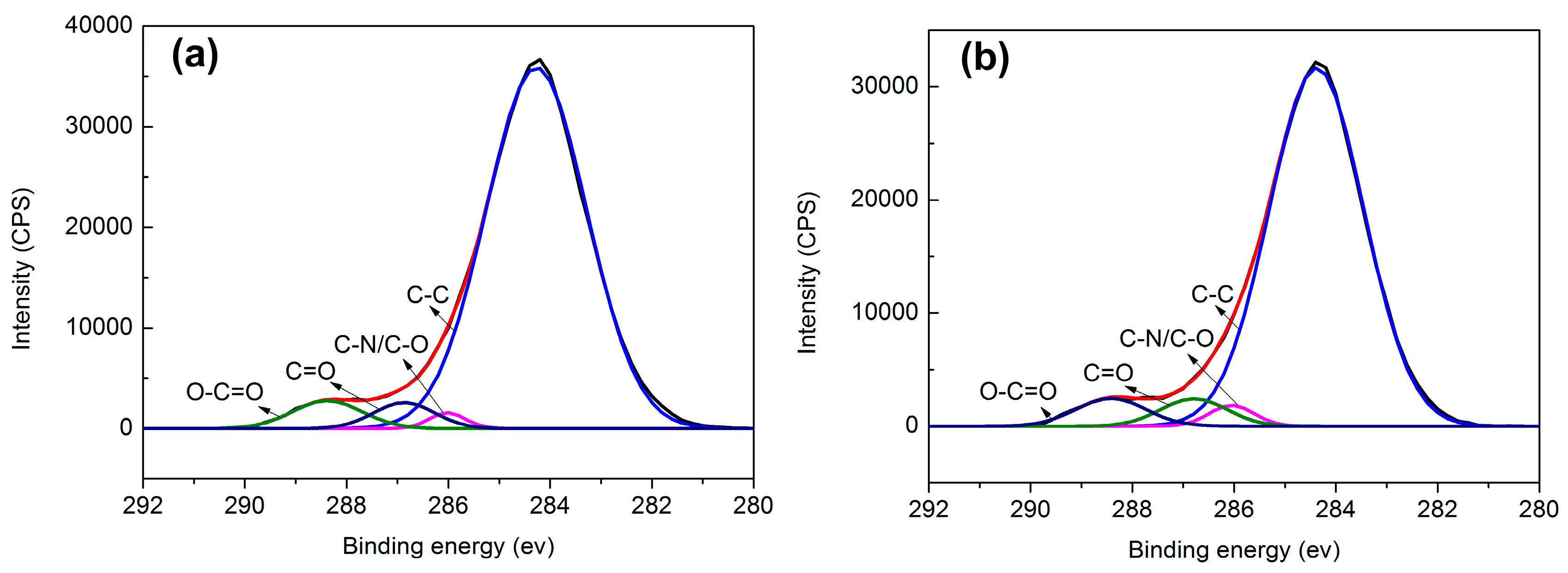
3.6. Surface Chemical Composition of the Aramid Fibers
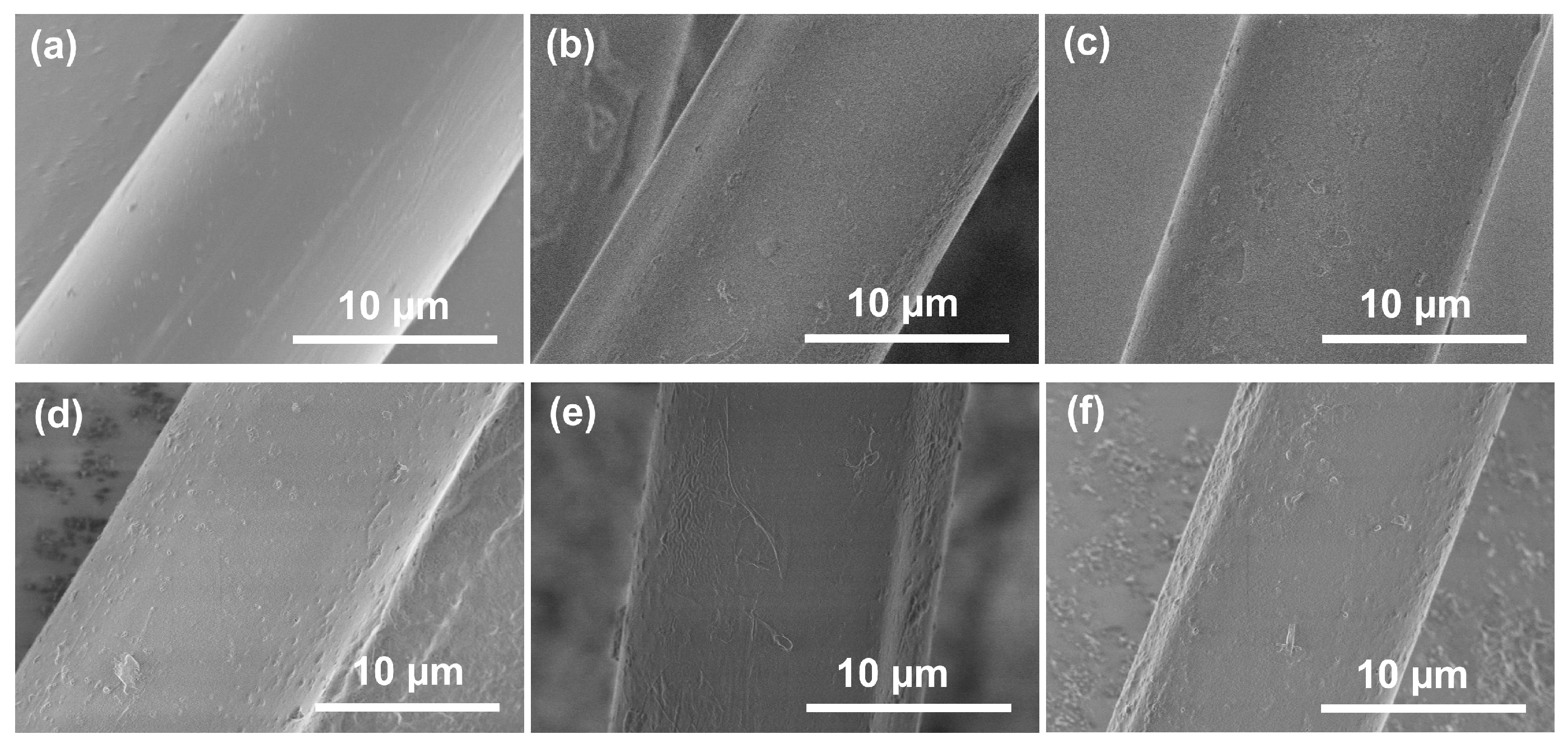
3.7. Surface Morphologies of Aramid Fibers
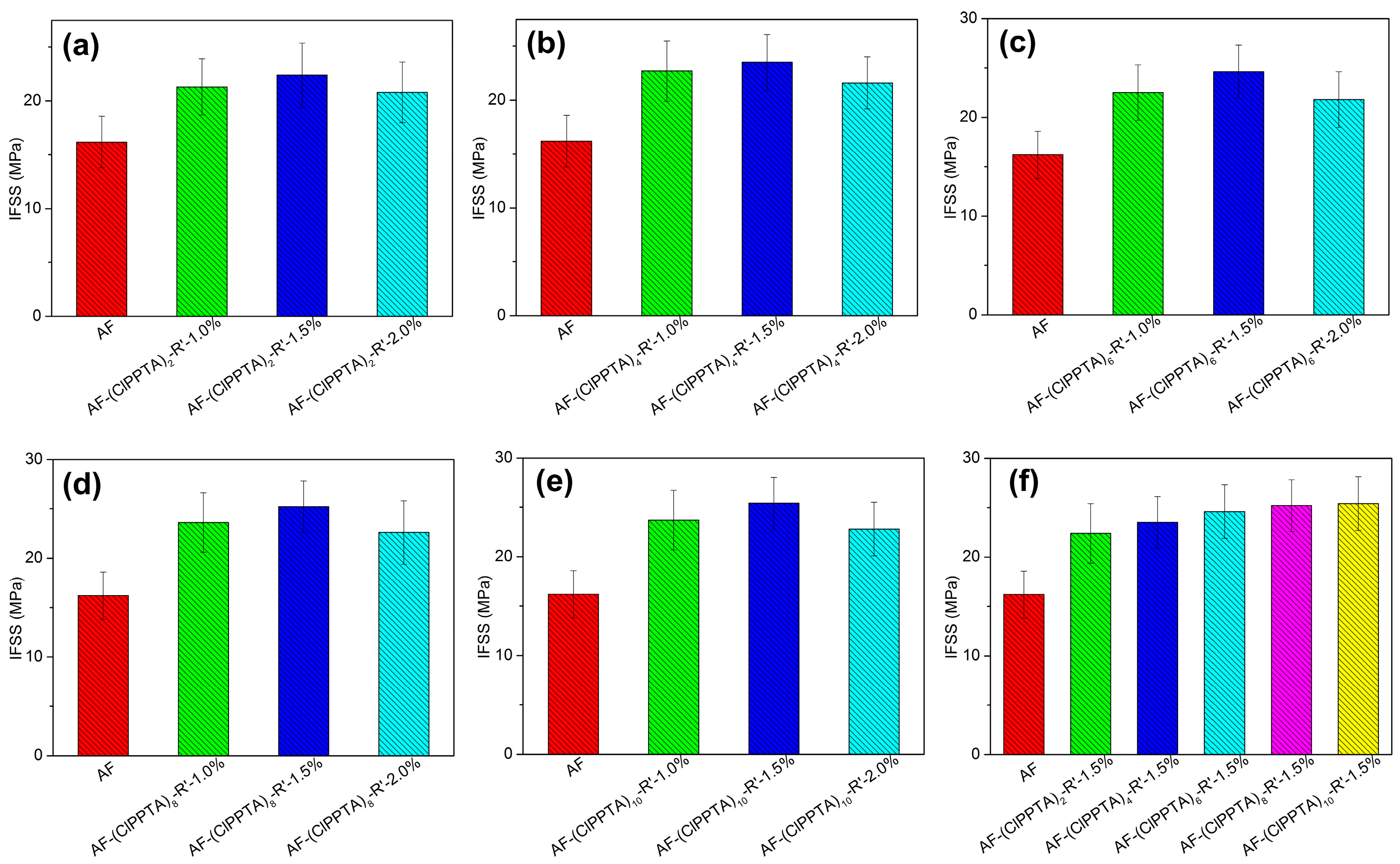
3.8. Interfacial Adhesion of Vinyl Epoxy Resin to Aramid Fibers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kang, S.J.; Hong, S.I.; Park, C.R. Preparation and properties of a new aromatic polyamide with an ethoxycarbonyl pendant group: Poly(4,4′-diamino-2′-ethoxycarbonyl-benzanilide terephthalamide). J. Polym. Sci. Part A Polym. Chem. 2000, 38, 936–942. [Google Scholar] [CrossRef]
- Guan, Y.; Zheng, Y.J.; Cui, J.X.; Wan, X.H. Synthesis and characterization of graft copolymers based on poly(p-phenylene terephthalamide) backbone and well-defined polystyrene side chains. Chin. J. Polym. Sci. 2010, 28, 257–267. [Google Scholar] [CrossRef]
- Du, S.M.; Zhang, J.; Guan, Y.; Wan, X.H. Sequence effects on properties of the poly(p-phenylene terephthalamide)-based macroinitiators and their comb-like copolymers grafted by polystyrene side chains. Aust. J. Chem. 2013, 67, 39–48. [Google Scholar] [CrossRef]
- Agarwal, U.S.; Khakhar, D.V. Enhancement of polymerization rates for rigid rod-like molecules by shearing. Nature 1992, 360, 53–55. [Google Scholar] [CrossRef]
- García, J.M.; García, F.C.; Serna, F.; de la Penã, J.L. High-performance aromatic polyamides. Prog. Polym. Sci. 2010, 35, 623–686. [Google Scholar] [CrossRef]
- García, J.M.; García, F.C.; Serna, F.; de la Penã, J.L. Handbook of Engineering and Specialty Thermoplastics; John Wiley & Sons, Inc.: New York, NY, USA, 2011; pp. 141–181. [Google Scholar]
- Xi, M.; Li, Y.L.; Shang, S.Y.; Li, D.H.; Yin, Y.X.; Dai, X.Y. Surface modification of aramid fiber by air DBD plasma at atmospheric pressure with continuous on-line processing. Surf. Coat. Technol. 2008, 202, 6029–6033. [Google Scholar] [CrossRef]
- Jia, C.; Chen, P.; Liu, W.; Li, B.; Wang, Q. Surface treatment of aramid fiber by air dielectric barrier discharge plasma at atmospheric pressure. Appl. Surf. Sci. 2011, 257, 4165–4170. [Google Scholar] [CrossRef]
- Gu, R.X.; Yu, J.R.; Hu, C.C.; Chen, L.; Zhu, J.; Hu, Z.M. Surface treatment of para-aramid fiber by argon dielectric barrier discharge plasma at atmospheric pressure. Appl. Surf. Sci. 2012, 258, 10168–10174. [Google Scholar] [CrossRef]
- Kong, H.J.; Yang, P.; Teng, C.Q.; Yu, M.H. Surface modification of poly(p-phenylene terephthalamide) fibers with HDI assisted by supercritical carbon dioxide. RSC Adv. 2015, 5, 58916–58920. [Google Scholar]
- Gu, H. Tensile behaviours of quartz, aramid and glass filaments after NaCl treatment. Mater. Des. 2009, 30, 867–870. [Google Scholar] [CrossRef]
- Fan, G.; Zhao, J.; Zhang, Y.; Guo, Z. Grafting modification of Kevlar fiber using horseradish peroxidase. Polym. Bull. 2006, 56, 507–515. [Google Scholar] [CrossRef]
- Hussain, S.; Yorucu, C.; Ahmed, I.; Hussain, R.; Chen, B.; Siddique, N.A.; Rehman, I.U. Surface modification of aramid fibres by graphene oxide nano-sheets for multiscale polymer composites. Surf. Coat. Technol. 2014, 258, 458–466. [Google Scholar] [CrossRef]
- Shirazi, M.; de Rooij, M.B.; Talma, A.G.; Noordermeer, K.W.M. Adhesion of RFL-coating aramid fibers to elastomers: The role of elastomer-latex compatibility. J. Adhes. Sci. Technol. 2013, 27, 1886–1898. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.X.; Qiu, Y.P. Influence of aramid fiber moisture regain during atmospheric plasma treatment on aging of treatment effects on surface wettability and bonding strength to epoxy. Appl. Surf. Sci. 2007, 253, 9283–9289. [Google Scholar] [CrossRef]
- De Lange, P.J.; Akker, P.G.; Mass, A.J.H.; Knoester, A.; Brongerama, H.H. Adhesion activation of Twaron® aramid fibres studied with low-energy ion scattering and X-ray photoelectron spectroscopy. Surf. Interface Anal. 2010, 31, 1079–1084. [Google Scholar] [CrossRef]
- De Lange, P.J.; Akker, P.G.; Maeder, E.; Gao, S.L.; Prasithphol, W.; Young, R.J. Controlled interfacial adhesion of Twaron®; aramid fibres in composites by the finish formulation. Compos. Sci. Technol. 2007, 67, 2027–2035. [Google Scholar] [CrossRef]
- De Lange, P.J.; Akker, P.G. Adhesion Activation of Twaron Aramid Fibers for Application in Rubber: Plasma versus Chemical Treatment. J. Adhes. Sci. Technol. 2012, 26, 827–839. [Google Scholar]
- Wu, J.; Cheng, X.H.; Xie, C.Y. Influence of rare earth surface treatment on tensile properties of aramid fiber reinforced epoxy composites. J. Mater. Sci. 2004, 39, 289–290. [Google Scholar] [CrossRef]
- Sa, R.; Yan, Y.; Wei, Z.H.; Zhang, L.Q.; Wang, W.C.; Ting, M. Surface modification of aramid fibers by bio-inspired poly(dopamine) and epoxy functionalized silane grafting. ACS Appl. Mater. Interfaces 2014, 6, 21730–21738. [Google Scholar] [CrossRef] [PubMed]
- Du, S.M.; Wang, W.B.; Yan, Y.; Zhang, J.; Tian, M.; Zhang, L.Q.; Wan, X.H. A facile synthetic route to poly(p-phenylene terephthalamide) with dual functional groups. Chem. Commun. 2014, 50, 9929–9931. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.B.; Qi, X.; Guan, Y.; Zhang, F.; Zhang, J.; Yan, C.; Zhu, Y.D.; Wan, X.H. Synthesis and properties of poly(p-phenylene terephthalamide) bearing both polar and unsaturated substituents introduced via claisen rearrangement reaction. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2050–2059. [Google Scholar] [CrossRef]
- Steuer, M.; Hörth, M.; Ballauff, M. Rigid rod polymers with flexible side chains. X. thermotropic mesophases from aromatic stiff-chain polyamides bearing n-alkoxy side chains. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1609–1619. [Google Scholar] [CrossRef]
- Bair, T.I.; Morgan, P.W.; Killian, F.L. Poly(1,4-phenyleneterephthalamides). Polymerization and novel liquid-crystalline solutions1. Macromolecules 1977, 19, 1396–1400. [Google Scholar] [CrossRef]
- Viale, S.; Best, A.S.; Mendes, E.; Jager, W.F.; Picken, S.J. A supramolecular nematic phase in sulfonated polyaramides. Chem. Commun. 2004, 1596–1597. [Google Scholar] [CrossRef] [PubMed]
- Viale, S.; Li, N.; Schotman, A.H.M.; Best, A.S.; Picken, J. Synthesis and formation of a supramolecular nematic liquid crystal in poly(p-phenylene-sulfoterephth)-H2O. Macromolecules 2005, 38, 3647–3652. [Google Scholar] [CrossRef]
- Lee, K.S. Dihydroxy Aramid Polymers. U.S. Patent 20070015896-A1, 18 June 2007. [Google Scholar]
- Glomm, B.H.; Oertli, A.G.; Rickert, C.; Neuenschwander, P.; Suter, U.W. An investigation of novel approaches in order to provide crosslinked fully aromatic polyamide chains. Macromol. Chem. Phys. 2000, 201, 1374–1385. [Google Scholar] [CrossRef]
- Trigo-Lopez, M.J.; Pablos, L.; Garcia, F.C.; Serna, F.; Garcia, J.M. Functional aramids: Aromatic polyamides with reactive azido and amino groups in the pendant structure. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1469–1477. [Google Scholar] [CrossRef]
- Kang, S.J.; Hong, S.I.; Park, C.R. Preparation and properties of aromatic polyamide homologs containing chlorine substituents. J. Polym. Sci. Part A Polym Chem. 2000, 77, 1387–1392. [Google Scholar]
- Wei, X.; Zhang, L.; Wang, W.; Yue, D. Homogeneous catalytic hydrogenation of hydroxyl-terminated liquid nitrile rubber. J. Appl. Polym. Sci. 2014, 124, 1716–1722. [Google Scholar] [CrossRef]
- Ruijter, C.; Jager, W.; Groenewold, J.; Picken, S. Synthesis and characterization of rod−coil poly(amide-block-aramid) alternating block copolymers. Macromolecules 2006, 39, 3824–3829. [Google Scholar] [CrossRef]
- Skrovanek, D.; Howe, S.; Painter, P.; Coleman, M. Hydrogen bonding in polymers: Infrared temperature studies of an amorphous polyamide. Macromolecules 1985, 18, 1676–1683. [Google Scholar] [CrossRef]
- Kapuscinski, M.; Pearce, E.M. Aromatic polyamides. XI. Effect of the halogen substitution on the thermal and flammability behavior of poly (1,4-phenylene terephthalamide). J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 3989. [Google Scholar] [CrossRef]
- Li, S.; Han, K.Q.; Rong, H.P.; Li, X.Z.; Yu, M.H. Surface modification of aramid fibers via ammonia-plasma treatment. J. Appl. Polym. Sci. 2014, 40250, 1–6. [Google Scholar] [CrossRef]

| Sample | PPTA | ((ClPPTA)m-R′) |
|---|---|---|
| H2SO4 | ++ | − |
| NMP | − | +− |
| DMAc | − | +− |
| DMSO | − | +− |
| DMF | − | +− |
| THF | − | − |
| CHCl3 | − | − |
| NMP + 0.5% LiCl | − | ++ |
| DMAc + 0.5% LiCl | − | ++ |
| DMF + 0.5% LiCl | − | ++ |
| DMSO + 0.5% LiCl | − | ++ |
| Samples | Chemical composition (at %) | Atomic ratio | |||
|---|---|---|---|---|---|
| C | N | O | O/C | N/C | |
| Unsized fibers | 78.4 | 1.2 | 20.4 | 0.26 | 0.015 |
| Sized fibers | 76.6 | 1.4 | 22.0 | 0.29 | 0.018 |
| Sample | Content of functional groups (%) | |||
|---|---|---|---|---|
| C–C | C–N/C–O | C=O | O–C=O | |
| Unsized fibers | 89.8 | 1.4 | 3.9 | 4.8 |
| Sized fibers | 87.8 | 2.5 | 4.7 | 5.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, M.; Kong, H.; Zhang, K.; Teng, C.; Yu, M.; Liao, Y. Simple Synthesis of Hydroxyl and Ethylene Functionalized Aromatic Polyamides as Sizing Agents to Improve Adhesion Properties of Aramid Fiber/Vinyl Epoxy Composites. Polymers 2017, 9, 143. https://doi.org/10.3390/polym9040143
Qin M, Kong H, Zhang K, Teng C, Yu M, Liao Y. Simple Synthesis of Hydroxyl and Ethylene Functionalized Aromatic Polyamides as Sizing Agents to Improve Adhesion Properties of Aramid Fiber/Vinyl Epoxy Composites. Polymers. 2017; 9(4):143. https://doi.org/10.3390/polym9040143
Chicago/Turabian StyleQin, Minglin, Haijuan Kong, Kang Zhang, Cuiqing Teng, Muhuo Yu, and Yaozu Liao. 2017. "Simple Synthesis of Hydroxyl and Ethylene Functionalized Aromatic Polyamides as Sizing Agents to Improve Adhesion Properties of Aramid Fiber/Vinyl Epoxy Composites" Polymers 9, no. 4: 143. https://doi.org/10.3390/polym9040143






