Development of Defatted Soy Flour-Based Adhesives by Acid Hydrolysis of Carbohydrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Separation of Carbohydrate and Protein Components
2.3. Preparation of SBA
2.4. Characterization
3. Results and Discussion
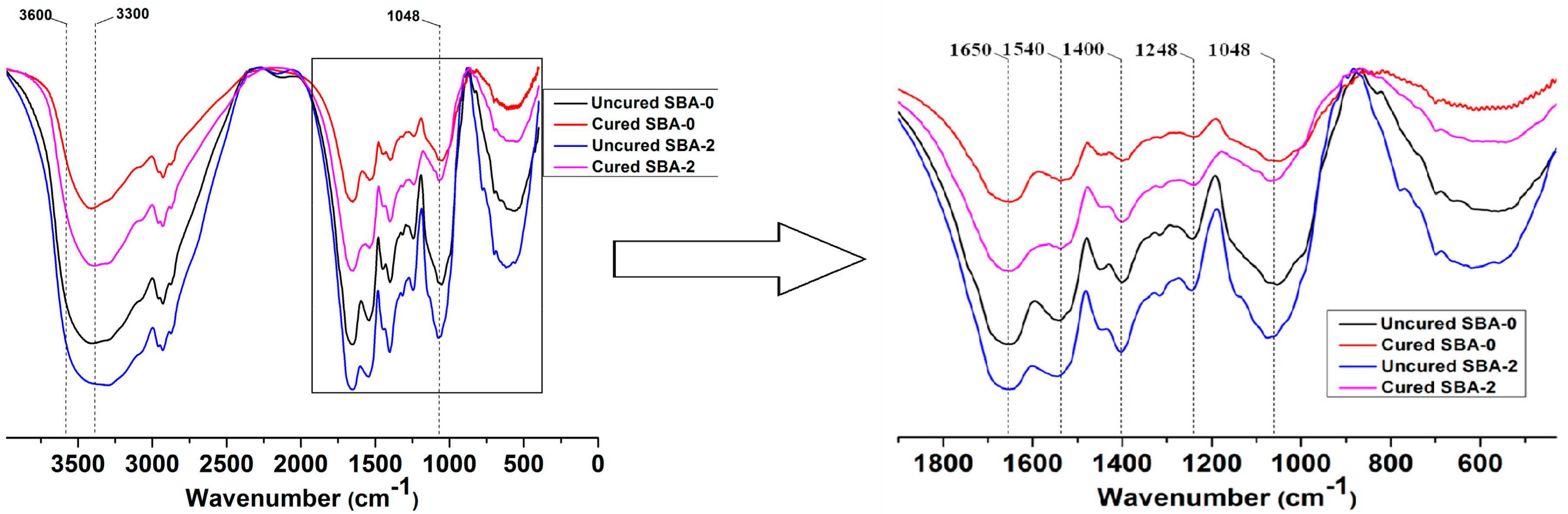
3.1. FTIR Analysis
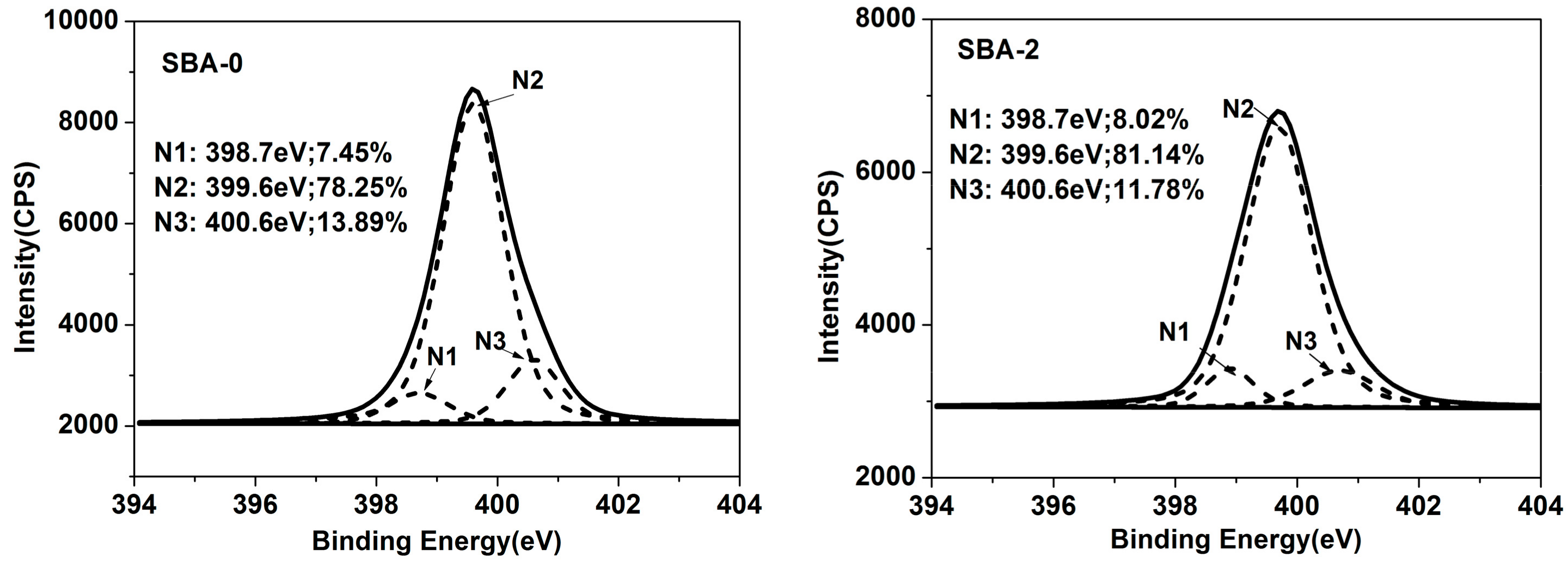
3.2. XPS Analysis
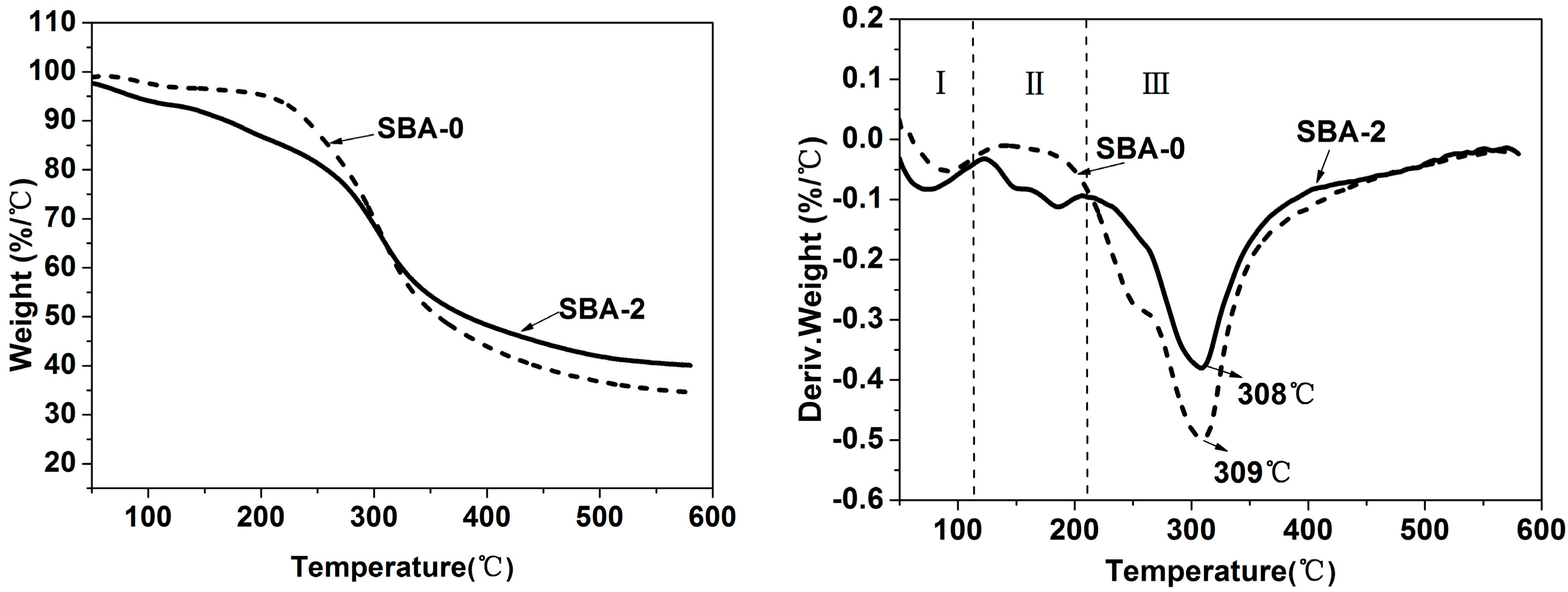
3.3. Thermal Stability of SBA
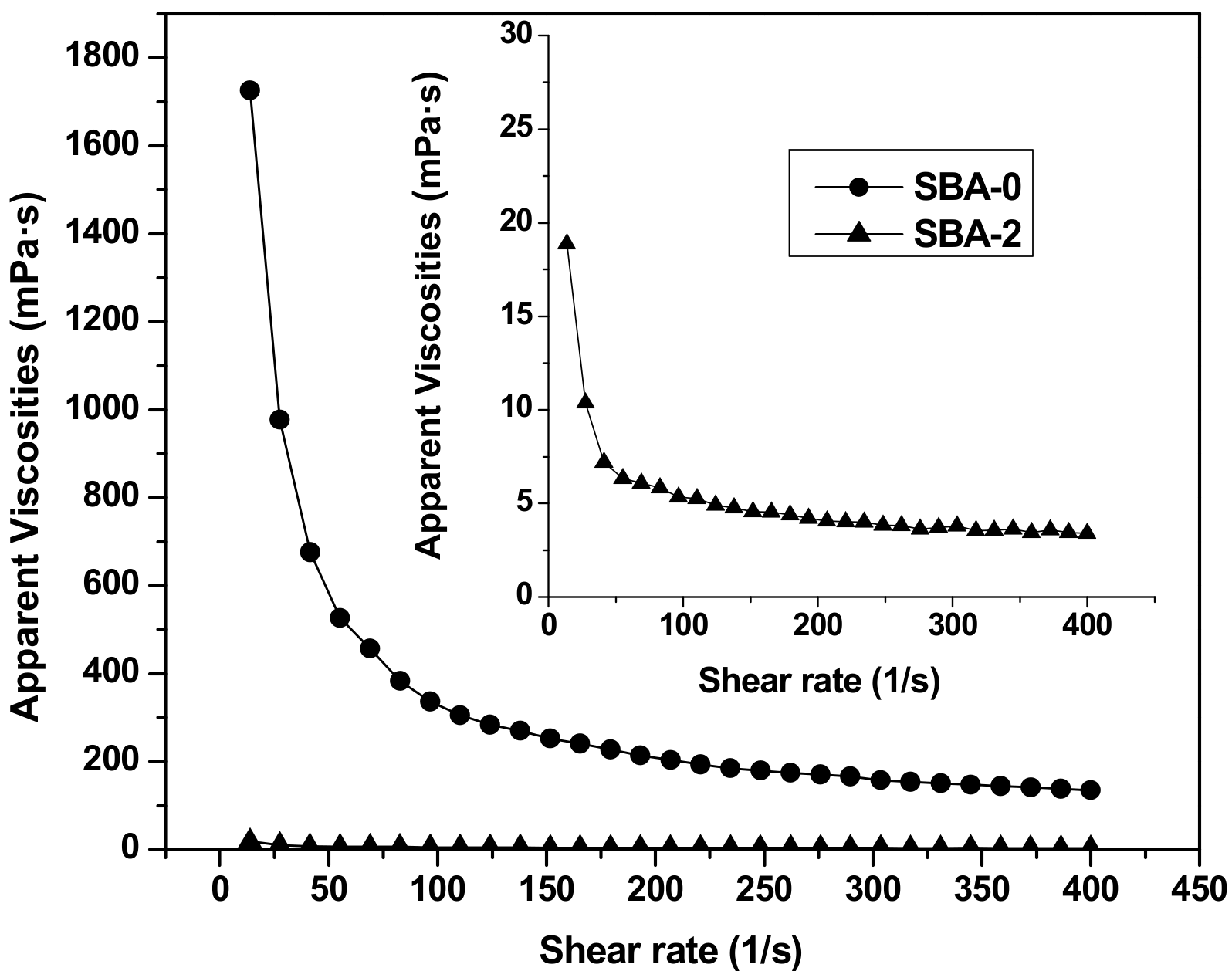
3.4. Rheological Properties of the SBA
3.5. Effect of Acid Hydrolysis on Carbohydrates
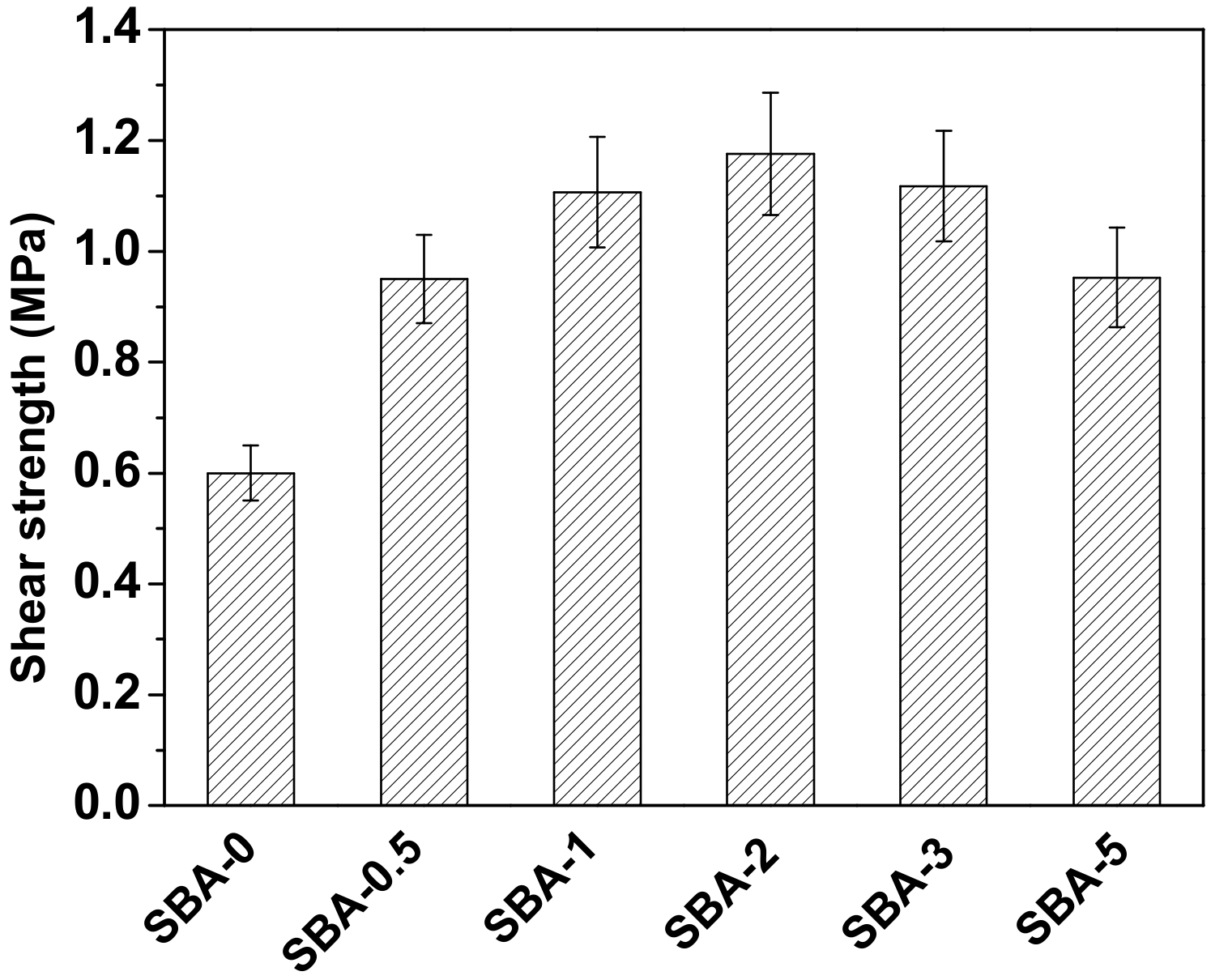
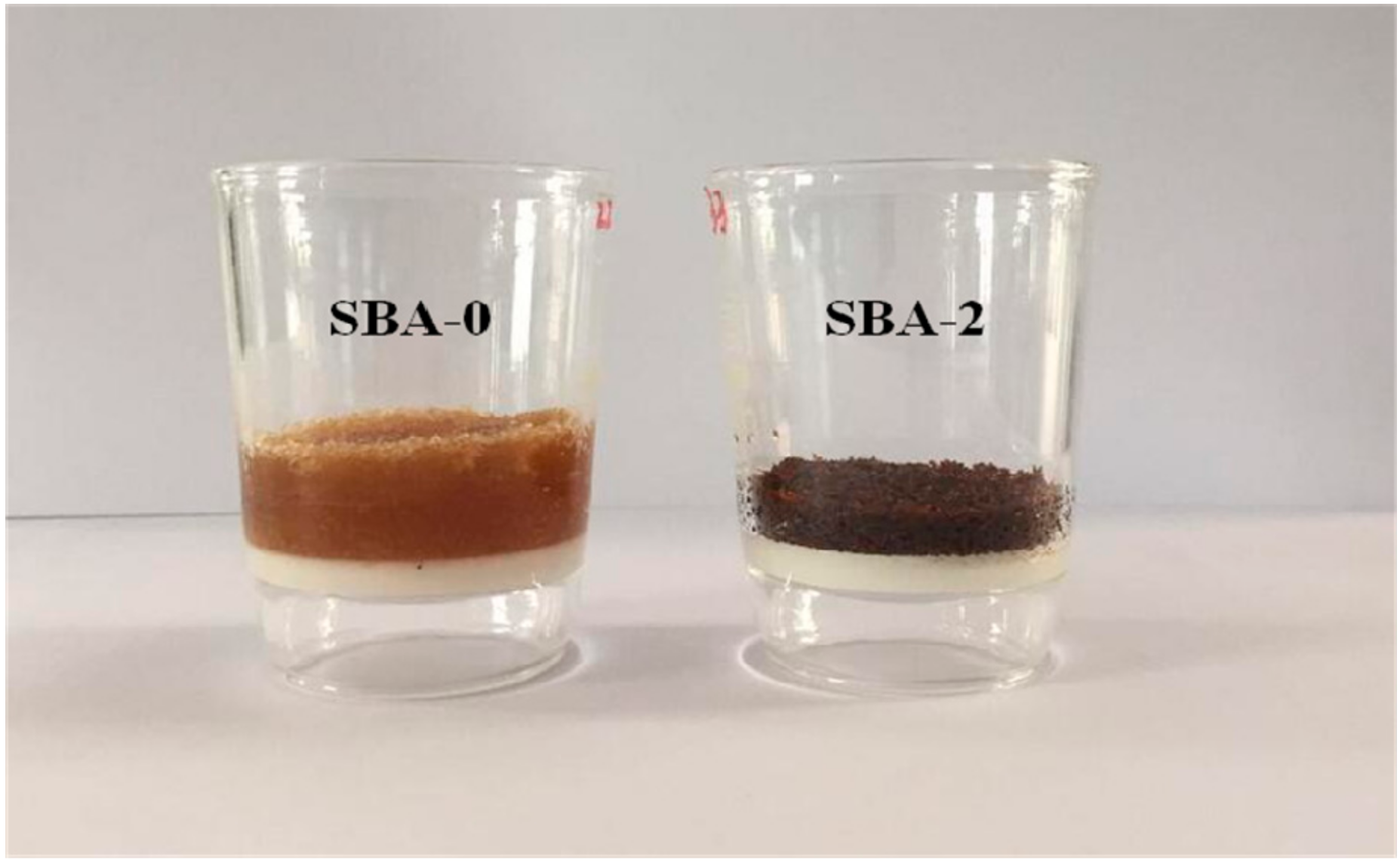
3.6. Gluability and Solubility of the SBA
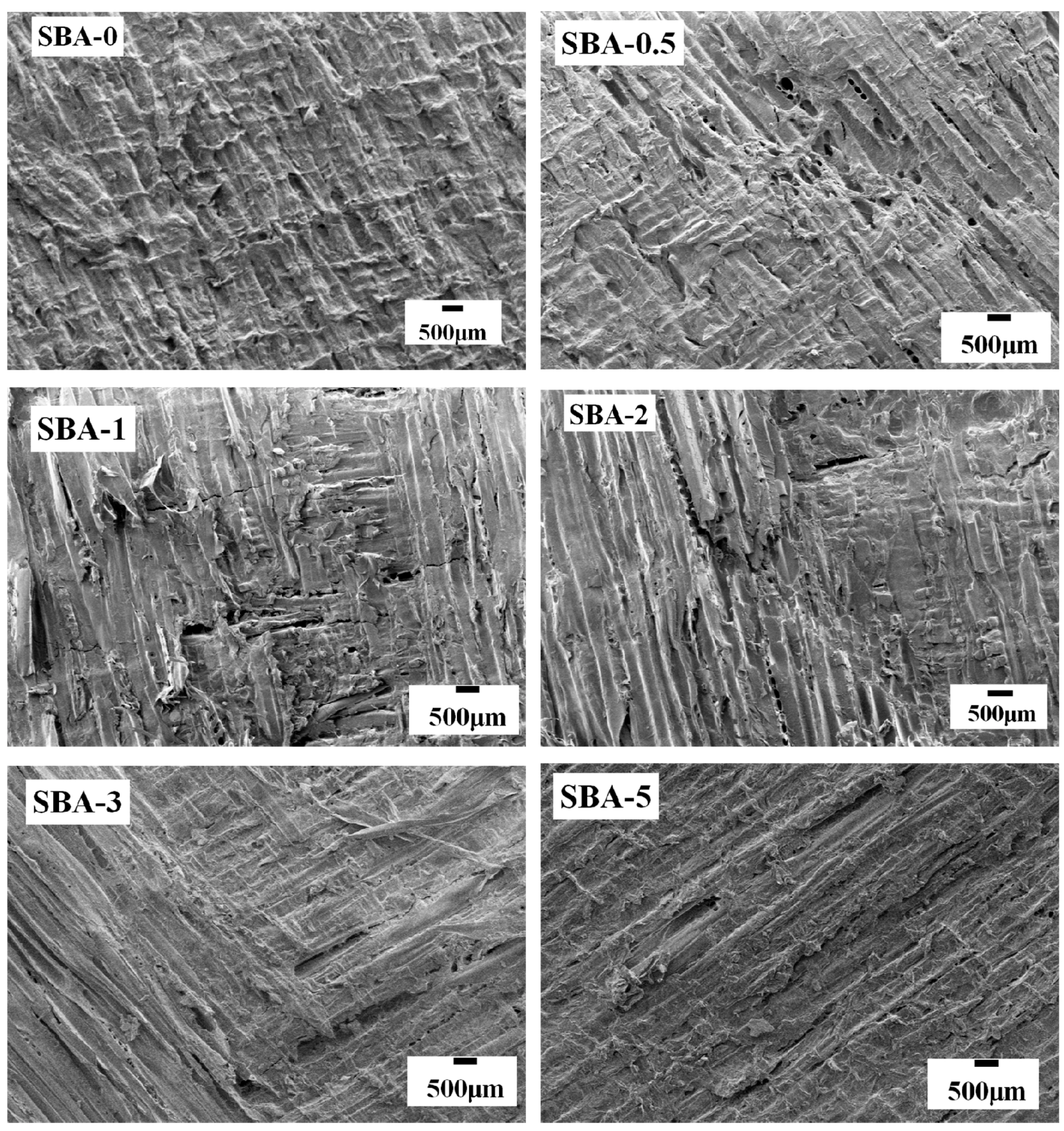
3.7. Fracture Surface Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haag, A.P.; Geesey, G.G.; Mittleman, M.W. Bacterially derived wood adhesive. Int. J. Adhes. Adhes. 2006, 26, 177–183. [Google Scholar] [CrossRef]
- Moubarik, A.; Mansouri, H.R.; Pizzi, A.; Allal, A.; Charrier, F.; Badia, M.A.; Charrier, B. Evaluation of mechanical and physical properties of industrial particleboard bonded with a corn flour-urea formaldehyde adhesive. Compos. Part B 2013, 44, 48–51. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Medium-density particleboards from modified rice husks and soybean protein concentrate-based adhesives. Bioresour. Technol. 2010, 101, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, H.; Chang, P.R.; Wu, Q.; Li, K.; Guan, L. Biomimetic soy protein nanocomposites with calcium carbonate crystalline arrays for use as wood adhesive. Bioresour. Technol. 2010, 101, 6235–6241. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.-J. Curing behavior and viscoelastic properties of pine andwattle tannin-based adhesive. J. Adhes. Sci. Technol. 2003, 17, 1369–1383. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, L.; Gu, J.; Tan, H.; Zhu, L. Preparation and properties of a starch-based wood adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2015, 115, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Coltelli, M.-B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the Art in the Development and Properties of Protein-Based Films and Coatings and Their Applicability to Cellulose Based Products: An Extensive Review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Hammann, F.; Schmid, M. Determination and Quantification of Molecular Interactions in Protein Films: A Review. Materials 2014, 7, 7975–7996. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, D.; Zhang, L. Advances in Proteinous Biomaterials. J. Biobased Mater. Bioenergy 2008, 2, 1–24. [Google Scholar] [CrossRef]
- Huang, J.; Li, K. A New Soy Flour-Based Adhesive for Making Interior Type II Plywood. J. Am. Oil Chem. Soc. 2007, 85, 63–70. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K. Chemical Modification of Soy Protein for Wood Adhesives. Macromol. Rapid Commun. 2002, 23, 739–742. [Google Scholar] [CrossRef]
- Qin, Z.; Gao, Q.; Zhang, S.; Li, J. Glycidyl methacrylate grafted onto enzyme-treated soybean meal Adhesive with Improved Wet Shear Strength. BioResources 2013, 8, 5369–5379. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, N.; Bian, L.; Fan, M. Development and mechanism characterization of high performance soy-based bio-adhesives. Int. J. Adhes. Adhes. 2012, 34, 11–16. [Google Scholar] [CrossRef]
- Huang, W.; Sun, X. Adhesive Properties of Soy Proteins Modified by Sodium Dodecyl Sulfate and Sodium Dodecylbenzene Sulfonate. J. Am. Oil Chem. Soc. 2000, 77, 705–708. [Google Scholar] [CrossRef]
- Huang, W.; Sun, X. Adhesive Properties of Soy Proteins Modified by Urea and Guanidine Hydrochloride. J. Am. Oil Chem. Soc. 2000, 77, 101–104. [Google Scholar] [CrossRef]
- Qi, G.; Li, N.; Wang, D.; Sun, X. Physicochemical properties of soy protein adhesives modified by 2-octen-1-ylsuccinic anhydride. Ind. Crops Prod. 2013, 46, 165–172. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Zhang, J.; Bai, Y.; Gao, Q.; Li, J.; Li, L. A New Flexible Soy-Based Adhesive Enhanced with Neopentyl Glycol Diglycidyl Ether: Properties and Application. Polymers 2016, 8, 346. [Google Scholar] [CrossRef]
- Lei, H.; Wu, Z.; Cao, M.; Du, G. Study on the Soy Protein-Based Wood Adhesive Modified by Hydroxymethyl Phenol. Polymers 2016, 8, 256. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Bainy, E.M.; Tosh, S.M.; Corredig, M.; Poysa, V.; Woodrow, L. Varietal differences of carbohydrates in defatted soybean flour and soy protein isolate by-products. Carbohydr. Polym. 2008, 72, 664–672. [Google Scholar] [CrossRef]
- Karr-Lilienthal, L.K.; Kadzere, C.T.; Grieshop, C.M.; Fahey, G.C. Chemical and nutritional properties of soybean carbohydrates as related to nonruminants: A review. Livest. Prod. Sci. 2005, 97, 1–12. [Google Scholar] [CrossRef]
- Lorenz, L.; Birkeland, M.; Daurio, C.; Frihart, C.R. Soy Flour Adhesive Strength Compared with That of Purified Soy Proteins. For. Prod. J. 2015, 65, 26–30. [Google Scholar] [CrossRef]
- Thakker, C.; San, K.Y.; Bennett, G.N. Production of succinic acid by engineered E. coli strains using soybean carbohydrates as feedstock under aerobic fermentation conditions. Bioresour. Technol. 2013, 130, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Frihart, C.R.; Birkeland, M.J. Soy Properties and Soy Wood Adhesives. ACS Symp. Ser. 2014, 1178, 167–192. [Google Scholar]
- Chen, N.; Zeng, Q.; Lin, Q.; Rao, J. Development of defatted soy flour based bio-adhesives using Viscozyme L. Ind. Crops Prod. 2015, 76, 198–203. [Google Scholar] [CrossRef]
- Wijaya, Y.P.; Putra, R.D.; Widyaya, V.T.; Ha, J.M.; Suh, D.J.; Kim, C.S. Comparative study on two-step concentrated acid hydrolysis for the extraction of sugars from lignocellulosic biomass. Bioresour. Technol. 2014, 164, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Corredor, D.Y.; Sun, X.S.; Salazar, J.M.; Hohn, K.L.; Wang, D. Enzymatic Hydrolysis of Soybean Hulls Using Dilute Acid and Modified Steam-Explosion Pretreatments. J. Biobased Mater. Bioenergy 2008, 2, 43–50. [Google Scholar] [CrossRef]
- Cassales, A.; de Souza-Cruz, P.B.; Rech, R.; Záchia Ayub, M.A. Optimization of soybean hull acid hydrolysis and its characterization as a potential substrate for bioprocessing. Biomass Bioenergy 2011, 35, 4675–4683. [Google Scholar] [CrossRef]
- Luján-Rhenals, D.; Morawicki, R.O.; Mendez-Montealvo, G.; Wang, Y.-J. Production of a high-protein meal and fermentable sugars from defatted soybean meal, a co-product of the soybean oil industry. Int. J. Food Sci. Technol. 2014, 49, 904–910. [Google Scholar] [CrossRef]
- Kumar, R.; Choudhary, V.; Mishra, S.; Varma, I.K.; Mattiason, B. Adhesives and plastics based on soy protein products. Ind. Crops Prod. 2002, 16, 155–172. [Google Scholar] [CrossRef]
- Al Loman, A.; Ju, L.K. Towards complete hydrolysis of soy flour carbohydrates by enzyme mixtures for protein enrichment: A modeling approach. Enzym. Microb. Technol. 2016, 86, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xiao, Y.; Niu, K.; Zhao, Y.; Zhang, B.; Hu, B. Functional ionic liquids for hydrolysis of lignocellulose. Carbohydr. Polym. 2013, 97, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, T.H.; Mussone, P.G.; Choi, P.; Bressler, D.C. Adhesives from waste protein biomass for oriented strand board composites: development and performance. Macromol. Mater. Eng. 2014, 299, 1003–1012. [Google Scholar] [CrossRef]
- Chen, N.; Lin, Q.; Rao, J.; Zeng, Q. Water resistances and bonding strengths of soy-based adhesives containing different carbohydrates. Ind. Crops Prod. 2013, 50, 44–49. [Google Scholar] [CrossRef]
- Nooshkam, M.; Madadlou, A. Maillard conjugation of lactulose with potentially bioactive peptides. Food Chem. 2016, 192, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Dastidar, T.; Netravali, A.N. A soy flour based thermoset resin without the use of any external crosslinker. Green Chem. 2013, 15, 3243–3251. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Zhu, Q.; Du, F.; Ao, Q.; Liu, J. Surface characterization of 7 S and 11 S globulin powders from soy protein examined by X-ray photoelectron spectroscopy and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 86, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Huang, J.; Li, K. A new formaldehyde-free wood adhesive from renewable materials. Int. J. Adhes. Adhes. 2011, 31, 754–759. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Li, J.; Gao, Q. Investigating the use of peanut meal: A potential new resource for wood adhesives. RSC Adv. 2015, 5, 80136–80141. [Google Scholar] [CrossRef]
- Frihart, C.R.; Lorenz, L. Protein Modifiers Generally Provide Limited Improvement in Wood Bond Strength of Soy Flour Adhesives. For. Prod. J. 2013, 63, 138–142. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Maurya, R.; Bhattacharya, S.; Bachani, P.; Mishra, S. Comparative evaluation of chemical and enzymatic saccharification of mixotrophically grown de-oiled microalgal biomass for reducing sugar production. Bioresour. Technol. 2016, 204, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Maki-Arvela, P.; Salmi, T.; Holmbom, B.; Willfor, S.; Murzin, D.Y. Synthesis of sugars by hydrolysis of hemicelluloses—A review. Chem. Rev. 2011, 111, 5638–5666. [Google Scholar] [CrossRef] [PubMed]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajo, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, M.P.; Cerisuelo, J.P.; Gavara, R.; Hernandez-Muñoz, P. Mass transport properties of gliadin films: Effect of cross-linking degree, relative humidity, and temperature. J. Membr. Sci. 2013, 428, 380–392. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Gomez-Estaca, J.; Gavara, R.; Hernandez-Munoz, P. Functional properties of bioplastics made from wheat gliadins modified with cinnamaldehyde. J. Agric. Food Chem. 2011, 59, 6689–6695. [Google Scholar] [CrossRef] [PubMed]

| HCl Concentration (%) | Reducing Sugars (%) | Insoluble Substances (%) |
|---|---|---|
| 0 | 2.13 ± 0.18 | 8.70 ± 0.19 |
| 0.5 | 5.27 ± 0.36 | 5.53 ± 0.15 |
| 1.0 | 8.94 ± 0.41 | 3.74 ± 0.23 |
| 2.0 | 12.36 ± 0.35 | 2.79 ± 0.14 |
| 3.0 | 11.89 ± 0.38 | 2.56 ± 0.12 |
| 5.0 | 10.24 ± 0.35 | 2.45 ± 0.07 |
| Samples | Sol Fraction (%) |
|---|---|
| SBA-0 | 35.49 ± 0.46 |
| SBA-2 | 33.52 ± 0.37 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, P.; Li, Y.; Li, F.; Ou, Y.; Lin, Q.; Chen, N. Development of Defatted Soy Flour-Based Adhesives by Acid Hydrolysis of Carbohydrates. Polymers 2017, 9, 153. https://doi.org/10.3390/polym9050153
Zheng P, Li Y, Li F, Ou Y, Lin Q, Chen N. Development of Defatted Soy Flour-Based Adhesives by Acid Hydrolysis of Carbohydrates. Polymers. 2017; 9(5):153. https://doi.org/10.3390/polym9050153
Chicago/Turabian StyleZheng, Peitao, Yuqi Li, Feng Li, Yangting Ou, Qiaojia Lin, and Nairong Chen. 2017. "Development of Defatted Soy Flour-Based Adhesives by Acid Hydrolysis of Carbohydrates" Polymers 9, no. 5: 153. https://doi.org/10.3390/polym9050153
APA StyleZheng, P., Li, Y., Li, F., Ou, Y., Lin, Q., & Chen, N. (2017). Development of Defatted Soy Flour-Based Adhesives by Acid Hydrolysis of Carbohydrates. Polymers, 9(5), 153. https://doi.org/10.3390/polym9050153







