Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids
Abstract
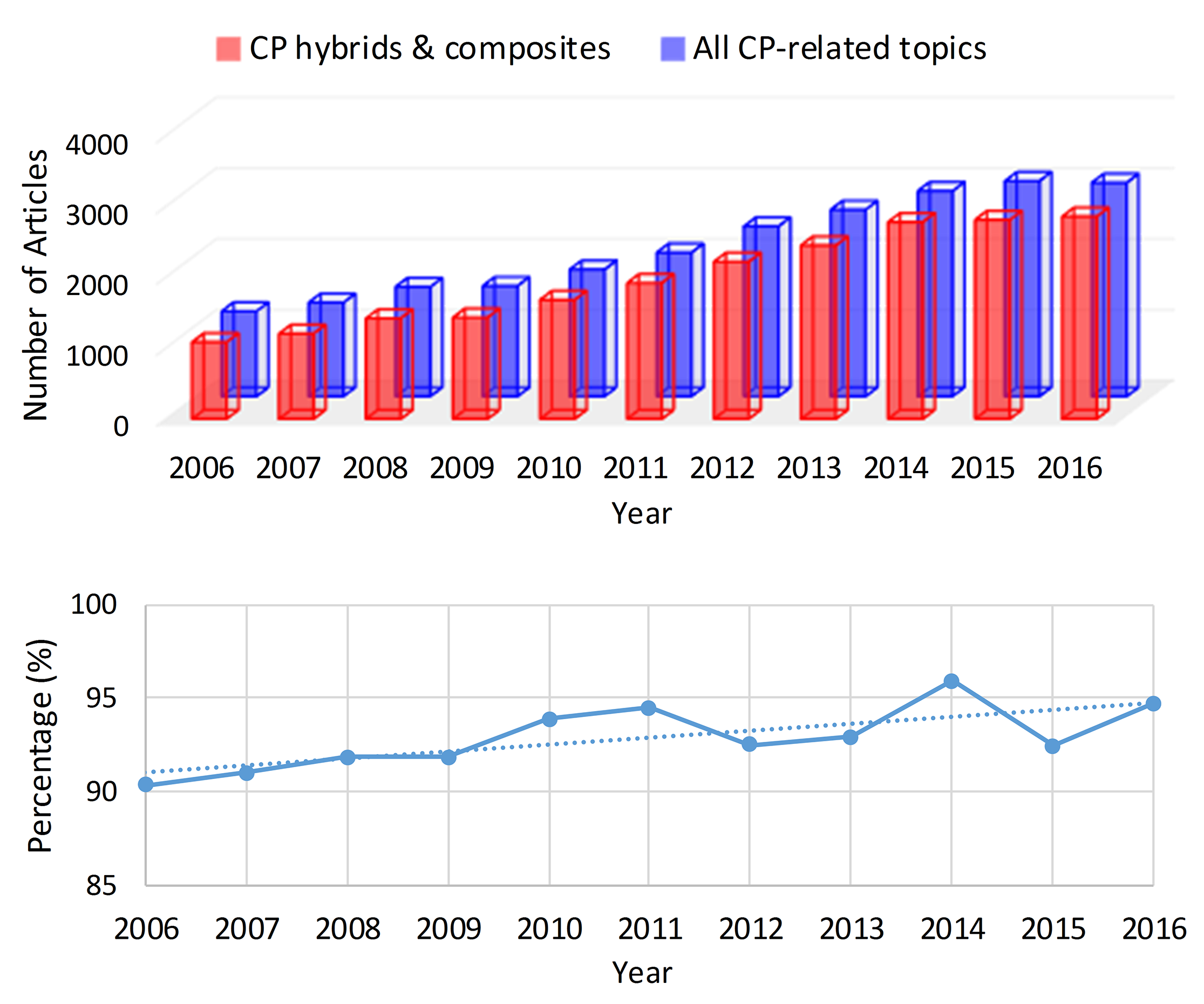
:1. Introduction
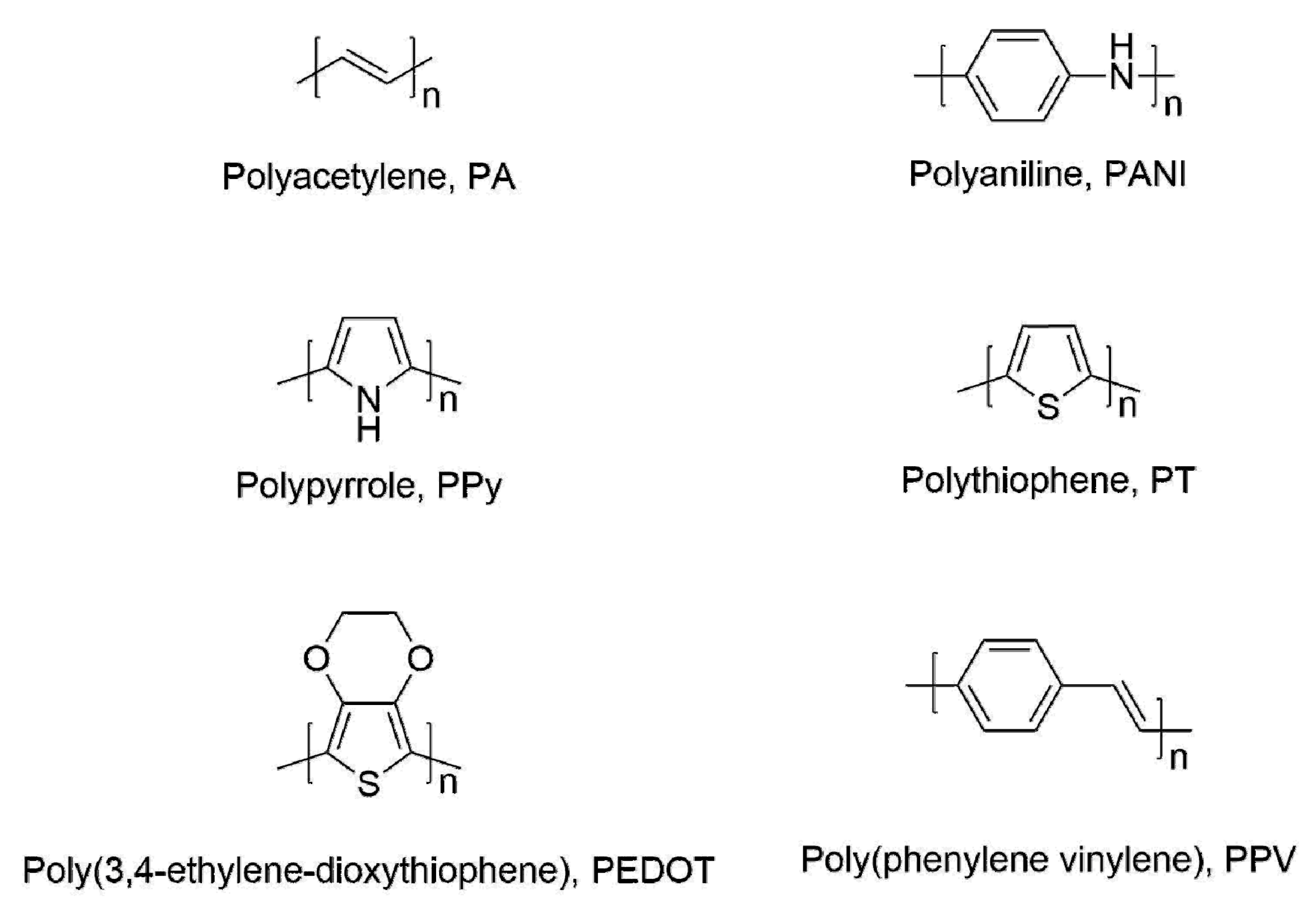
2. CP Chemosensors
3. CP Hybrids
3.1. Inorganic-CP Hybrids
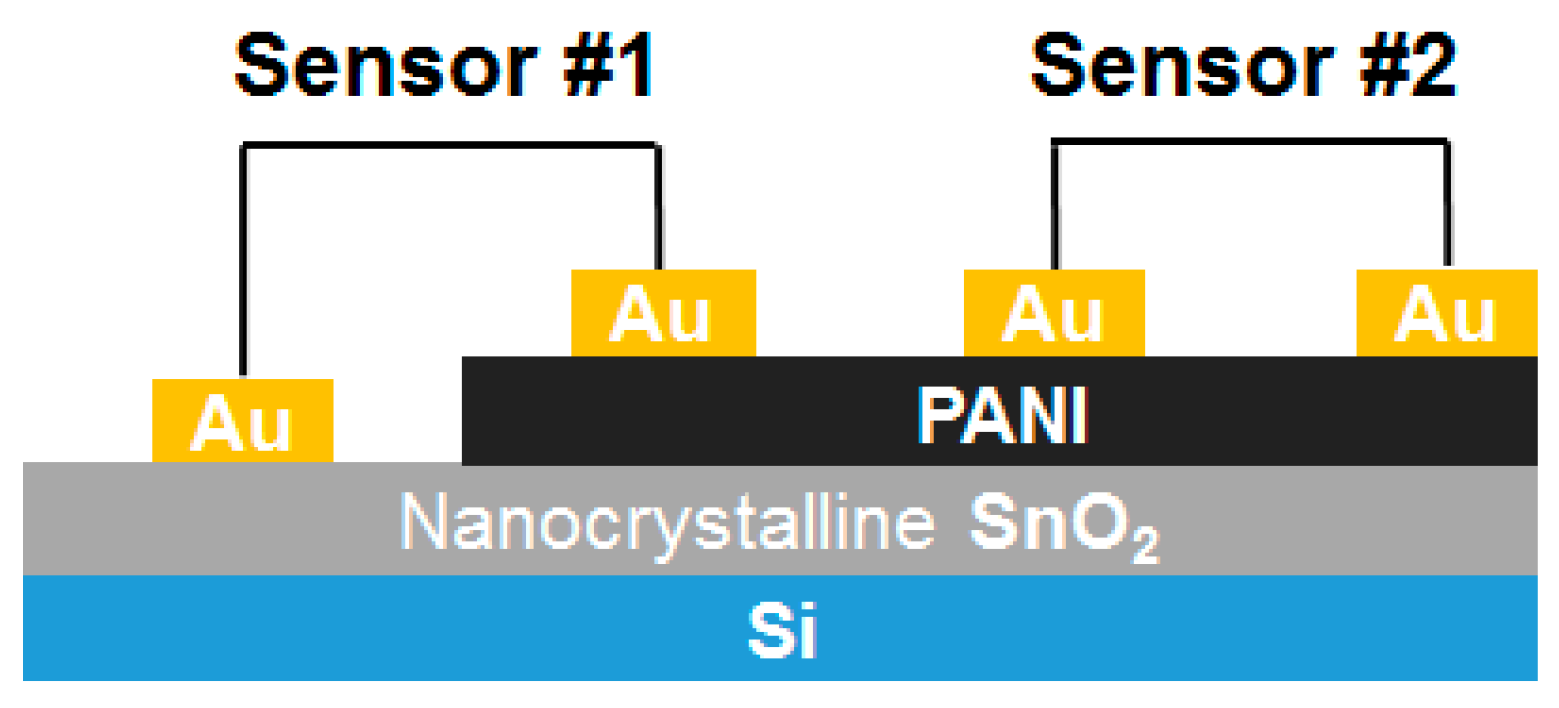
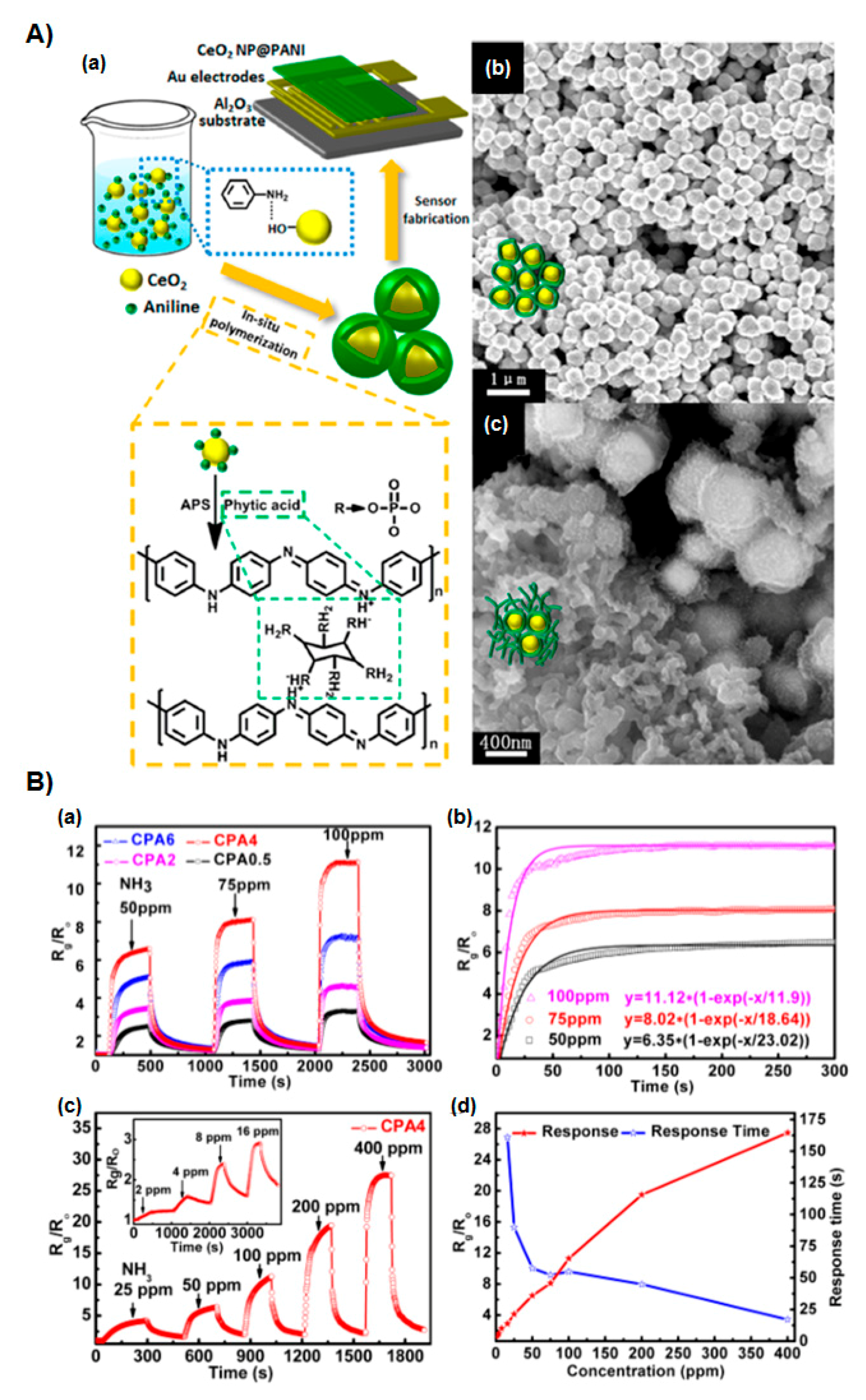
3.1.1. Metal Oxide Hybrids
3.1.2. Metal Hybrids
3.2. Organic-CP Hybrids
3.3. CNT/Graphene-CP Hybrids
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yoon, H. Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Fincher, C.R.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1978, 40, 1472–1483. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Yoon, H. Recent advances in nanostructured conducting polymers: From synthesis to practical applications. Polymers 2016, 8, 118. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, H.; Kim, M.-S.; Noh, S.; Ahn, K.-J.; Im, K.; Kwon, O.S.; Yoon, H. Nanoparticle-mediated physical exfoliation of aqueous-phase graphene for fabrication of three-dimensionally structured hybrid electrodes. Sci. Rep. 2016, 6, 19761. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Park, C.S.; Park, S.J.; Noh, S.; Kim, S.; Kong, H.J.; Bae, J.; Lee, C.-S.; Yoon, H. Carboxylic acid-functionalized conducting-polymer nanotubes as highly sensitive nerve-agent chemiresistors. Sci. Rep. 2016, 6, 33724. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, R.; De, A. Conducting polymer nanocomposites: A brief overview. Chem. Mater. 2000, 12, 608–622. [Google Scholar] [CrossRef]
- Hassanien, R.; Almaky, M.M.; Houlton, A.; Horrocks, B.R. Preparation and electrical properties of a copper conductive polymer hybrid nanostructure. RSC Adv. 2016, 6, 99422–99432. [Google Scholar] [CrossRef]
- Wu, Z.; Parvez, K.; Li, S.; Yang, S.; Liu, Z.; Liu, S.; Feng, X.; Mullen, K. Alternating stacked graphene-conducting polymer compact films with ultrahigh areal and volumetric capacitances for high-energy micro-supercapacitors. Adv. Mater. 2015, 27, 4054–4061. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jung, S.; Jeong, S.; Kim, G.; Lee, K. Polymer-metal hybrid transparent electrodes for flexible electronics. Nat. Commun. 2015, 6, 6503. [Google Scholar]
- Park, S.J.; Lee, S.H.; Yang, H.; Park, C.S.; Lee, C.-S.; Kwon, O.S.; Park, T.H.; Jang, J. Human dopamine receptor-conjugated multidimensional conducting polymer nanofiber membrane for dopamine detection. ACS Appl. Mater. Interfaces 2016, 8, 28897–28903. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Lee, C.; Kwon, O.S. Conducting polymer based nanobiosensors. Polymers 2016, 8, 249. [Google Scholar] [CrossRef]
- Hangarter, C.M.; Chartuprayoon, N.; Hernandez, S.C.; Choa, Y.; Myung, N.V. Hybridized conducting polymer chemiresistive nano-sensors. Nano Today 2013, 8, 39–55. [Google Scholar] [CrossRef]
- Park, S.J.; Kwon, O.S.; Lee, J.E.; Jang, J.; Yoon, H. Conducting polymer-based nanohybrid transducers: A potential route to high sensitivity and selectivity sensors. Sensors 2014, 14, 3604–3630. [Google Scholar] [CrossRef] [PubMed]
- Weathers, A.; Khan, Z.U.; Brooke, R.; Evans, D.; Pettes, M.T.; Andreasen, J.W.; Crispin, X.; Shi, L. Significant electronic thermal transport in the conducting polymer poly(3,4-ethylenedioxythiophene). Adv. Mater. 2015, 27, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Kouame, N.A.; Ramos, L.; Remita, S.; Dazzi, A.; Deniset-Besseau, A.; Beaunier, P.; Goubard, F.; Aubert, P.; Remita, H. Conducting polymer nanostructures for photocatalysis under visible light. Nat. Mater. 2015, 14, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, H.; Meng, Y.; Wei, Z. Conducting polymer nanowire arrays for high performance supercapacitors. Small 2014, 10, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Du, J.; Zhao, R.; Wang, H.; Yao, H. Facile synthesis of hexagonal brick-shaped SnO2 and its gas sensing toward triethylamine. J. Environ. Chem. Eng. 2013, 1, 1380–1384. [Google Scholar] [CrossRef]
- Ju, D.; Xu, H.; Qiu, Z.; Guo, J.; Zhang, J.; Cao, B. Highly sensitive and selective triethylamine-sensing properties of nanosheets directly grown on ceramic tube by forming NiO/ZnO p–n heterojunction. Sens. Actuators B 2014, 200, 288–296. [Google Scholar] [CrossRef]
- Ju, D.; Xu, H.; Xu, Q.; Gong, H.; Qiu, Z.; Guo, J.; Zhang, J.; Cao, B. High triethylamine-sensing properties of NiO/SnO2 hollow sphere p–n heterojunction sensors. Sens. Actuators B 2015, 215, 39–44. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Cheng, X.-L.; Zhang, X.-F.; Zheng, Z.-K.; Tang, X.-F.; Xu, Y.-M.; Gao, S.; Zhao, H.; Huo, L.-H. A novel sensor for fast detection of triethylamine based on rutile TiO2 nanorod arrays. Sens. Actuators B 2014, 205, 322–328. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, H.; Xie, X.; Li, L.; Zhang, L.; Yu, J.; Zhao, H.; Liu, B. Controlled synthesis and gas-sensing properties of hollow sea urchin-like α-Fe2O3 nanostructures and α-Fe2O3 nanocubes. Sens. Actuators B 2009, 141, 381–389. [Google Scholar] [CrossRef]
- Sui, L.-L.; Xu, Y.-M.; Zhang, X.-F.; Cheng, X.-L.; Gao, S.; Zhao, H.; Cai, Z.; Huo, L.-H. Construction of three-dimensional flower-like α-MoO3 with hierarchical structure for highly selective triethylamine sensor. Sens. Actuators B 2015, 208, 406–414. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Kolmakov, A.; Moskovits, M. Chemical sensing and catalysis by one-dimensional metal-oxide nanostructures. Rev. Mater. Res. 2004, 34, 151–180. [Google Scholar] [CrossRef]
- Al-Mashat, L.; Debiemme-Chouvy, C.; Borensztajn, S.; Wlodarski, W. Electropolymerized polypyrrole nanowires for hydrogen gas sensing. J. Phys. Chem. C 2012, 116, 13388–13394. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Kailasa Ganapathi, S.; Kaur, M.; Datta, N.; Muthe, K.P.; Aswal, D.K.; Gupta, S.K.; Yakhmi, J.V. Sub-ppm H2S sensing at room temperature using CuO thin films. Sens. Actuators B 2010, 151, 90–96. [Google Scholar] [CrossRef]
- Kneer, J.; Knobelspies, S.; Bierer, B.; Wöllenstein, J.; Palzer, S. New method to selectively determine hydrogen sulfideconcentrations using CuO layers. Sens. Actuators B 2016, 222, 625–631. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Comini, E. Metal oxide nanowire chemical sensors: innovation and quality of life. Mater. Today 2016, 19, 559–567. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.G.; Cho, S.; Jang, J. Porous palladium coated conducting polymer nanoparticles for ultrasensitive hydrogen sensors. Nanoscale 2015, 7, 20665–20673. [Google Scholar] [CrossRef] [PubMed]
- Volanti, D.P.; Felix, A.A.; Orlandi, M.O.; Whitfield, G.; Yang, D.-J.; Longo, E.; Tuller, H.L.; Varela, J.A. The role of hierarchical morphologies in the superior gas sensing performance of CuO-based chemiresistors. Adv. Funct. Mater. 2013, 23, 1759–1766. [Google Scholar] [CrossRef]
- Hosono, K.; Matsubara, I.; Murayama, N.; Shin, W.; Izu, N. The sensitivity of 4-ethylbenzenesulfonic acid-doped plasma polymerized polypyrrole films to volatile organic compounds. Thin Solid Films 2005, 484, 396–399. [Google Scholar] [CrossRef]
- Liao, Y.; Li, X.-G.; Kaner, R.B. Facile synthesis of water-dispersible conducting polymer nanospheres. ACS Nano 2010, 4, 5193–5202. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Hong, J.Y.; Jang, J. Charge-transport behavior in shape-controlled poly(3,4-ethylenedioxythiophene) nanomaterials: Intrinsic and extrinsic factors. Small 2007, 3, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Park, E.; Kwon, O.Y.; Park, S.J.; Jang, J. Novel flexible chemical gas sensor based on poly(3,4-ethylenedioxythiophene) nanotube membrane. Talanta 2010, 82, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Sun, J.; Bai, S.; Luo, R.; Li, D.; Chen, A.; Liu, C.C. A flexible sensor based on polyaniline hybrid using ZnO as template and sensing properties to triethylamine at room temperature. Appl. Surf. Sci. 2017, 399, 583–591. [Google Scholar] [CrossRef]
- Bai, S.; Zhao, Y.; Sun, J.; Tong, Z.; Luo, R.; Li, D.; Chen, A. Preparation of conducting films based on α-MoO3/PANI hybrids and their sensing properties to triethylamine at room temperature. Sens. Actuators B 2017, 239, 131–138. [Google Scholar] [CrossRef]
- Sen, T.; Shimpi, N.; Mishra, S. Room temperature CO sensing by polyaniline/Co3O4 nanocomposite. J. Appl. Polym. Sci. 2016, 133, 1–8. [Google Scholar] [CrossRef]
- Betty, C.A.; Choudhury, S.; Arora, S. Tin oxide–polyaniline heterostructure sensors for highly sensitive and selective detection of toxic gases at room temperature. Sens. Actuators B 2015, 220, 288–294. [Google Scholar] [CrossRef]
- Hicks, S.M.; Killard, A.J. Electrochemical impedance characterisation of tungsten trioxide-polyaniline nanocomposites for room temperature acetone sensing. Sens. Actuators B 2014, 194, 283–289. [Google Scholar] [CrossRef]
- Talwar, V.; Singh, O.; Singh, R.C. ZnO assisted polyaniline nanofibers and its application as ammonia gas sensor. Sens. Actuators B 2014, 191, 276–282. [Google Scholar] [CrossRef]
- Wang, L.; Huang, H.; Xiao, S.; Cai, D.; Liu, Y.; Liu, B.; Wang, D.; Wang, C.; Li, H.; Wang, Y. Enhanced sensitivity and stability of room-temperature NH3 sensors using core-shell CeO2 nanoparticles cross-linked PANI with p–n heterojunctions. ACS Appl. Mater. Interfaces 2014, 6, 14131–14140. [Google Scholar] [CrossRef] [PubMed]
- Zampetti, E.; Pantalei, S.; Muzyczuk, A.; Bearzotti, A.; De Cesare, F.; Spinella, C.; Macagnano, A. A high sensitive NO2 gas sensor based on PEDOT-PSS/TiO2 nanofibres. Sens. Actuators B 2013, 176, 390–398. [Google Scholar] [CrossRef]
- Taccola, S.; Greco, F.; Zucca, A.; Innocenti, C.; de Julián Fernández, C.S.; Campo, G.; Sangregorio, C.; Mazzolai, B.; Mattoli, V. Characterization of free-standing PEDOT:PSS/iron oxide nanoparticle composite thin films and application as conformable humidity sensors. ACS Appl. Mater. Interfaces 2013, 5, 6324–6332. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Lee, J.S.; Jun, J.; Kim, S.G.; Jang, J. Detection of hazardous gas using multidemensional porous iron oxide nanorods-decorated carbon nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shin, D.H.; Jun, J.; Jang, J. Multidimensional polypyrrole/iron oxyhydroxide hybrid nanoparticles for chemical nerve gas agent sensing application. ACS Nano 2013, 7, 10139–10147. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Jiang, D.; Zou, Y.; Chu, H.; Qiu, S.; Zhang, H.; Xu, F.; Sun, L.; Zheng, L. Ammonia sensor based on polypyrrole-graphene nanocomposite decorated with titania nanoparticles. Ceram. Int. 2015, 41, 6432–6438. [Google Scholar] [CrossRef]
- Lee, J.S.; Jun, J.; Shin, D.H.; Jang, J. Urchin-like polypyrrole nanoparticles for highly sensitive and selective chemiresistive sensor application. Nanoscale 2014, 6, 4188–4194. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kang, Y.; Yang, T.; Wang, Y.; Wang, S. Preparation of polythiophene/WO3 organic-inorganic hybrids and their gas sensing properties for NO2 detection at low temperature. J. Nat. Gas Chem. 2011, 20, 403–407. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, J.; Wang, S.; Guo, X.; Xia, H.; Wang, Y.; Zhang, S.; Huang, W.; Wu, S. Gas sensing properties of SnO2 hollow spheres/polythiophene inorganic-organic hybrids. Sens. Actuators B 2010, 146, 8–13. [Google Scholar] [CrossRef]
- Guo, X.-Z.; Kang, Y.-F.; Yang, T.-L.; Wang, S.-R. Low-temperature NO2 sensors based on polythiophene/WO3 organic-inorganic hybrids. Trans. Nonferr. Met. Soc. China 2012, 22, 380–385. [Google Scholar] [CrossRef]
- Patil, U.; Ramgir, N.S.; Karmakar, N.; Bhogale, A.; Debnath, A.; Aswal, D.; Gupta, S.; Kothari, D. Room temperature ammonia sensor based on copper nanoparticle intercalated polyaniline nanocomposite thin films. Appl. Surf. Sci. 2015, 339, 69–74. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S.; Jun, J.; Jang, J. High-sensitivity hydrogen gas sensors based on Pd-decorated nanoporous poly(aniline-co-aniline-2-sulfonic acid):poly(4-styrenesulfonic acid). J. Mater. Chem. A 2014, 2, 1955–1966. [Google Scholar] [CrossRef]
- Bai, S.; Sun, C.; Wan, P.; Wang, C.; Luo, R.; Li, Y.; Liu, J.; Sun, X. Transparent conducting films of hierarchically nanostructured polyaniline networks on flexible substrates for high-performance gas sensors. Small 2015, 11, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hayashi, K.; Toko, K. Au nanoparticles decorated polyaniline nanofiber sensor for detecting volatile sulfur compounds in expired breath. Sens. Actuators B 2012, 161, 504–509. [Google Scholar] [CrossRef]
- Tiwari, D.; Sharma, R.; Vyas, K.; Boopathi, M.; Singh, V.V.; Pandey, P. Electrochemical incorporation of copper phthalocyanine in conducting polypyrrole for the sensing of DMMP. Sens. Actuators B 2010, 151, 256–264. [Google Scholar] [CrossRef]
- Hong, L.; Li, Y.; Yang, M. Fabrication and ammonia gas sensing of palladium/polypyrrole nanocomposite. Sens. Actuators B 2010, 145, 25–31. [Google Scholar] [CrossRef]
- Gaikwad, N.; Bhanoth, S.; More, P.V.; Jain, G.; Khanna, P. Chemically designed Pt/PPy nano-composite for effective LPG gas sensor. Nanoscale 2014, 6, 2746–2751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Wu, S.; Xu, H.; Cao, B. One-pot fabrication of uniform polypyrrole/Au nanocomposites and investigation for gas sensing. Sens. Actuators B 2013, 186, 695–700. [Google Scholar] [CrossRef]
- Park, S.J.; Kwon, O.S.; Jang, J. A high-performance hydrogen gas sensor using ultrathin polypyrrole-coated CNT nanohybrids. Chem. Commun. 2013, 49, 4673–4675. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kwon, O.S.; Park, S.J.; Lee, J.S.; You, S.; Jang, J. One-pot synthesis of silver nanoparticles decorated poly(3,4-ethylenedioxythiophene) nanotubes for chemical sensor application. J. Mater. Chem. 2012, 22, 1521–1526. [Google Scholar] [CrossRef]
- Zhao, D.; Li, L.; Niu, W.; Chen, S. Highly conductive polythiophene films doped with chloroauric acid for dual-mode sensing of volatile organic amines and thiols. Sens. Actuators B 2017, 243, 380–387. [Google Scholar] [CrossRef]
- Srinives, S.; Sarkar, T.; Mulchandani, A. Primary amine-functionalized polyaniline nanothin film sensor for detecting formaldehyde. Sens. Actuators B 2014, 194, 255–259. [Google Scholar] [CrossRef]
- Sicard, L.; Navarathne, D.; Skalski, T.; Skene, W. On-substrate preparation of an electroactive conjugated polyazomethine from solution-processable monomers and its application in electrochromic devices. Adv. Funct. Mater. 2013, 23, 3549–3559. [Google Scholar] [CrossRef]
- Işık, D.; Santato, C.; Barik, S.; Skene, W. Charge-carrier transport in thin films of π-conjugated thiopheno-azomethines. Org. Electron. 2012, 13, 3022–3031. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Hsieh, P.-R.; Lin, C.-H.; Chou, P.-C.; Fu, L.-M.; Chiang, C.-M. MEMS-based formaldehyde gas sensor integrated with a micro-hotplate. Microsyst. Technol. 2006, 12, 893–898. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.; Lu, Y.; Jiang, T.; Liu, B.; Lin, Y. Visible-light-assisted HCHO gas sensing based on Fe-doped flowerlike ZnO at room temperature. J. Phys. Chem. C 2011, 115, 22939–22944. [Google Scholar] [CrossRef]
- Feng, L.; Musto, C.J.; Suslick, K.S. A simple and highly sensitive colorimetric detection method for gaseous formaldehyde. J. Am. Chem. Soc. 2010, 132, 4046–4047. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Jung, S.; Jeon, S. Detection of formaldehyde vapor using mercaptophenol-coated piezoresistive cantilevers. Sens. Actuators B 2007, 126, 522–526. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Lee, J.S.; Park, E.; Kim, T.; Park, H.-W.; You, S.A.; Yoon, H.; Jang, J. Multidimensional conducting polymer nanotubes for ultrasensitive chemical nerve agent sensing. Nano Lett. 2012, 12, 2797–2802. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.; Oh, J.; Shin, D.H.; Kim, S.G.; Lee, J.S.; Kim, W.; Jang, J. Wireless, room temperature volatile organic compound sensor based on polypyrrole nanoparticle immobilized ultrahigh frequency radio frequency identification tag. ACS Appl. Mater. Interfaces 2016, 8, 33139–33147. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Yoon, H.; Jang, J. Kinetic study of the formation of polypyrrole nanoparticles in water-soluble polymer/metal cation systems: A light-scattering analysis. Small 2010, 6, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Mahato, M.; Adhikari, B. Vapor phase sensing response of doped polyaniline-poly(vinyl alcohol) composite membrane to different aliphatic alcohols. Synth. Met. 2016, 220, 410–420. [Google Scholar] [CrossRef]
- Qin, Y.; Pan, S.; Howlader, M.M.; Ghosh, R.; Hu, N.-X.; Deen, M.J. Paper-based, hand-drawn free chlorine sensor with poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate). Anal. Chem. 2016, 88, 10384–10389. [Google Scholar] [CrossRef] [PubMed]
- Esteves, C.H.; Iglesias, B.A.; Li, R.W.; Ogawa, T.; Araki, K.; Gruber, J. New composite porphyrin-conductive polymer gas sensors for application in electronic noses. Sens. Actuators B 2014, 193, 136–141. [Google Scholar] [CrossRef]
- Littler, B.J.; Ciringh, Y.; Lindsey, J.S. Investigation of conditions giving minimal scrambling in the synthesis of trans-porphyrins from dipyrromethanes and aldehydes. J. Org. Chem. 1999, 64, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hui, L.; Foster, D.A.; Drain, C.M. Efficient synthesis and photodynamic activity of porphyrin-saccharide conjugates: Targeting and incapacitating cancer cells. Biochemistry 2004, 43, 10918–10929. [Google Scholar] [CrossRef] [PubMed]
- Brückner, C.; Posakony, J.J.; Johnson, C.K.; Boyle, R.W.; James, B.R.; Dolphin, D. Novel and improved syntheses of 5,15-diphenylporphyrin and its dipyrrolic precursors. J. Porphyr. Phthalocya. 1998, 2, 455–465. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Jiang, T.; Zhao, Z.; Li, Y.; Wang, Z.; Wang, C. Sulfonated poly(ether ether ketone)/polypyrrole core–shell nanofibers: A novel polymeric adsorbent/conducting polymer nanostructures for ultrasensitive gas sensors. ACS Appl. Mater. Interfaces 2012, 4, 6080–6084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, J.; Kwong, G.; Li, J.; Fan, Z.; Deng, X.; Tang, G. All-carbon sp–sp2 hybrid structures: Geometrical properties, current rectification, and current amplification. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Song, H.S.; Park, S.J.; Lee, S.H.; An, J.H.; Park, J.W.; Yang, H.; Yoon, H.; Bae, J.; Park, T.H. An ultrasensitive, selective, multiplexed superbioelectronic nose that mimics the human sense of smell. Nano Lett. 2015, 15, 6559–6567. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, T.; Kumar, D. Recent progress in the development of nano-structured conducting polymers/nanocomposites for sensor applications. Sens. Actuators B 2009, 136, 275–286. [Google Scholar]
- Park, C.S.; Yoon, H.; Kwon, O.S. Graphene-based nanoelectronic biosensors. J. Ind. Eng. Chem. 2016, 38, 13–22. [Google Scholar] [CrossRef]
- Im, K.; Nguyen, D.N.; Kim, S.; Kong, H.J.; Kim, Y.; Park, C.S.; Kwon, O.S.; Yoon, H. Graphene-embedded hydrogel nanofibers for detection and removal of aqueous-phase dyes. ACS Appl. Mater. Interfaces 2017, 9, 10768–10776. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Ahn, J.-H.; Barone, P.W.; Yum, K.; Sharma, R.; Boghossian, A.A.; Han, J.-H.; Strano, M.S. Periplasmic binding proteins as optical modulators of single-walled carbon nanotube fluorescence: amplifying a nanoscale. Angew. Chem. Int. Ed. 2011, 50, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- Brennan, B.; Spencer, S.J.; Belsey, N.A.; Faris, T.; Cronin, H.; Silva, S.R.P.; Sainsbury, T.; Gilmore, I.S.; Stoeva, Z.; Pollard, A.J. Structural, chemical and electrical characterisation of conductive graphene-polymer composite films. Appl. Surf. Sci. 2017, 403, 403–412. [Google Scholar] [CrossRef]
- Huang, X.; Hu, N.; Gao, R.; Yu, Y.; Wang, Y.; Yang, Z.; Kong, E.S.-W.; Wei, H.; Zhang, Y. Reduced graphene oxide–polyaniline hybrid: Preparation, characterization and its applications for ammonia gas sensing. J. Mater. Chem. 2012, 22, 22488–22495. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, S.; Kong, H.J.; Kwon, O.S.; Yoon, H. Tunable electrical-sensing performance of random-alternating layered graphene/polyaniline nanoarchitectures. J. Phys. Chem. C 2016, 120, 18289–18295. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Liu, A.; Shi, G. Electrosynthesis of graphene oxide/polypyrene composite films and their applications for sensing organic vapors. J. Mater. Chem. 2012, 22, 8438–8443. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Zengin, H.; Zhou, W.; Jin, J.; Czerw, R.; Smith, D.W.; Echegoyen, L.; Carroll, D.L.; Foulger, S.H.; Ballato, J. Carbon nanotube doped polyaniline. Adv. Mater. 2002, 14, 1480–1483. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Feng, P.; Alhokbany, N.; Al-Farraj, E.; Alshehri, S.M.; Ahamad, T. Synthesis and characterization of hybrid nanocomposites as highly-efficient conducting CH4 gas sensor. Spectrochim. Acta Part A 2017, 173, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Hussain, S.; Singh, S.; Islam, S. MWCNT-conducting polymer composite based ammonia gas sensors: A new approach for complete recovery process. Sens. Actuators B 2014, 194, 213–219. [Google Scholar] [CrossRef]
- Macagnano, A.; Perri, V.; Zampetti, E.; Bearzotti, A.; De Cesare, F. Humidity effects on a novel eco-friendly chemosensor based on electrospun PANI/PHB nanofibres. Sens. Actuators B 2016, 232, 16–27. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.Y.; Du, S.; Guo, W.J.; Pei, M.S. Micro/nanostructures of PANI obtained in the presence of water soluble polymers and their electrochemical sensing properties. RSC Adv. 2015, 5, 69067–69074. [Google Scholar] [CrossRef]
- Badhulika, S.; Tlili, C.; Mulchandani, A. Poly(3-aminophenylboronic acid)-functionalized carbon nanotubes-based chemiresistive sensors for detection of sugars. Analyst 2014, 139, 3077–3082. [Google Scholar] [CrossRef] [PubMed]
- Sizun, T.; Patois, T.; Bouvet, M.; Lakard, B. Microstructured electrodeposited polypyrrole-phthalocyanine hybrid material, from morphology to ammonia sensing. J. Mater. Chem. 2012, 22, 25246–25253. [Google Scholar] [CrossRef]
- Badhulika, S.; Myung, N.V.; Mulchandani, A. Conducting polymer coated single-walled carbon nanotube gas sensors for the detection of volatile organic compounds. Talanta 2014, 123, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Singh, A.K.; Balasubramanian, S.; Takashima, W.; Prakash, R. Poly-3-hexylthiophene (P3HT)/graphene nanocomposite field-effect-transistor as ammonia detector. J. Nanosci. Nanotechnol. 2016, 16, 9634–9641. [Google Scholar] [CrossRef]
- Gusain, A.; Joshi, N.J.; Varde, P.; Aswal, D. Flexible no gas sensor based on conducting polymer poly(N-9′-heptadecanyl-2,7-carbazole-ALT-5,5-(4′,7′-di-2-thienyl-2′,1′,3′-benzothiadiazole))(PCDTBT). Sens. Actuators B Chem. 2017, 239, 734–745. [Google Scholar] [CrossRef]

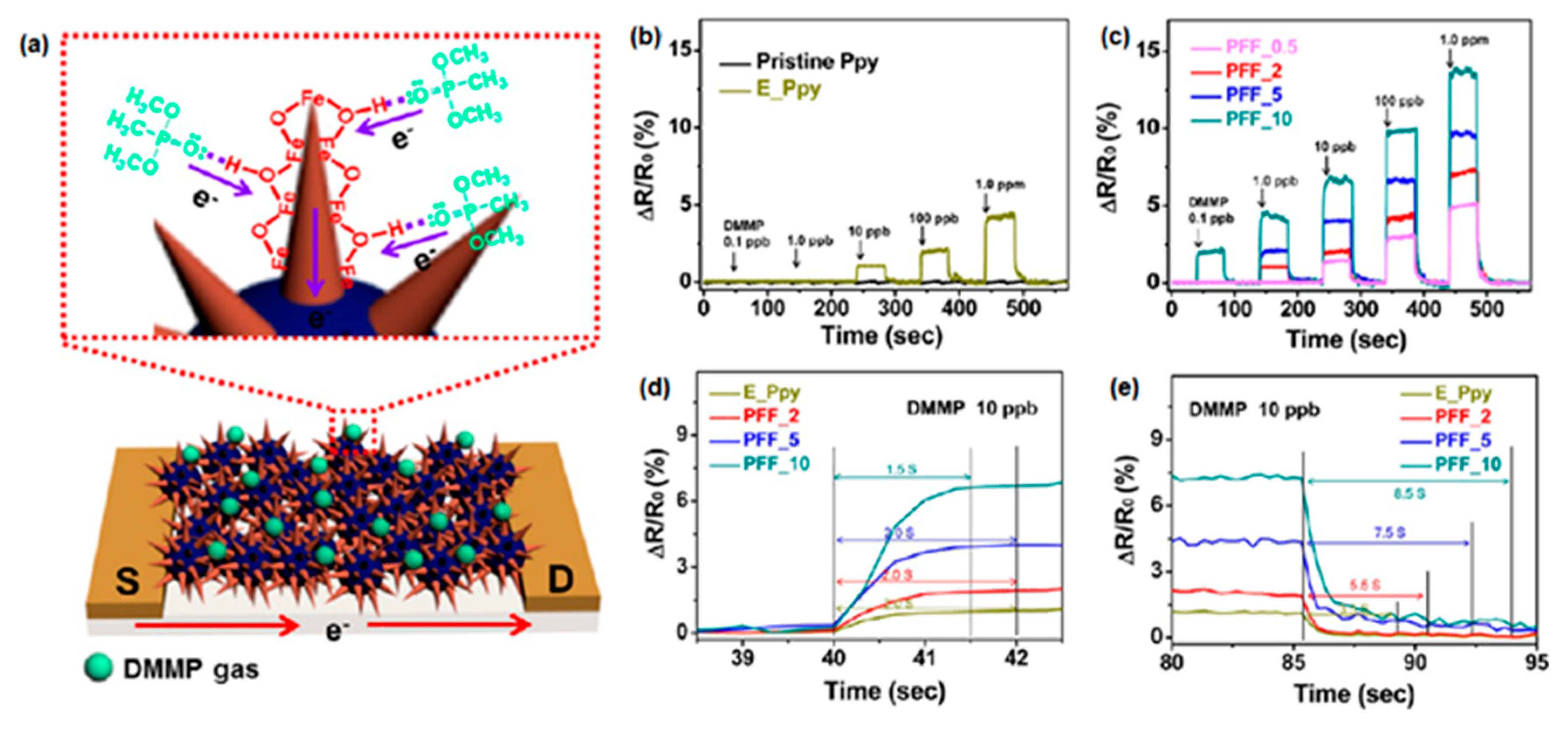
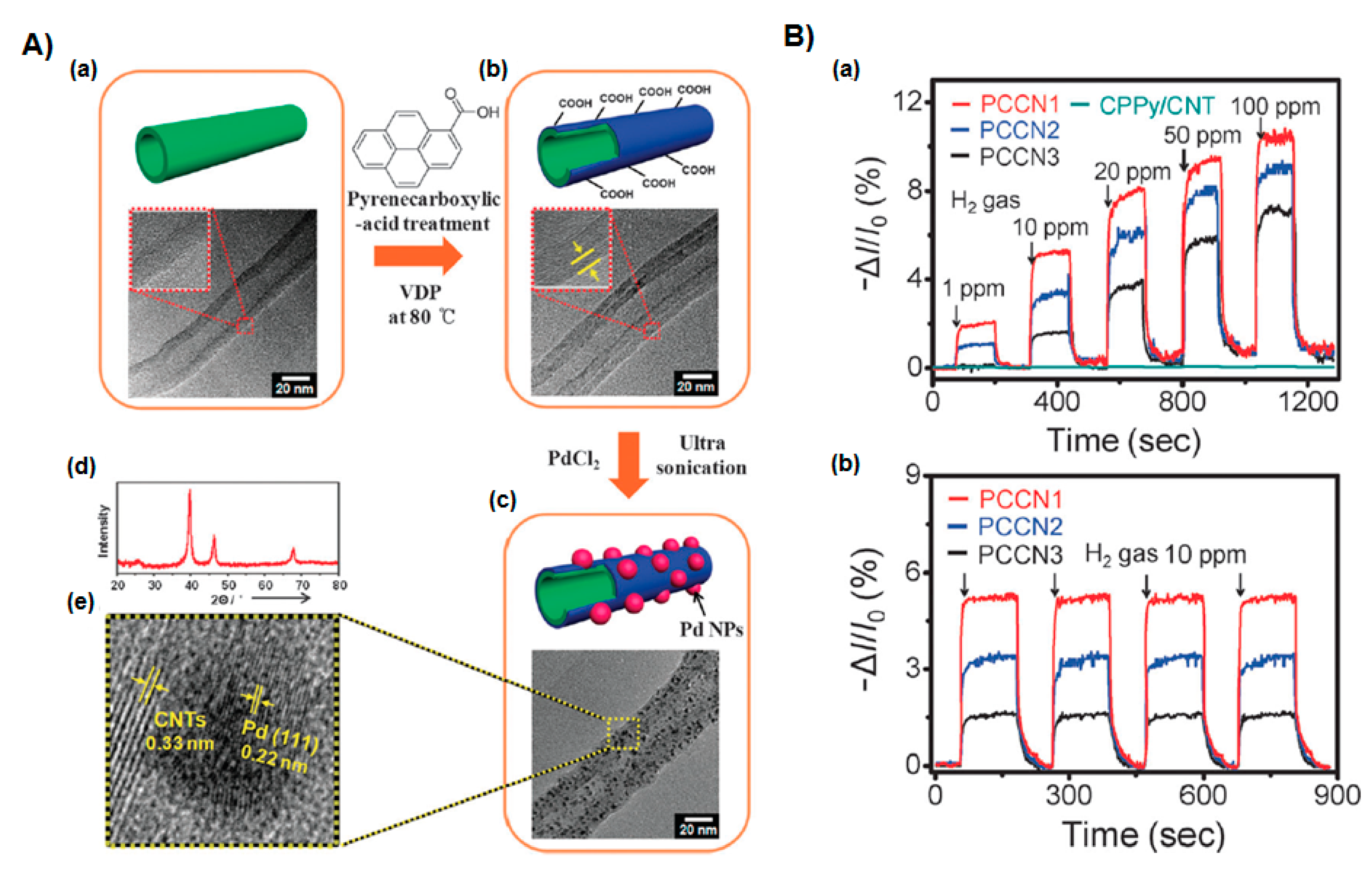
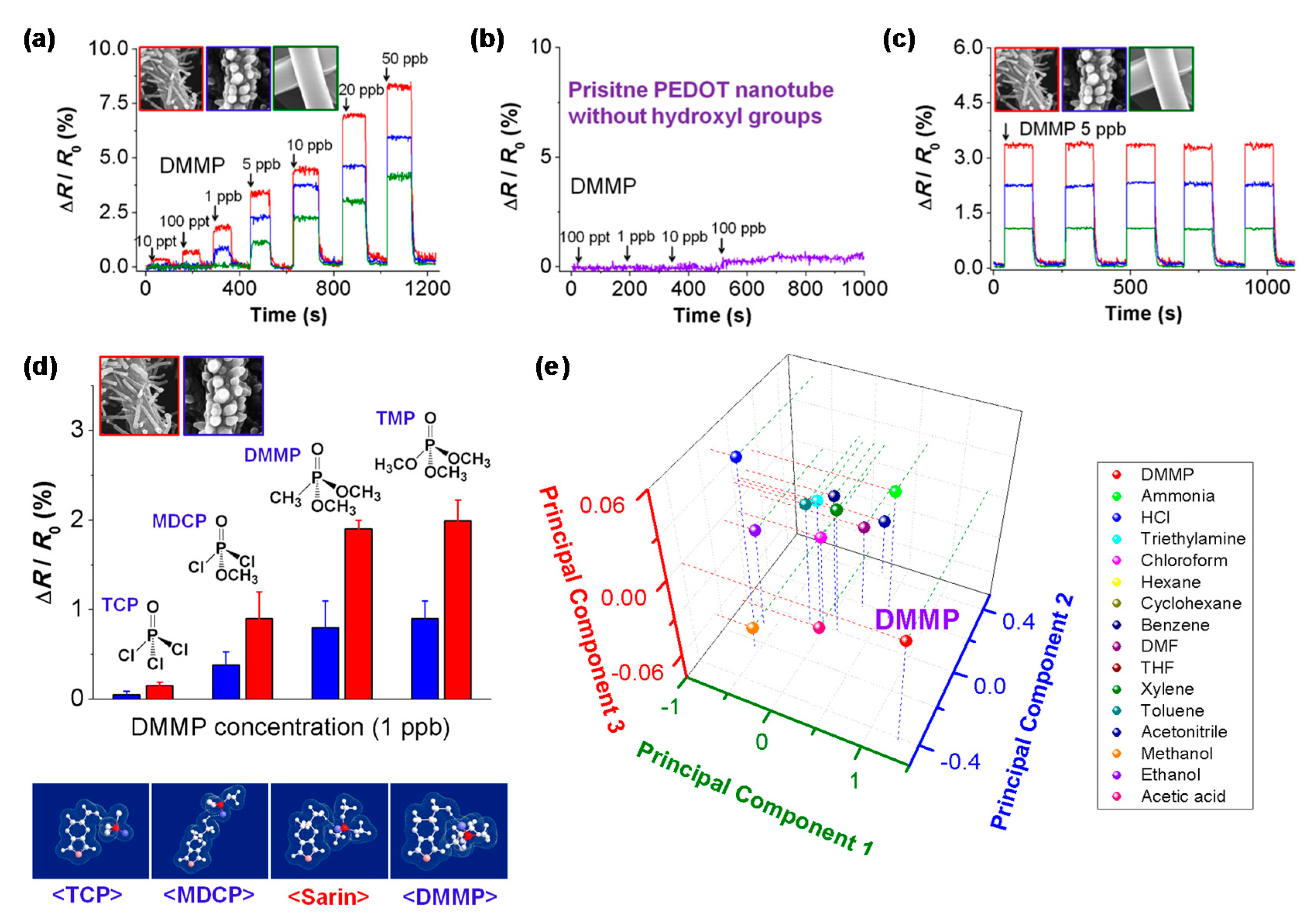
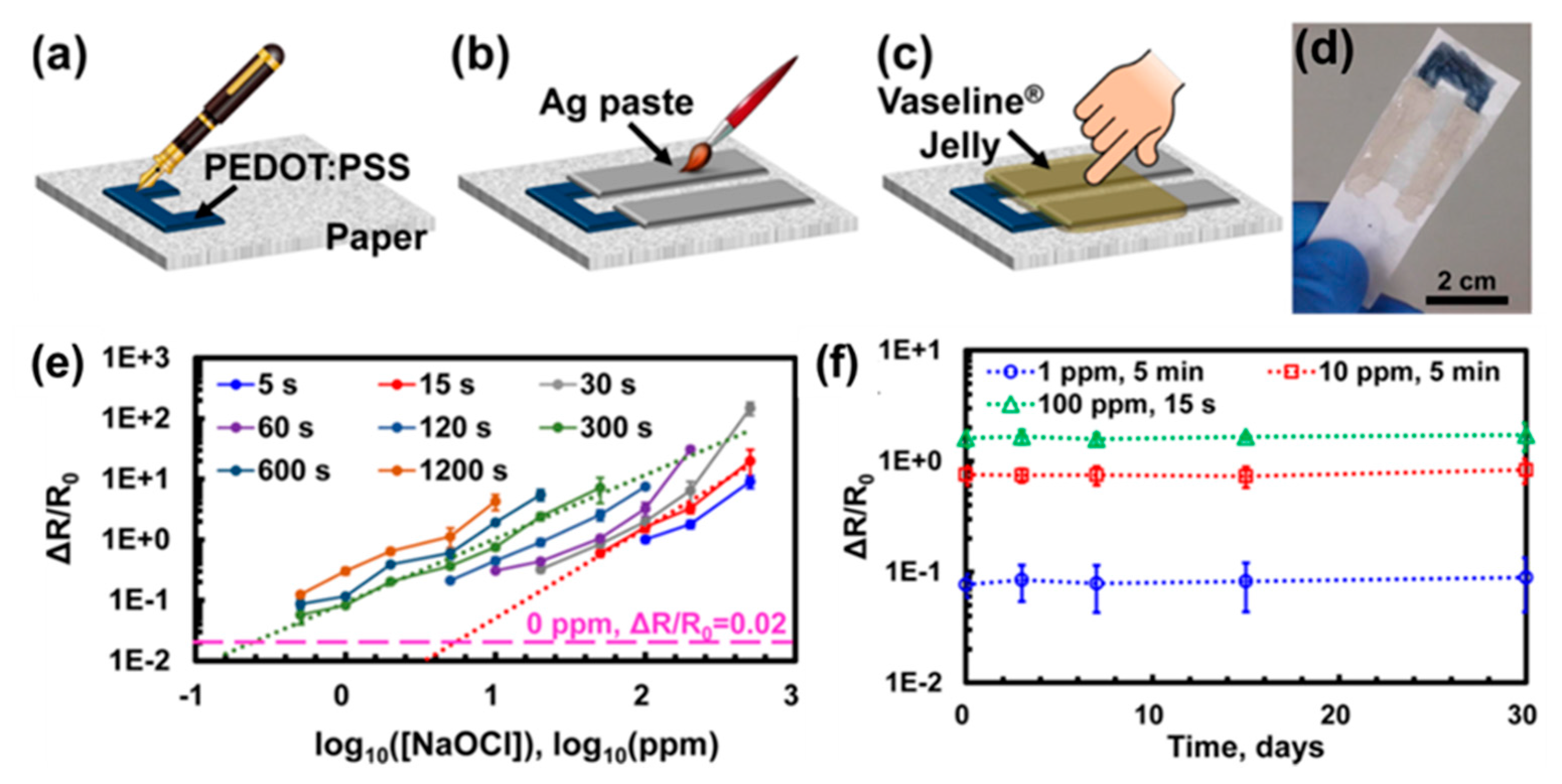
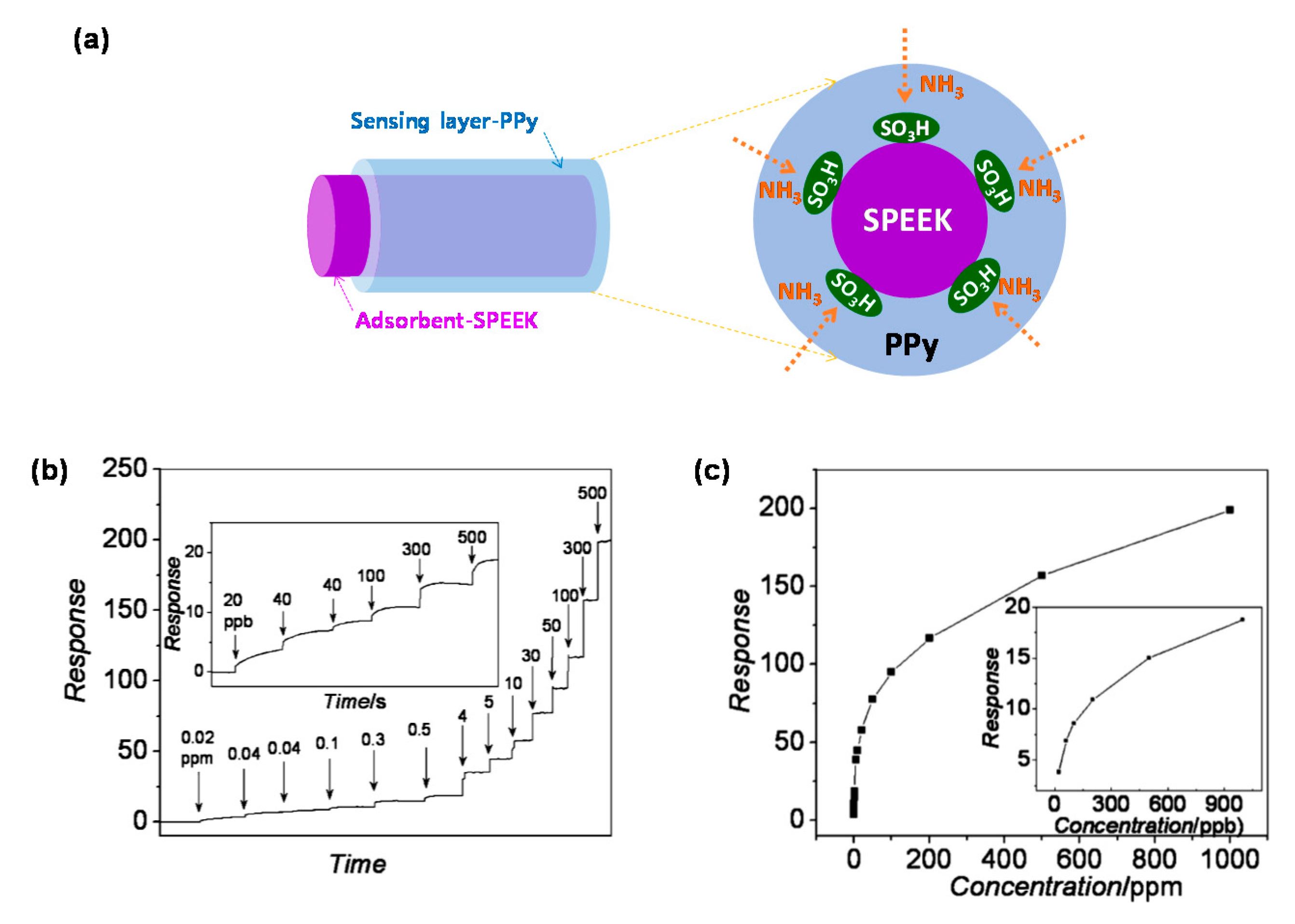
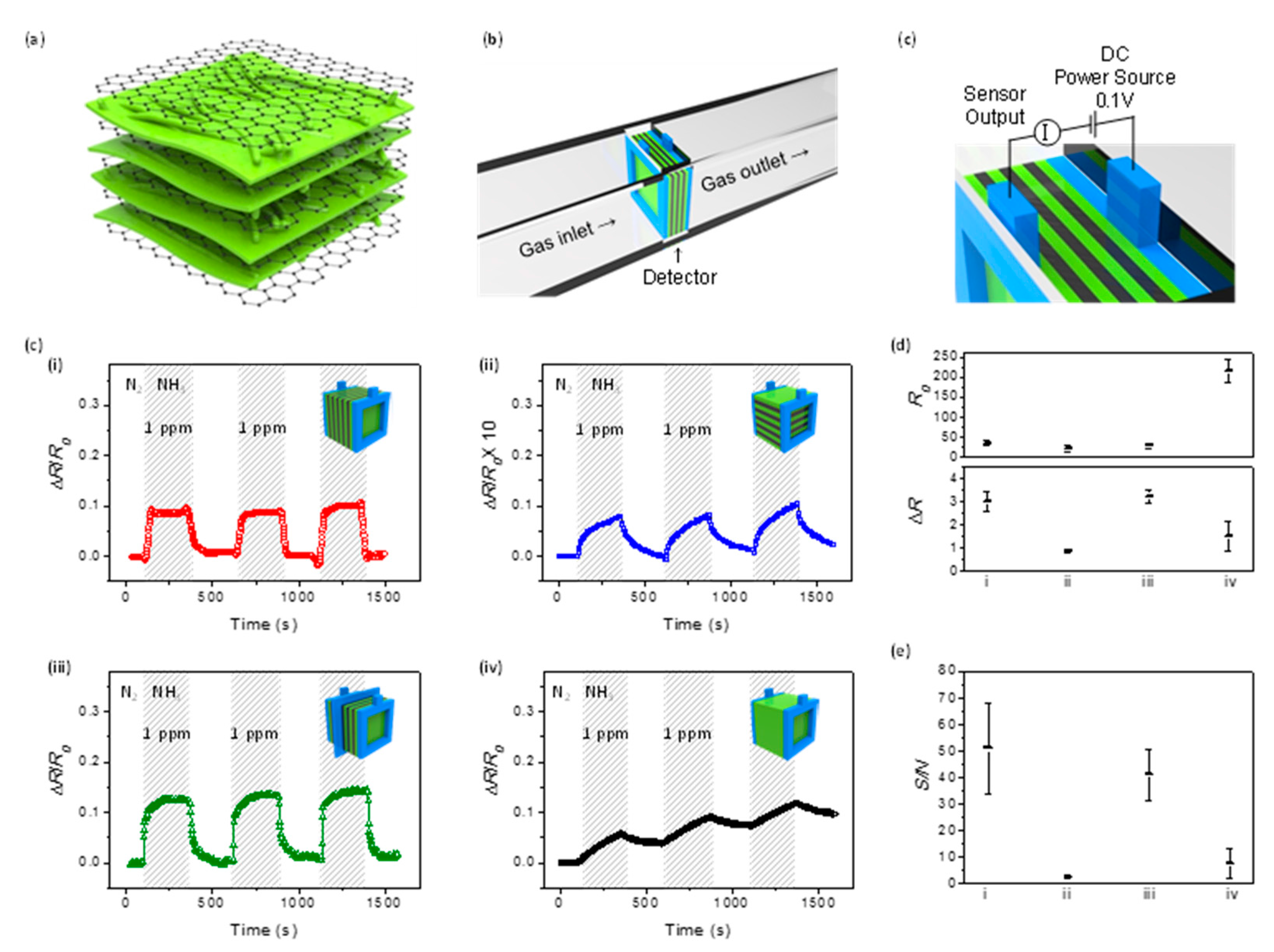
| CP | Hybrid material 1 | Analyte | Response time | Sensing temperature 2 | Detection limit | Ref. |
|---|---|---|---|---|---|---|
| PANI | ZnO | TEA | 65−130 s | RT | 10 ppm | [37] |
| α-MoO3 | TEA | 35 s | RT | 0.55 ppm | [38] | |
| Co3O4 | CO | 40 s | RT | 75 ppm | [39] | |
| SnO2 | SO2 and NO2 | SO2: –, NO2: 5 min | RT | SO2: 2ppm and NO2: 50ppb | [40] | |
| WO3 | Acetone | - | RT | 10 ppm | [41] | |
| ZnO NPs | NH3 | 50 s | RT | 25−100 ppm | [42] | |
| CeO2 NPs | NH3 | 57.6 s | RT | 6.5−50 ppm | [43] | |
| Cu NPs | NH3 | 72 s | RT | 1−50 ppm | [53] | |
| Pd NPs | H2 | <90 s | RT | 5 ppm | [54] | |
| Ag NWs | NH3 | - | RT | 5 ppm | [55] | |
| Au NPs | H2S and CH3SH | - | RT | H2S: 1 ppm and CH3SH: 1.5 ppm | [56] | |
| PPy | Fe2O3 nanorod | NO2 | - | RT | 1 ppm | [46] |
| FeOOH | DMMP | 1.5−2.0 s | RT | 0.1 ppb | [47] | |
| Graphene-TiO2 NPs | NH3 | 36 s | RT | 1 ppm | [48] | |
| FeOOH-Cu2+ | NH3 | <1 s | RT | 0.01 ppm | [49] | |
| Pd NPs | H2 | 4.5−12.5 s | RT | 0.1 ppm | [31] | |
| Pt | LPG | - | 170 °C | 40 ppm | [59] | |
| Au NPs | NH3 | - | RT | 100 ppm | [60] | |
| Pd NPs | H2 | <1 s | RT | 1 ppm | [61] | |
| PEDOT | TiO2 nanofiber | NO2 | 5−17 min | RT | 1 ppb | [44] |
| Fe3O4/γ-Fe2O3 NPs | humidity | 1 s | RT | relative humidity 30−70% | [45] | |
| Ag NPs | NH3 | 2−3 s | RT | 1 ppm | [62] | |
| PTh | WO3 | NO2 | - | 40–90 °C | 10−100 ppm | [50] |
| SnO2 | NO2 | - | 90 °C | 10−100 ppm | [51] | |
| WO3 | NO2 | - | RT–90 °C | - | [52] | |
| HAuCl4 | amines and thiols | - | - | 1 ppm | [63] |
| CP | Hybrid material 1 | Analyte | Response time | Sensing temperature 2 | Detection limit | Ref. |
|---|---|---|---|---|---|---|
| PANI | RGO | NH3 | - | RT | 5−50 ppm | [88] |
| PVA | Aliphatic alcohols | 4.6−7.8 s | RT | - | [74] | |
| Poly-3-hydroxybutirrate | NH3 | 100 s | RT | 834 ppb | [95] | |
| HEC, PAA | H2O2 | - | RT | 0.5 mM | [96] | |
| PDA | Formaldehyde | - | RT | 750 ppb | [64] | |
| polymer/MWCNT | CH4 | <5 s | RT−60 °C | 5–15 ppm | [93] | |
| MWCNT | NH3 | < 6 s | RT | 2–10 ppm | [91] | |
| Graphene | sugar | 4 min | RT | D-fructose: 2.92 mM and D-glucose: 3.46 mM | [97] | |
| Graphene | NH3 | <40 s | RT | 1 ppm | [89] | |
| PPy | Phthalocyanine | NH3 | 4−10 min | RT | 25–90 ppm | [98] |
| PPy-COOH NTs | DMMP | - | RT | 0.5 ppb | [6] | |
| PPy-COOH NPs | NH3 | 2.0−3.5 s | RT | 0.1 ppm | [72] | |
| PEDOT | PSS | Chlorine | 15−300 s | RT | 0.5 ppm | [75] |
| SWCNT | VOCs | - | RT | Methanol: 1.3%, Ethanol: 5.95%, methylethylketone: 3% | [99] | |
| PEDOT-OH NTs | DMMP | - | RT | 10 ppt | [71] | |
| MWCNT | NH3 | <15 min | RT | 1 ppm | [94] | |
| PT | Graphene | NH3 | - | RT | 250 ppb | [100] |
| PCDTBT | PCDTBT | NO | 300 s | RT | 5 ppm | [101] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.J.; Park, C.S.; Yoon, H. Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers 2017, 9, 155. https://doi.org/10.3390/polym9050155
Park SJ, Park CS, Yoon H. Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers. 2017; 9(5):155. https://doi.org/10.3390/polym9050155
Chicago/Turabian StylePark, Seon Joo, Chul Soon Park, and Hyeonseok Yoon. 2017. "Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids" Polymers 9, no. 5: 155. https://doi.org/10.3390/polym9050155







