Effect of Interfacial Polarization and Water Absorption on the Dielectric Properties of Epoxy-Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Modification of the Nanoparticles
2.4. Compounding of the Nanocomposites
2.5. Preparation of the Test Specimens
3. Results
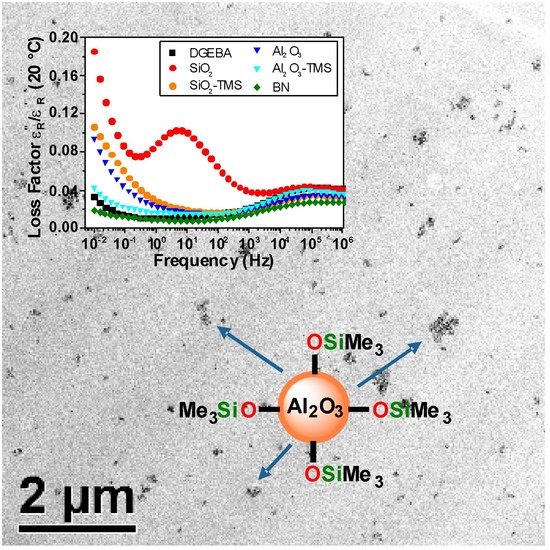
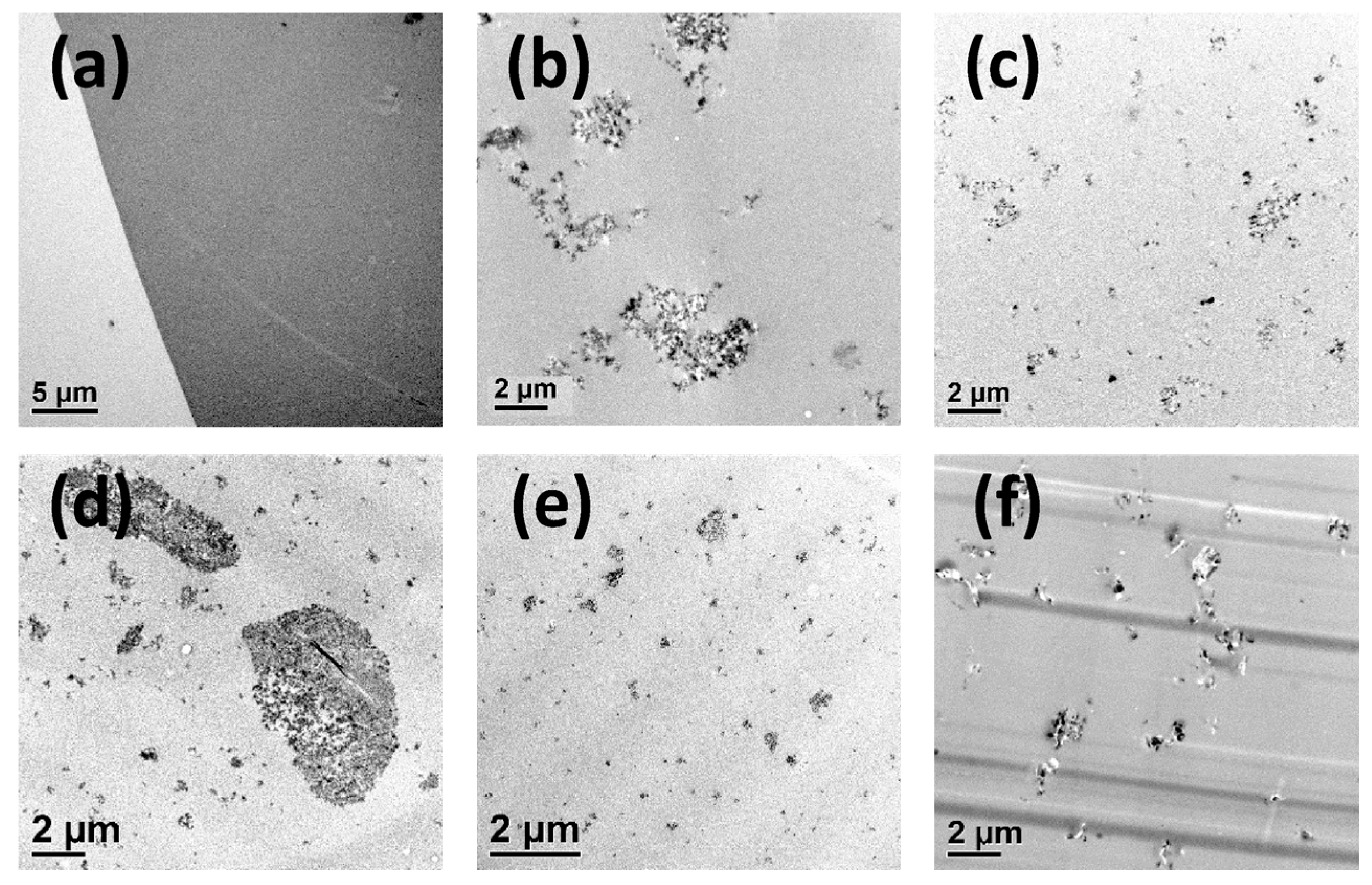
3.1. Surface Functionalization of the Nanoparticles and Preparation of the Nanocomposites
3.2. Permittivity Measurements of the Unfilled Epoxy Resin and the Nanocomposites
3.3. Breakdown Voltage
3.4. Water Uptake
3.5. Thermal Conductivity
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saeedi, I.A.; Vaughan, A.S.; Andritsch, T.; Virtanen, S. The Effect of Curing Conditions on the Electrical Properties of an Epoxy Resin. In Proceedings of the 2016 IEEE Conference on Electrical Insulation and Dielectric Phenomena, Toronto, ON, Canada, 16–19 October 2016; IEEE: New York City, NY, USA, 2016; pp. 461–464. [Google Scholar] [CrossRef]
- Saeedi, I.A.; Vaughan, A.S.; Andritsch, T. Change in the Electrical Properties Due to Modification of the Epoxy Network Structure Using Reactive Diluents. In Proceedings of the 2016 IEEE Conference on Electrical Insulation and Dielectric Phenomena, Toronto, ON, Canada, 16–19 October 2016; IEEE: New York City, NY, USA, 2016; pp. 663–666. [Google Scholar] [CrossRef]
- Ma, B.; Gubanski, S.M.; Krivda, A.; Schmidt, L.E.; Hollertz, R. Dielectric Properties and Resistance to Corona and Ozone of Epoxy Compositions Filled with Micro- and Nano-fillers. In Proceedings of the 2009 IEEE Conference on Electrical Insulation and Dielectric Phenomena, Virginia Beach, VA, USA, 18–21 October 2009; IEEE: New York City, NY, USA, 2009; pp. 672–677. [Google Scholar] [CrossRef]
- Gu, H.B.; Ma, C.; Gu, J.W.; Guo, J.; Yan, X.R.; Huang, J.N.; Zhang, Q.Y.; Guo, Z.H. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5896. [Google Scholar] [CrossRef]
- Pleşa, I.; Noţingher, P.V.; Schlögl, S.; Sumereder, C.; Muhr, M. Properties of Polymer Composites Used in High-Voltage Applications. Polymers 2016, 8, 173. [Google Scholar] [CrossRef]
- Moser, A.; Feuchter, M. Mechanical Properties of Composites Used in High-Voltage Applications. Polymers 2016, 8, 260. [Google Scholar] [CrossRef]
- Andraschek, N.; Wanner, A.J.; Ebner, C.; Riess, G. Mica/Epoxy-Composites in the Electrical Industry: Applications, Composites for Insulation, and Investigations on Failure Mechanisms for Prospective Optimizations. Polymers 2016, 8, 201. [Google Scholar] [CrossRef]
- Andritsch, T.; Kochetov, R.; Gebrekiros, Y.T.; Lafont, U.; Morshuis, P.H.F.; Smit, J.J. Synthesis and Dielectric Properties of Epoxy based Nanocomposites. In Proceedings of the 2009 IEEE Conference on Electrical Insulation and Dielectric Phenomena, Virginia Beach, VA, USA, 18–21 October 2009; IEEE: New York City, NY, USA, 2009; pp. 523–526. [Google Scholar] [CrossRef]
- Couderc, H.; Frechette, M.; Savoie, S.; Reading, M.; Vaughan, A.S. Dielectric and Thermal Properties of Boron Nitride and Silica Epoxy Composites. In Proceedings of the 2012 IEEE International Symposium on Electrical Insulation, San Juan, PR, USA, 10–13 June 2012; IEEE: New York City, NY, USA, 2012; pp. 64–68. [Google Scholar] [CrossRef]
- Danikas, M.G.; Varsamidou, K.; Cheng, Y.; Karlis, A.D. Epoxy Resin Insulation: The Influence of Nanoparticles on the Flashover Voltage and Possible Alternatives for Electrical Machines Insulation. In Proceedings of the 2016 XXII International Conference on Electrical Machines, Lausanne, Switzerland, 4–7 September 2016; IEEE: New York City, NY, USA, 2016; pp. 1668–1672. [Google Scholar] [CrossRef]
- Weng, Q.H.; Wang, X.B.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [PubMed]
- Ben Amor, I.; Rekik, H.; Kaddami, H.; Raihane, M.; Arous, M.; Kallel, A. Studies of dielectric relaxation in natural fiber-polymer composites. J. Electrost. 2009, 67, 717–722. [Google Scholar] [CrossRef]
- Smaoui, H.; Mir, L.E.L.; Guermazi, H.; Agnel, S.; Toureille, A. Study of dielectric relaxations in zinc oxide-epoxy resin nanocomposites. J. Alloys Compd. 2009, 477, 316–321. [Google Scholar] [CrossRef]
- Fimberger, M.; Tsekmes, I.-A.; Kochetov, R.; Smit, J.J.; Wiesbrock, F. Crosslinked Poly(2-oxazoline)s as “Green” Materials for Electronic Applications. Polymers 2016, 8, 6. [Google Scholar] [CrossRef]
- Sun, Z.; Dong, L.; Zhuang, Y.; Cao, L.; Ding, M.; Feng, Z. Beta relaxation in polyimides. Polymer 1992, 33, 4728–4731. [Google Scholar] [CrossRef]
- Kochetov, R.; Tsekmes, I.A.; Morshuis, P.H.F.; Smit, J.J.; Wanner, A.J.; Wiesbrock, F.; Kern, W. Effect of water absorption on the dielectric spectrum of nanocomposites. In Proceedings of the 2016 IEEE Electrical Insulation Conference, Montreal, QC, Canada, 19–22 June 2016; IEEE: New York City, NY, USA, 2016; pp. 579–582. [Google Scholar] [CrossRef]
- Zhang, Z.; Moser, A.; Feuchter, M.; Wieser, B.; Mühlbacher, I.; Pinter, G.; Schwarz, R.; Pukel, G.; Wiesbrock, F. BADGE/Aminhärter-Systeme für die Verklebung von Isolationskompositen. In Hocheffiziente Verbundwerkstoffe, 1st ed.; Schledjewski, R., Ed.; Eigenverlag: Leoben, AT, Austria, 2014; pp. 207–212. ISBN 978-3-9503248-3-9. [Google Scholar]
- Doszlop, S.; Vargha, V.; Horkay, F. Reactions of Epoxy with other Functional Groups and the Arising sec-Hydroxyl Groups. Period. Polytech. Chem. Eng. 1978, 22, 253–275. [Google Scholar]
- Bodner, T.; Behrendt, A.; Prax, E.; Wiesbrock, F. Correlation of Surface Roughness and Surface Energy of Silicon-based Materials with their Priming Reactivity. Chem. Mon. 2012, 143, 717–722. [Google Scholar] [CrossRef]
- Tanaka, T.; Kozako, M.; Fuse, N.; Ohki, Y. Proposal of a Multi-core Model for Polymer Nanocomposite Dielectrics. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 669–681. [Google Scholar] [CrossRef]
- Parida, S.K.; Dash, S.; Patel, S.; Mishra, B.K. Adsorption of organic molecules on silica surface. Adv. Colloid Interface Sci. 2006, 121, 77–110. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Yamazaki, A.; Watanabe, T.; Chikazawa, M. Water Adsorption Properties on Porous Silica Glass Surface Modified by Trimethylsilyl Groups. J. Colloid Interface Sci. 1997, 188, 409–414. [Google Scholar] [CrossRef]
- Shin, Y.-J.; Su, C.-C.; Shen, Y.-H. Dispersion of aqueous nano-sized alumina suspensions using cationic polyelectrolyte. Mater. Res. Bull. 2006, 41, 1964–1971. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003–2004; ISBN 978-0-8493048-4-2. [Google Scholar]
- Remunan-Lopez, C.; Bodmeier, R. Mechanical, water uptake and permeability properties of crosslinked chitosan glutamate and alginate films. J. Control. Release 1997, 44, 215–225. [Google Scholar] [CrossRef]
- Kumar, A.; Sundararaghavan, V.; Browning, A.R. Study of temperature dependence of thermal conductivity in cross-linked epoxies using molecular dynamics simulations with long range interactions. Model. Simul. Mater. Sci. Eng. 2014, 22, 025013. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Xiao, W.; Liua, Y.; Guo, S. Composites of graphene oxide and epoxy resin assuming a uniform 3D graphene oxide network structure. RSC Adv. 2016, 6, 86904–86908. [Google Scholar] [CrossRef]









| Specimen ↓/T → | −20 °C | 0 °C | 20 °C | 40 °C | 60 °C |
|---|---|---|---|---|---|
| DGEBA | 3.97 | 4.26 | 4.42 | 4.57 | 4.78 |
| SiO2 | 3.86 | 4.01 | 4.22 | 4.35 | 4.52 |
| SiO2-TMS | 4.17 | 4.43 | 4.59 | 4.76 | 5.02 |
| Al2O3 | 4.31 | 4.56 | 4.70 | 4.86 | 5.11 |
| Al2O3-TMS | 4.31 | 4.56 | 4.70 | 4.84 | 5.05 |
| BN | 4.20 | 4.42 | 4.55 | 4.69 | 4.89 |
| Specimen ↓/T → | −20 °C | 0 °C | 20 °C | 40 °C | 60 °C |
|---|---|---|---|---|---|
| DGEBA | 0.029 | 0.020 | 0.013 | 0.012 | 0.018 |
| SiO2 | 0.024 | 0.017 | 0.011 | 0.011 | 0.015 |
| SiO2-TMS | 0.025 | 0.016 | 0.012 | 0.014 | 0.024 |
| Al2O3 | 0.023 | 0.015 | 0.011 | 0.013 | 0.023 |
| Al2O3-TMS | 0.023 | 0.015 | 0.010 | 0.010 | 0.016 |
| BN | 0.020 | 0.014 | 0.010 | 0.010 | 0.015 |
| Specimen | AC Breakdown | DC Breakdown | ||||
|---|---|---|---|---|---|---|
| β | η | R2 | β | η | R2 | |
| DGEBA | 9.635 | 34.828 | 0.978 | 6.806 | 124.306 | 0.988 |
| SiO2 | 12.164 | 35.264 | 0.954 | 9.536 | 157.706 | 0.969 |
| SiO2-TMS | 8.098 | 32.470 | 0.994 | 7.970 | 162.367 | 0.971 |
| Al2O3 | 7.311 | 34.884 | 0.982 | 4.869 | 146.065 | 0.977 |
| Al2O3-TMS | 7.723 | 37.192 | 0.981 | 9.432 | 175.415 | 0.961 |
| BN | 21.620 | 32.198 | 0.928 | 6.188 | 143.252 | 0.946 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marx, P.; Wanner, A.J.; Zhang, Z.; Jin, H.; Tsekmes, I.-A.; Smit, J.J.; Kern, W.; Wiesbrock, F. Effect of Interfacial Polarization and Water Absorption on the Dielectric Properties of Epoxy-Nanocomposites. Polymers 2017, 9, 195. https://doi.org/10.3390/polym9060195
Marx P, Wanner AJ, Zhang Z, Jin H, Tsekmes I-A, Smit JJ, Kern W, Wiesbrock F. Effect of Interfacial Polarization and Water Absorption on the Dielectric Properties of Epoxy-Nanocomposites. Polymers. 2017; 9(6):195. https://doi.org/10.3390/polym9060195
Chicago/Turabian StyleMarx, Philipp, Andrea J. Wanner, Zucong Zhang, Huifei Jin, Ioannis-Alexandros Tsekmes, Johan J. Smit, Wolfgang Kern, and Frank Wiesbrock. 2017. "Effect of Interfacial Polarization and Water Absorption on the Dielectric Properties of Epoxy-Nanocomposites" Polymers 9, no. 6: 195. https://doi.org/10.3390/polym9060195
APA StyleMarx, P., Wanner, A. J., Zhang, Z., Jin, H., Tsekmes, I.-A., Smit, J. J., Kern, W., & Wiesbrock, F. (2017). Effect of Interfacial Polarization and Water Absorption on the Dielectric Properties of Epoxy-Nanocomposites. Polymers, 9(6), 195. https://doi.org/10.3390/polym9060195