Analysis of the Cross-Linking Reaction of Lignin with Triethyl Phosphate by MALDI-TOF and 13C NMR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Resins
2.2. Characterization of Samples
2.2.1. CP-MAS 13C NMR Analysis
2.2.2. MALDI-TOF Analysis
2.2.3. Wood Surface Coating Application
2.2.4. Metallic Surface Coating Application
3. Results
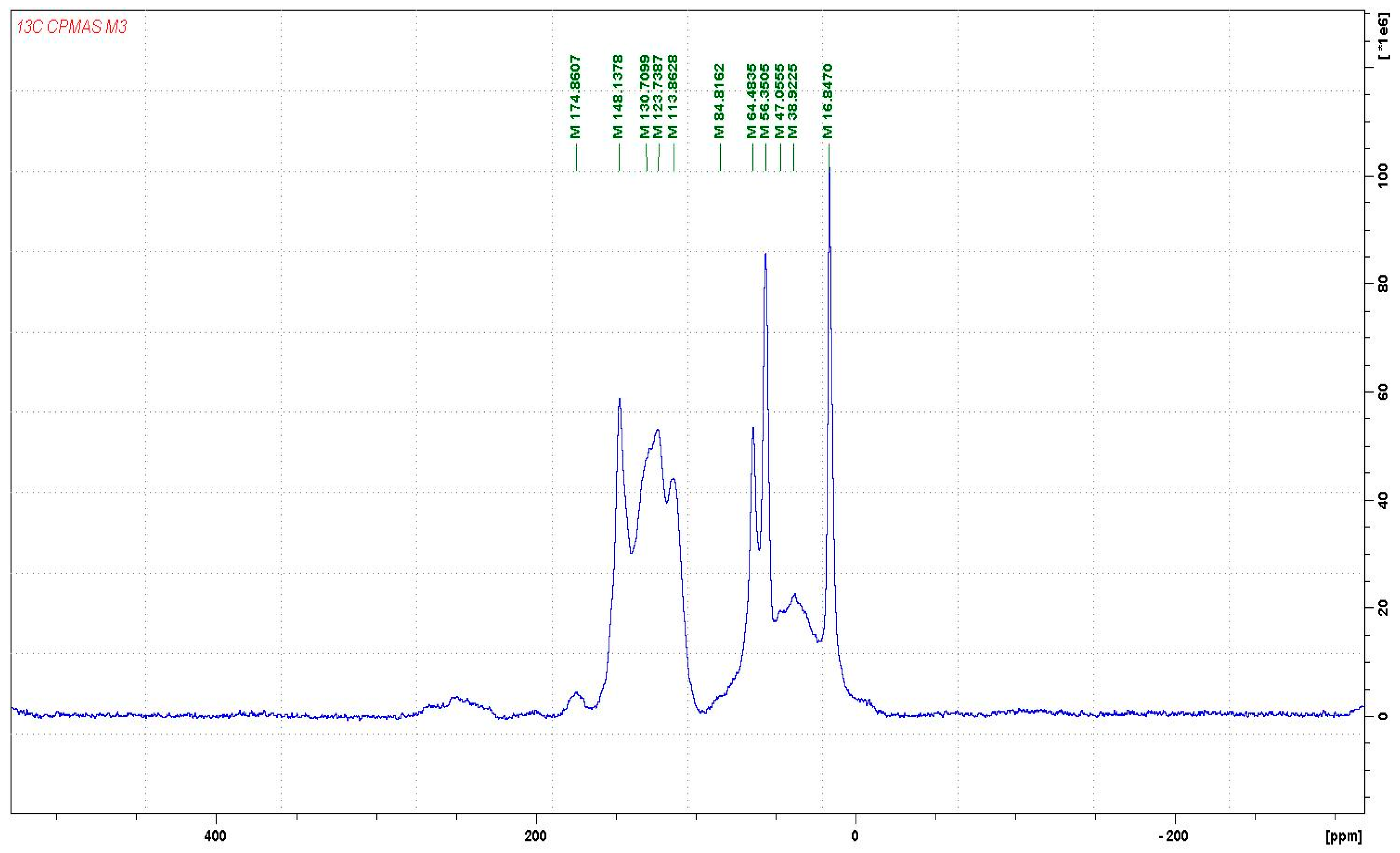
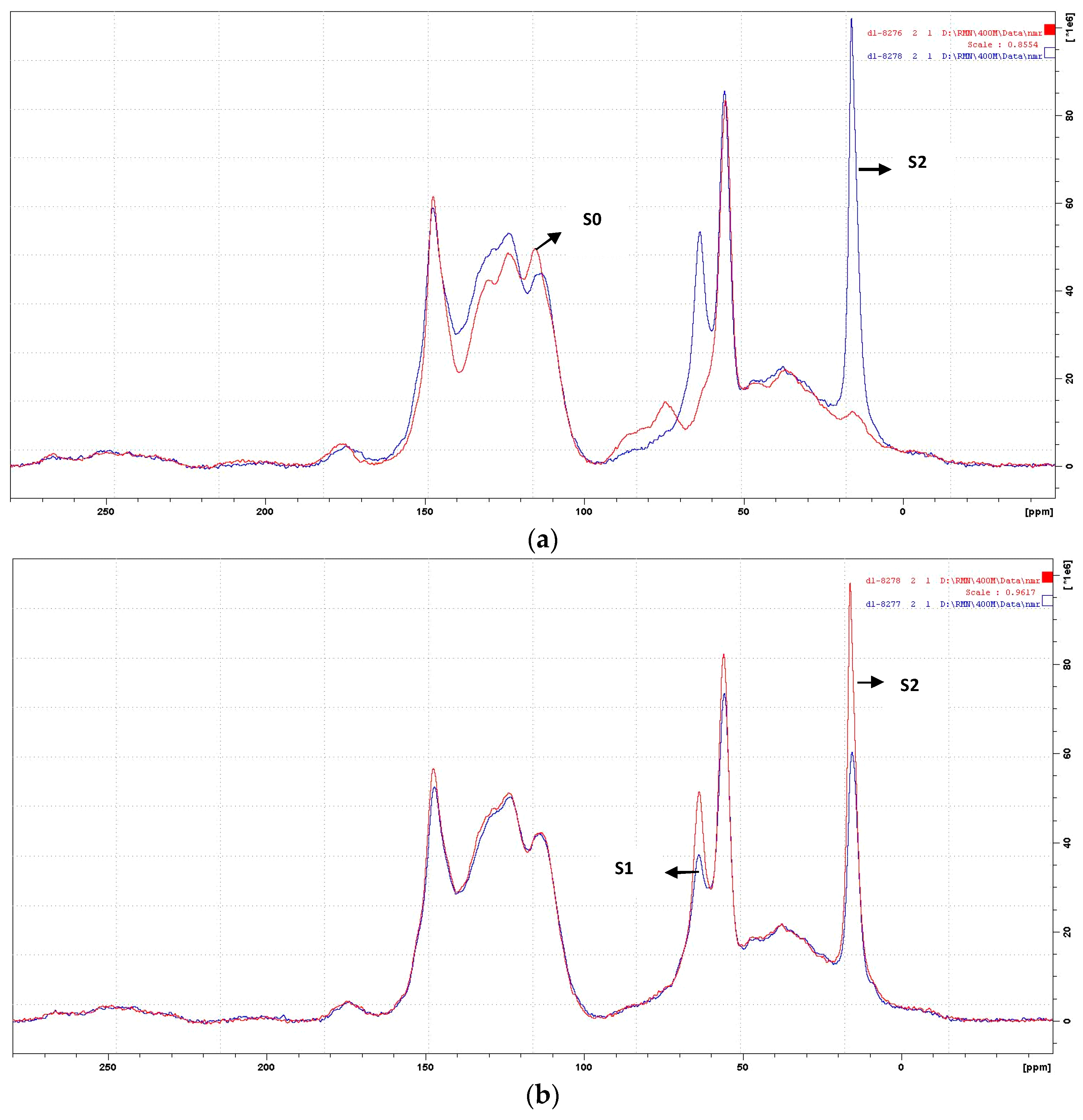
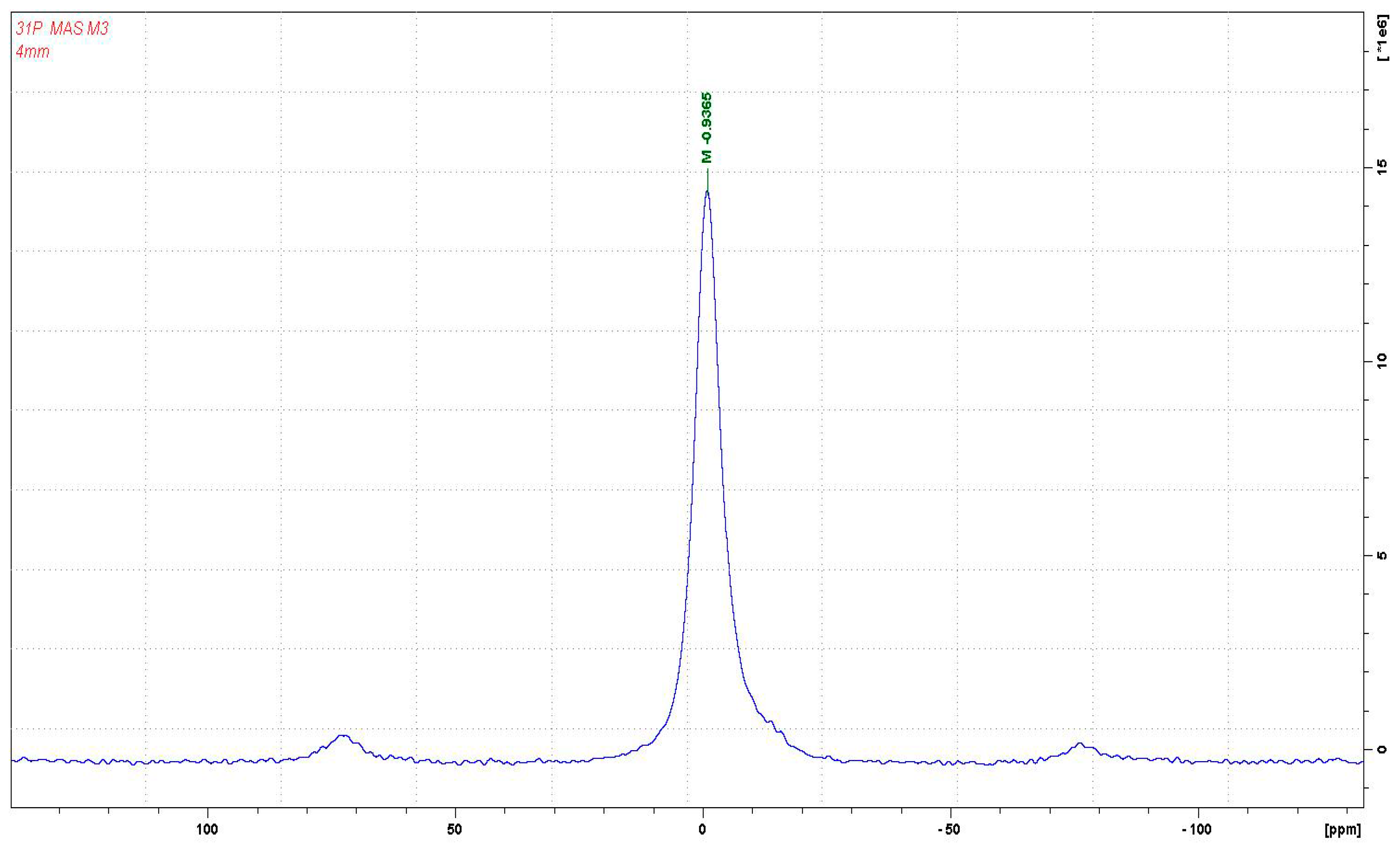
3.1. CP-MAS 13C NMR Analysis
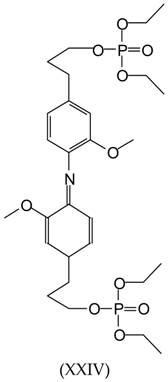
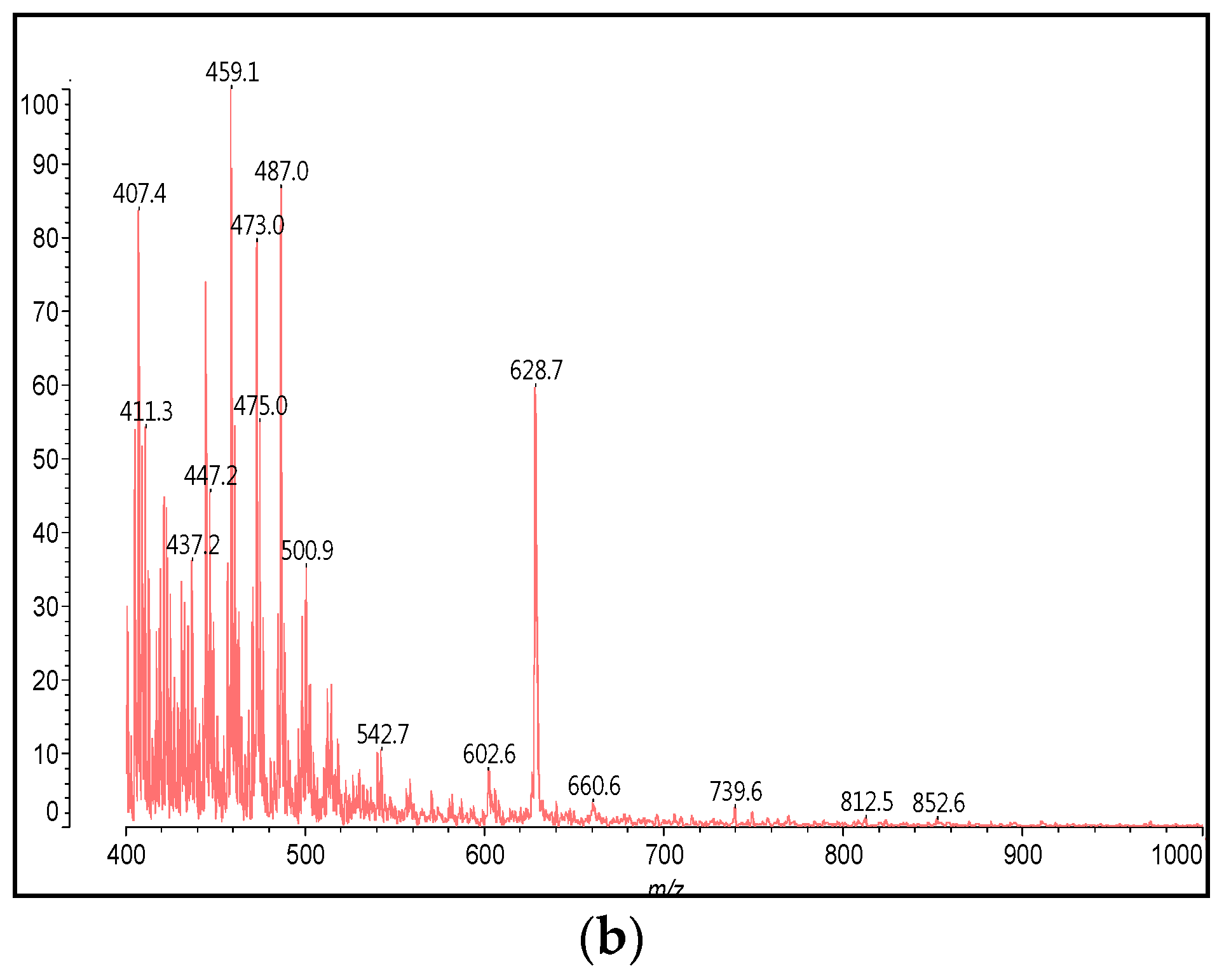
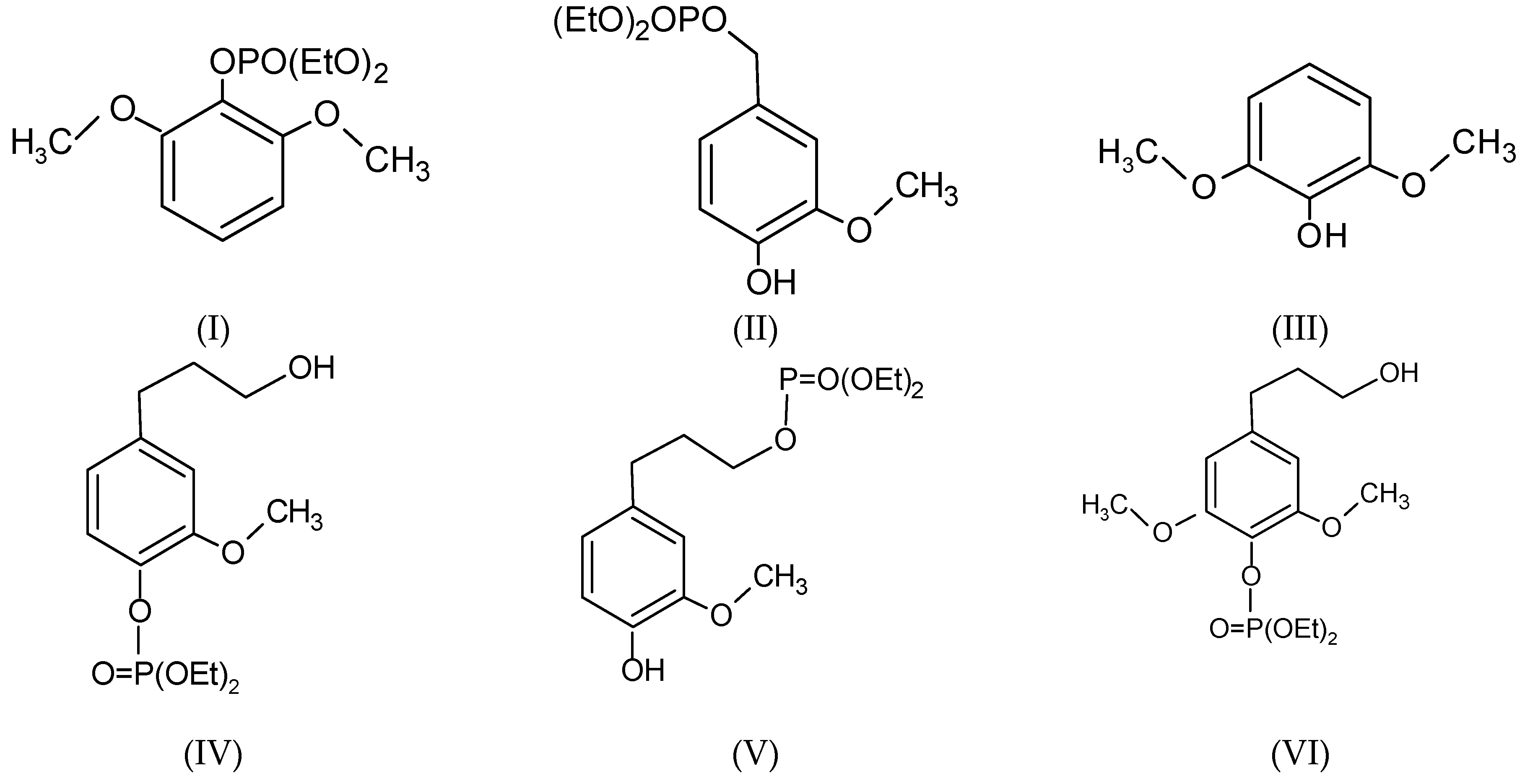
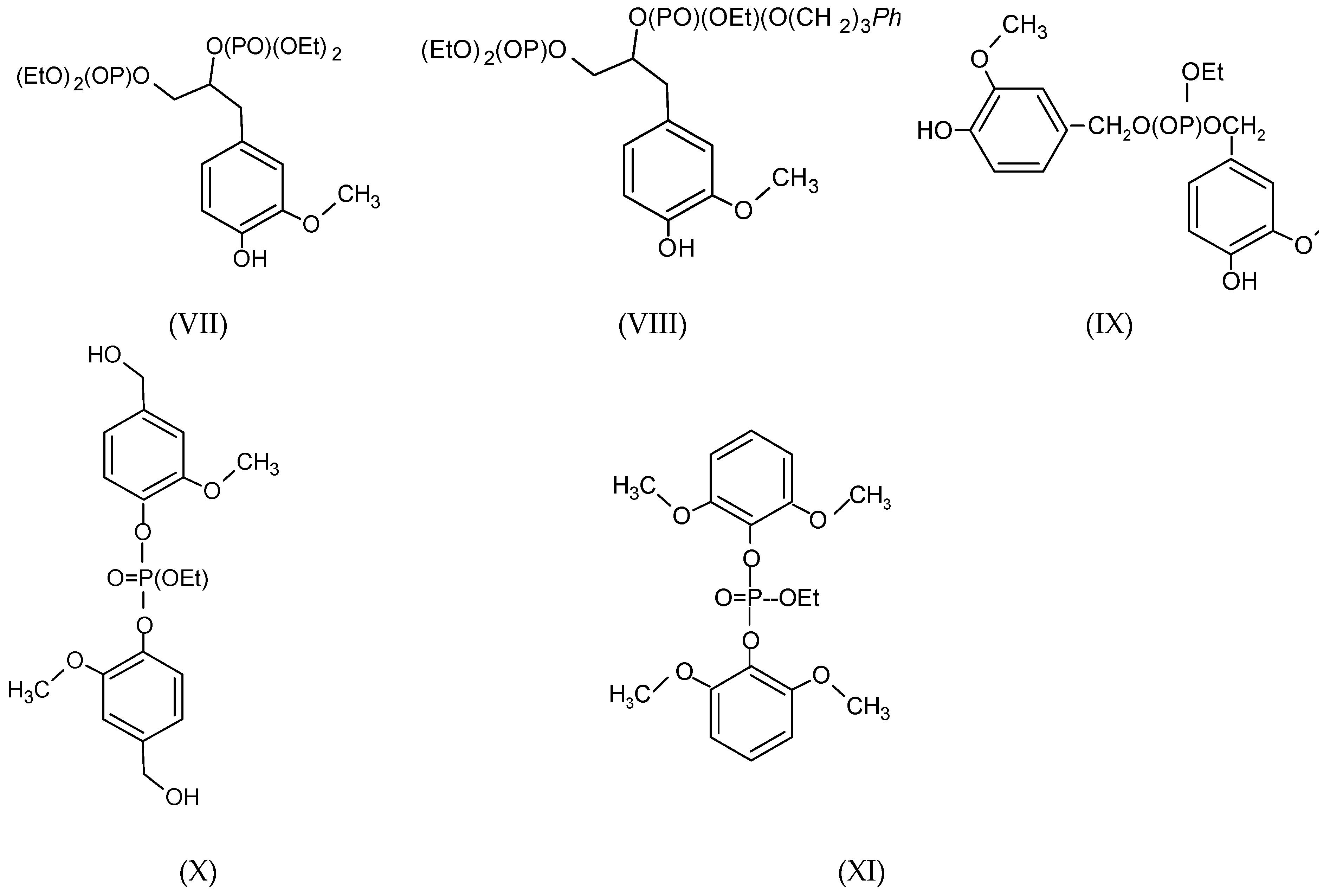
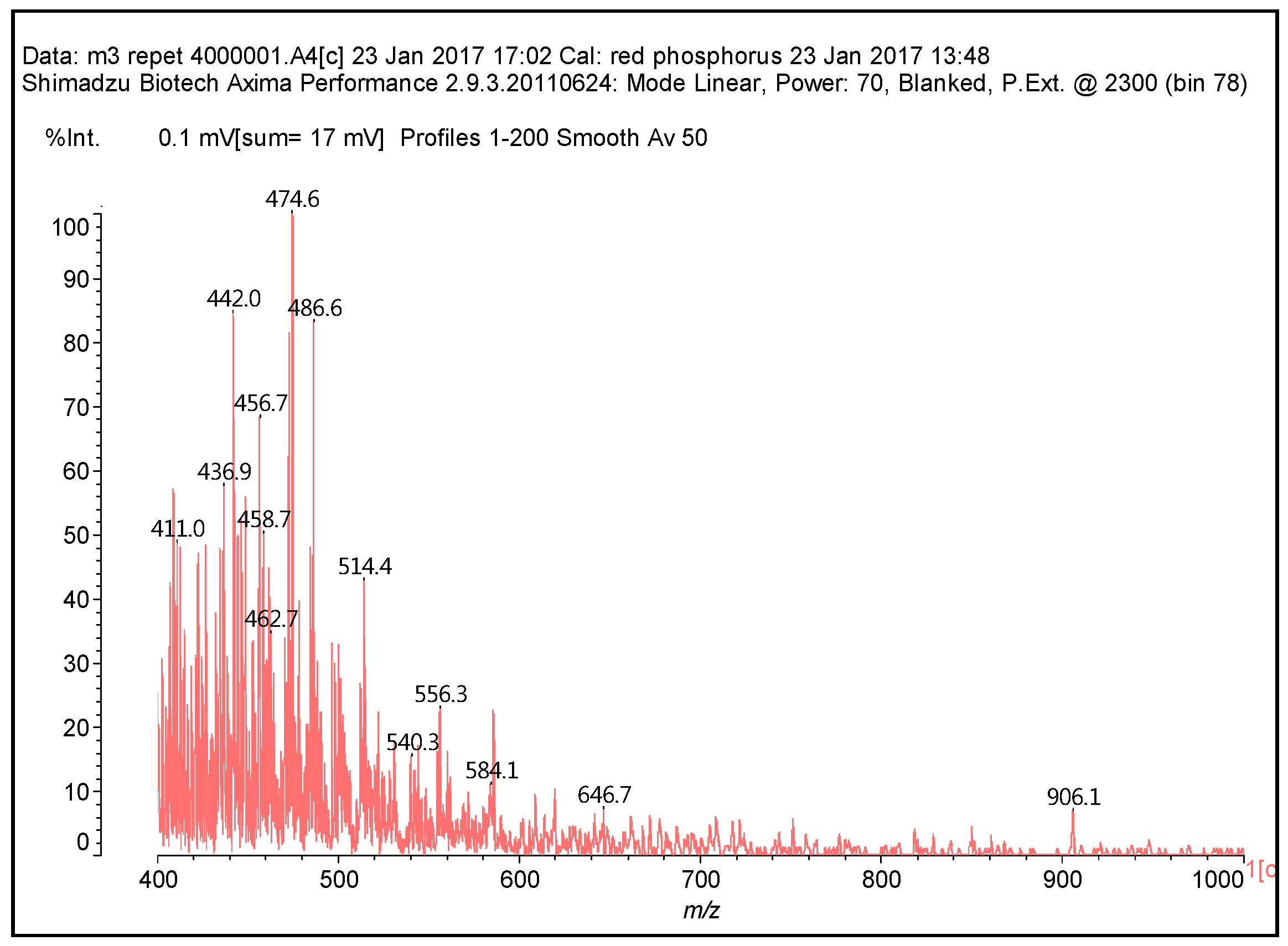
3.2. MALDI-TOF Analysis

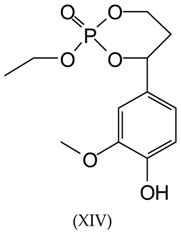

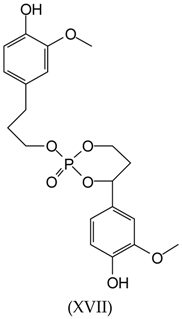
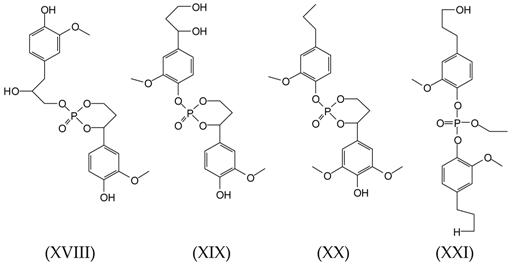
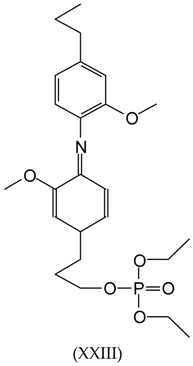
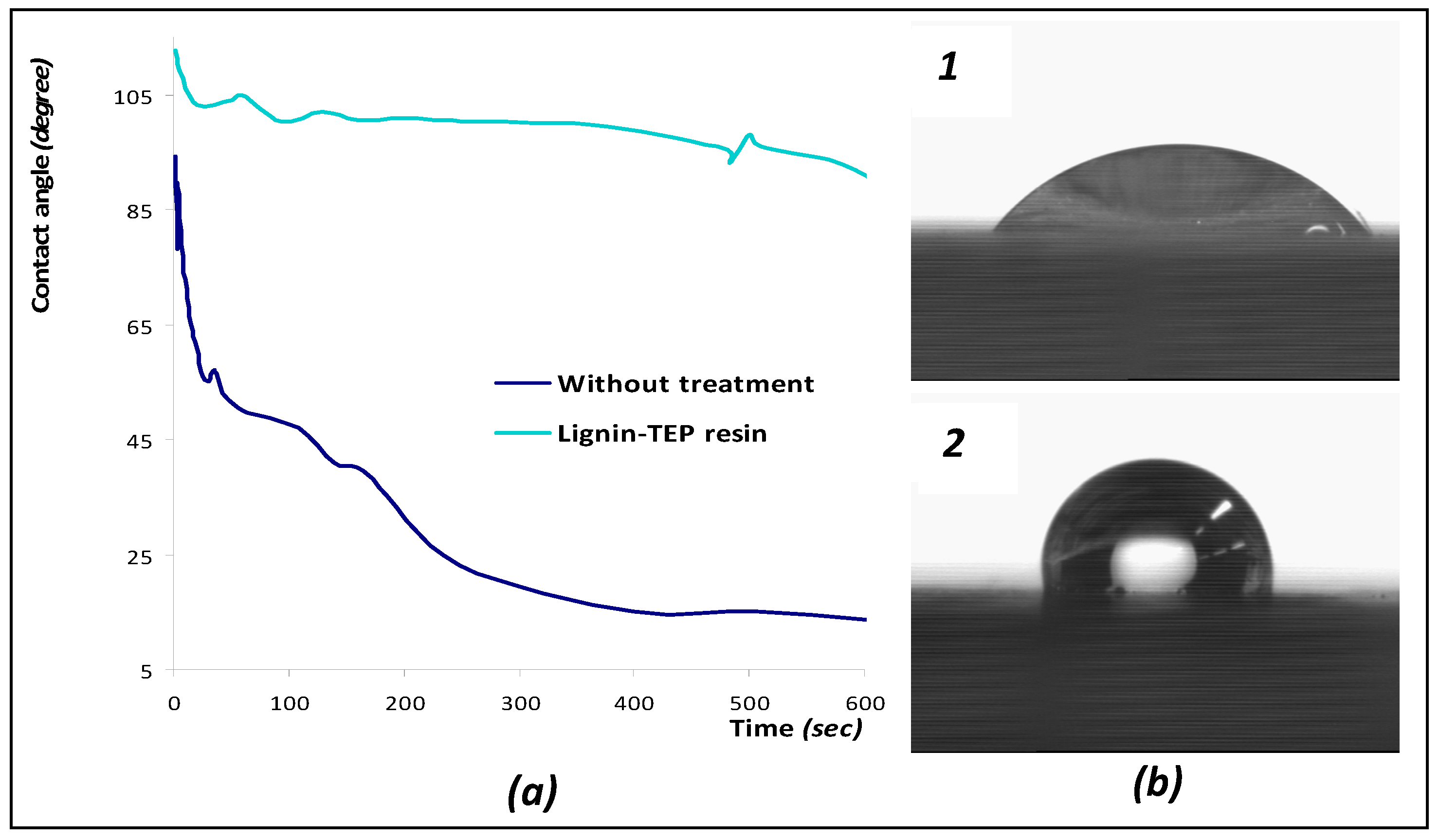
3.3. Wood Surface Coating Application: Contact Angle Measures with Water
3.4. Metallic Surface Coating Application: Adhesion Tests
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaplan, D.L. Introduction to biopolymers from renewable resources. In Biopolymers from Renewable Resources; Kaplan, D.L., Ed.; Springer: New York, NY, USA, 1998; pp. 1–29. ISBN 978-3-662-03680-8. [Google Scholar] [CrossRef]
- Raqueza, J.M.; Deleglise, M.; Lacrampea, M.F.; Krawczak, P. Thermosetting (bio) materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Berezina, N.; Martelli, S.M. Bio-based polymers and materials. In Renewable Resources for Biorefineries; Luque, R., Lin, C., Eds.; RSC Green Chemistry, The Royal Society of Chemistry: Cambridge, UK, 2014; pp. 1–28. ISBN 978-1-78262-018-1. [Google Scholar] [CrossRef]
- Kalita, H.; Kalita, D.; Alam, S.; Chemykh, A.; Tarnavchyk, I.; Bahr, J.; Satyabrata, S.; Jayasooriyama, A.; Shashi, F.; Selvakumar, S.; et al. Novel biobased polymers for coating applications. In Biobased and Environmentally Benign Coatings; Tiwari, A., Galanis, A., Soucek, M.D., Eds.; Scrivener Publishing: Wiley, NJ, USA, 2016; Chapter 1; ISBN 9781119185055. [Google Scholar] [CrossRef]
- Wool, R.P. Lignin, polymers and composites. In Bio-Based Polymers and Composites, 1st ed.; Sun, W.S., Wool, R., Eds.; Elsevier: Burlington, VT, USA, 2005; pp. 551–598. ISBN 978-0-12-763952-9. [Google Scholar] [CrossRef]
- Gellerstedt, G.; Henriksson, G. Lignin: Major sources, structure and properties. In Monomers, Polymers and Composites from Renewable Resources, 1st ed.; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Oxford, UK, 2008; pp. 201–224. ISBN 978-0-08-045316-3. [Google Scholar]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose-lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. 2012, 32, 452–463. [Google Scholar] [CrossRef]
- Bujanovic, B.; Amidon, T.; Driscoll, M.; Dongre, P. Lignin-furfural based adhesives. Energies 2015, 8, 7897–7914. [Google Scholar] [CrossRef]
- Wertz, J.-L.; Bédué, O. Lignocellulosic Biorefineries; EPFL Press, CRC Press: Lausanne, Switzerland, 2013; ISBN 13:978-1-4665-7307-9. [Google Scholar]
- Zamani, A. Introduction of lignocelluloses-based products. In Lignocellulose-Based Bioproducts; Karimi, K., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–36. [Google Scholar] [CrossRef]
- El Mansouri, N.E.; Pizzi, A.; Salvado, J. Lignin-based wood panel adhesives without formaldehyde. Holz Roh Werkst. 2007, 65, 65–70. [Google Scholar] [CrossRef]
- Lei, H.; Pizzi, A.; Du, G. Environmentally friendly mixed tannin/lignin wood resins. J. Appl. Polym. Sci. 2007, 107, 203–209. [Google Scholar] [CrossRef]
- El Mansouri, N.E.; Yuan, Q.; Huang, F. Study of chemical modification of alkaline lignin by the glyoxalation reaction. Bioresources 2011, 6, 4523–4536. [Google Scholar] [CrossRef]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C. Synthesis of lignin-based epoxy resins: Optimization of reaction parameters using reponse surface methodology. RSC Adv. 2014, 4, 31745–31753. [Google Scholar] [CrossRef]
- Xin, J.; Li, M.; Li, R.; Wolcott, M.P.; Zhang, J. Green epoxy resin system based on lignin and tung oil and ts application in epoxy asphalt. ACS Sustain. Chem. Eng. 2016, 4, 2754–2761. [Google Scholar] [CrossRef]
- Cateto, C.A.; Barreiro, M.F.; Ottati, C.; Lopretti, M.; Rodrigues, A.E.; Belgacem, M.N. Lignin-based rigid polyurethane foams with improved biodegradation. J. Cell. Plast. 2014, 50, 81–95. [Google Scholar] [CrossRef]
- Bernardini, J.; Cinelli, P.; Anguillesi, I.; Coltelli, M.B.; Lazzeri, A. Flexible polyurethane foams green production employing lignin or oxypropylated lignin. Eur. Polym. J. 2015, 64, 147–156. [Google Scholar] [CrossRef]
- Carvalho, G.; Frollini, E. Lignin in phenolic closed cell foams: Thermal stability and apparent density. J. Macromol. Sci. Pure Appl. Chem. 2002, 39, 643–656. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.; Wang, C.; Li, S.; Zhang, M.; Chu, F. Preparation and properties of lignin-phenol-formaldehyde resins based on different biorefinery residues of agricultural biomass. Ind. Crops Prod. 2013, 43, 326–333. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Polesel Maris, J.; Delmotte, L.; Colin, B.; Rogaume, Y. MALDI-TOF, 13C NMR and FTIR analysis of the cross-linking reaction of condensed tannins by triethyl phosphate. Ind. Crops. Prod. 2017, 95, 621–631. [Google Scholar] [CrossRef]
- Weil, E.D. Phosphorus-based flame retardants. In Flame Retardant Polymeric Materials; Lewis, M., Atlas, S.M., Pearce, E.M., Eds.; Springer: New York, NY, USA, 1978; Volume 2, pp. 103–131. ISBN 978-1-4684-6973-8. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood-Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1984. [Google Scholar] [CrossRef]
- Wang, K.; Xu, F.; Sun, R. Molecular characteristics of Kraft-AQ pulping lignin fractionated by sequential organic solvent extraction. Int. J. Mol. Sci. 2010, 11, 2988–3001. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.-L.; Sun, S.-L.; Xue, B.-L.; Sun, R.C. Recent advances in characterization of lignin polymer by solution-state nuclear magnetic resonance. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Tian, X.; Fang, Z.; Smith, R.L.; Wu, Z.; Liu, M. Properties, chemical characteristics and application of lignin and its derivatives. In Production of Biofuels and Chemical from Lignin; Fang, Z., Smith, R.L., Eds.; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Corbridge, D.E.C. Phosphorus: Chemistry, Biochemistry and Technology, 6th ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 13: 978-1-4398-4089-40. [Google Scholar]
- Braghiroli, F.; Fierro, V.; Izquierdo, M.T.; Parmentier, J.; Pizzi, A.; Celzard, A. Nitrogen-doped carbon materials produced from hydrothermally treated tannin. Carbon 2012, 50, 5411–5420. [Google Scholar] [CrossRef]


| Sample | Temperature (°C) | Composition | ||||
|---|---|---|---|---|---|---|
| Lignin 3 g | NH3 (25%) 1.5 g | TEP 3 g | Catechol 3 g | Glycerol 3 g | ||
| S0 | - | X | - | - | - | - |
| S1 | 180 | X | - | X | - | - |
| S2 | 180 | X | X | X | - | - |
| S3 | 180 | - | - | X | X | - |
| S4 | 180 | - | X | X | X | - |
| S5 | 180 | - | - | X | - | X |
| S6 | 90 | X | - | X | - | - |
| S7 | 90 | - | X | X | - | X |
| S8 | 220 | X | - | X | - | - |
| S9 | 220 | X | X | X | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso, M.C.; Pizzi, A.; Delmotte, L.; Abdalla, S. Analysis of the Cross-Linking Reaction of Lignin with Triethyl Phosphate by MALDI-TOF and 13C NMR. Polymers 2017, 9, 206. https://doi.org/10.3390/polym9060206
Basso MC, Pizzi A, Delmotte L, Abdalla S. Analysis of the Cross-Linking Reaction of Lignin with Triethyl Phosphate by MALDI-TOF and 13C NMR. Polymers. 2017; 9(6):206. https://doi.org/10.3390/polym9060206
Chicago/Turabian StyleBasso, María Cecilia, Antonio Pizzi, Luc Delmotte, and Soliman Abdalla. 2017. "Analysis of the Cross-Linking Reaction of Lignin with Triethyl Phosphate by MALDI-TOF and 13C NMR" Polymers 9, no. 6: 206. https://doi.org/10.3390/polym9060206







