Inducing β Phase Crystallinity in Block Copolymers of Vinylidene Fluoride with Methyl Methacrylate or Styrene
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Characterization
2.3. Synthesis of PVDF-I
2.4. Synthesis of the Block Copolymers (PVDF-b-PMMA and PVDF-b-PS)
3. Results and Discussion
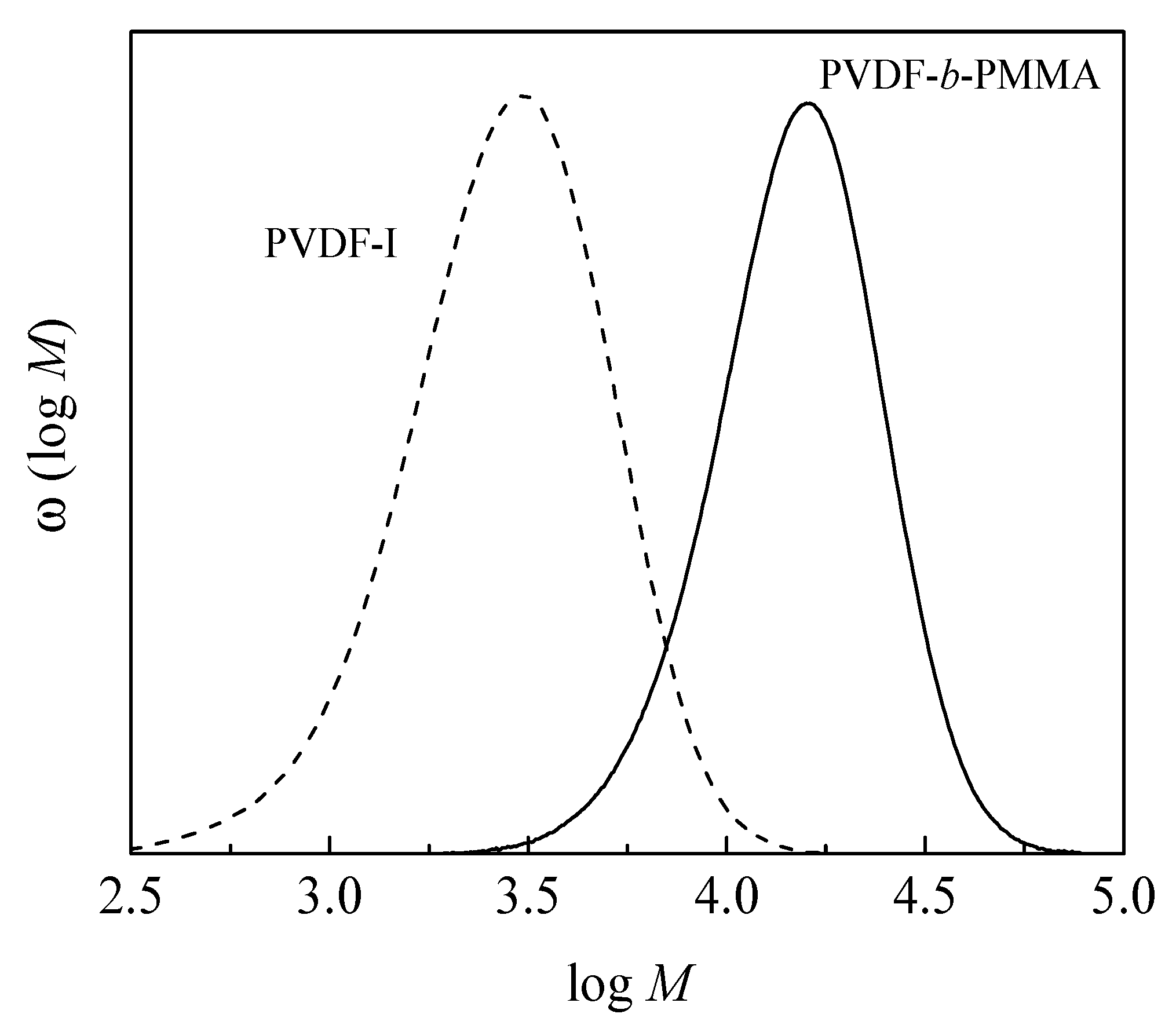
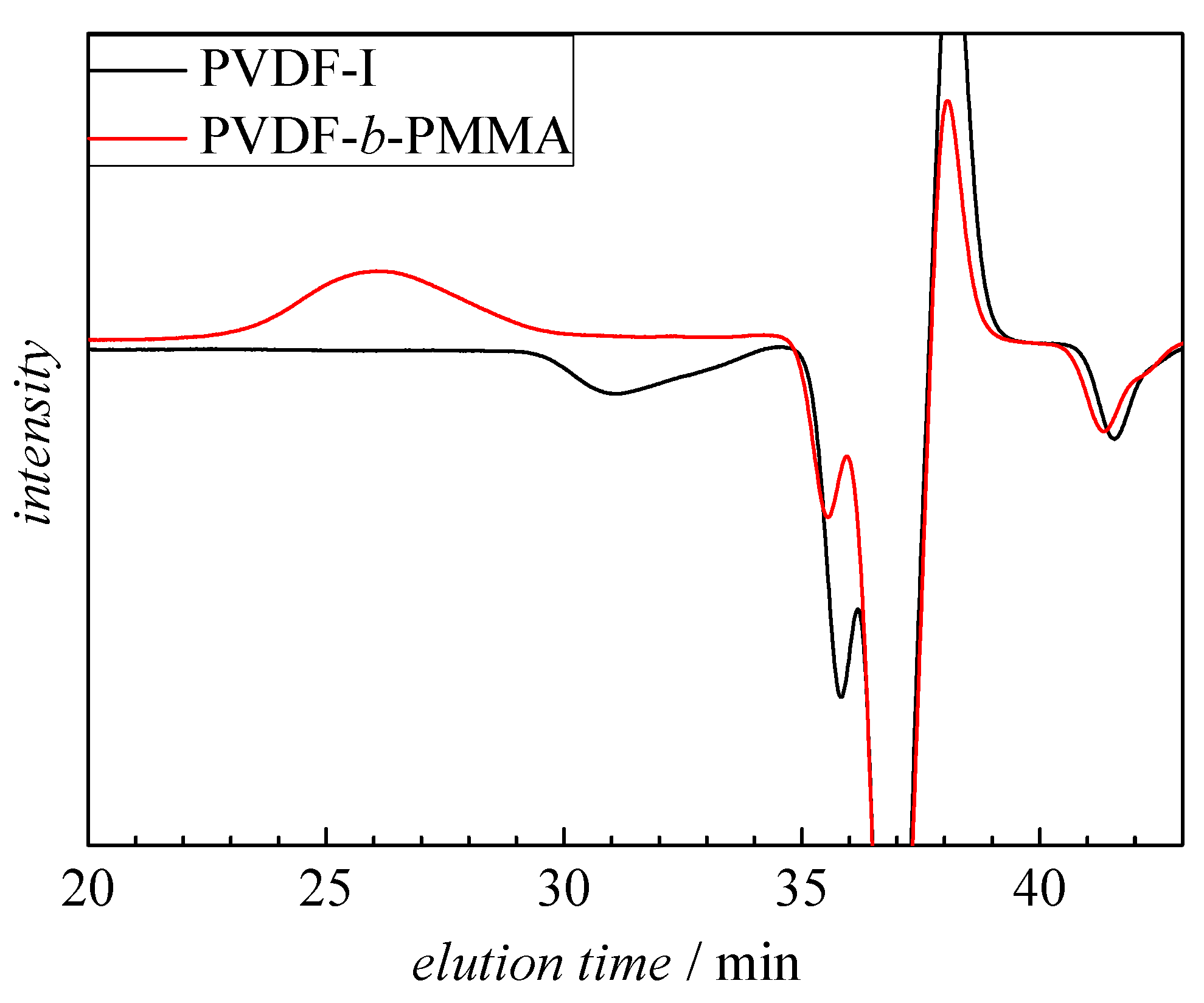
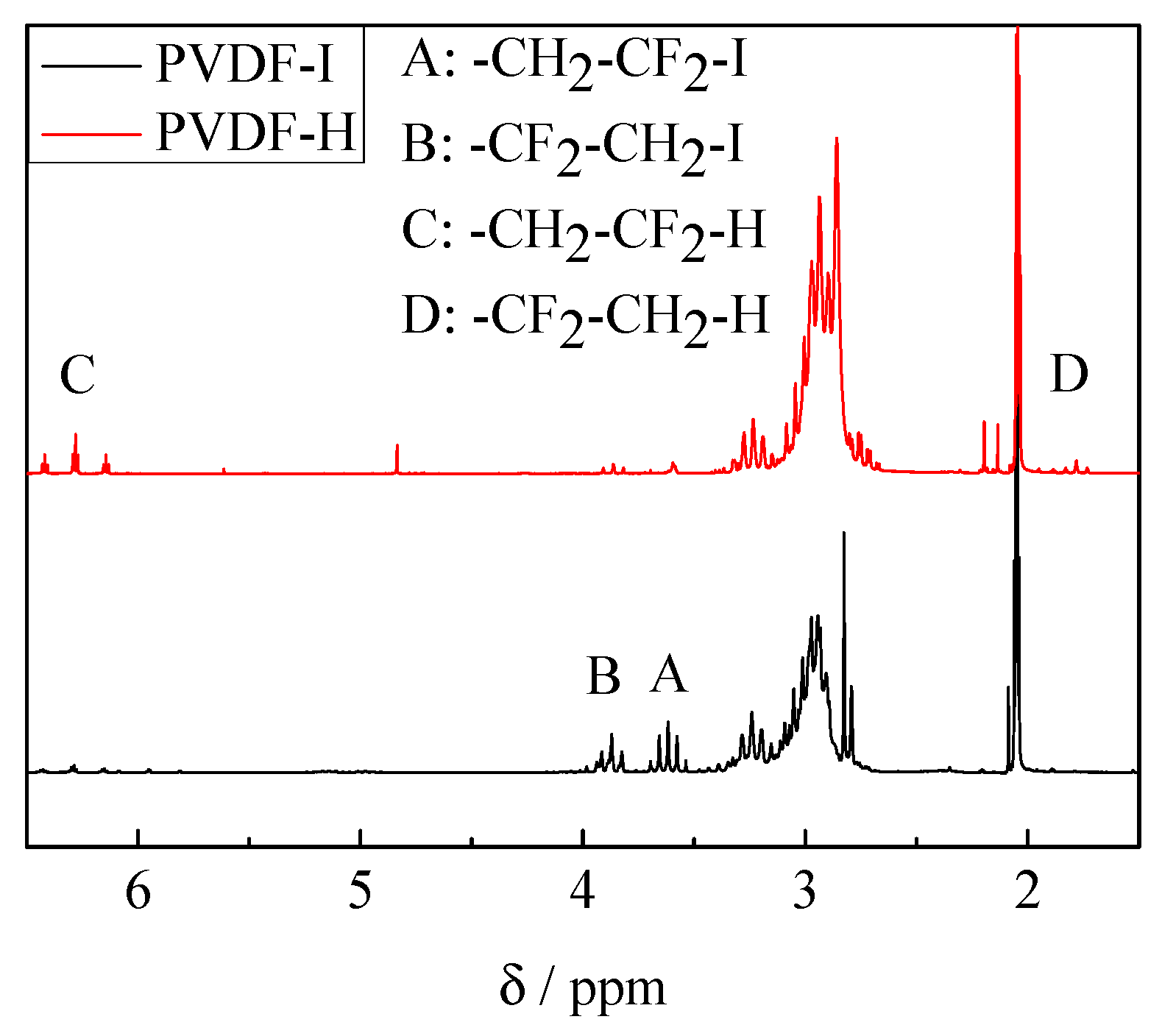
3.1. Chain Extension of PVDF
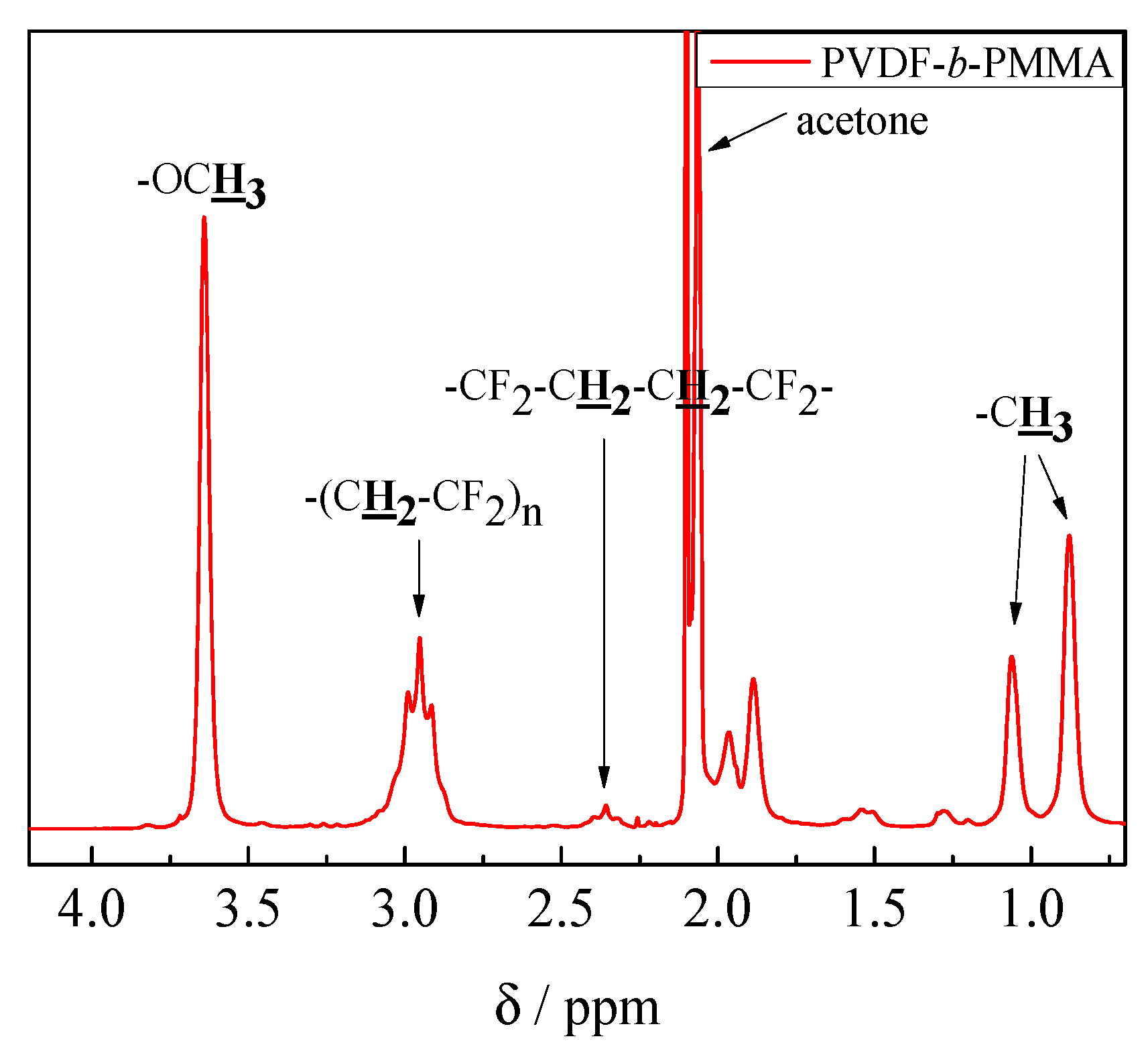
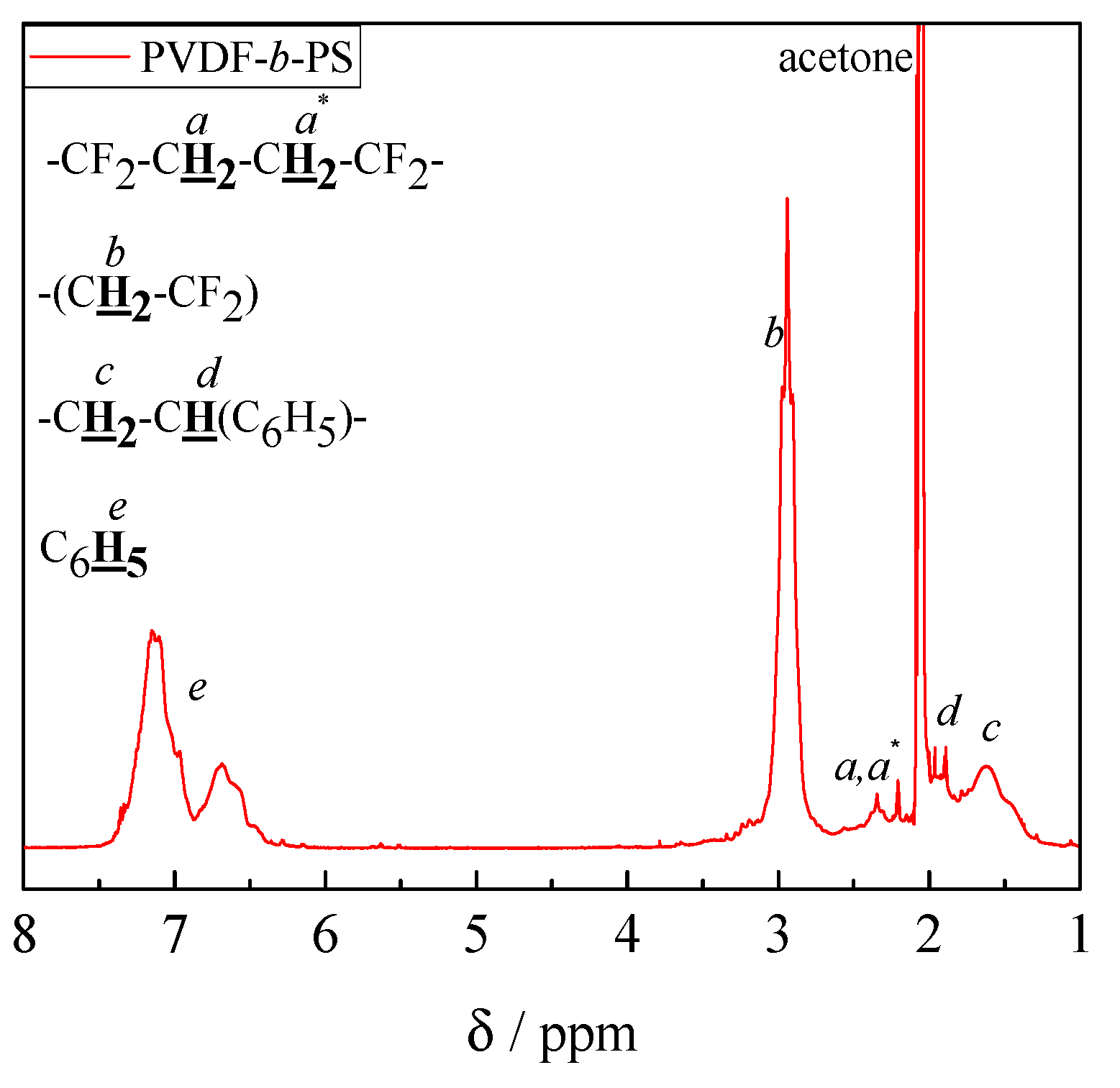
3.2. Block Copolymer Composition
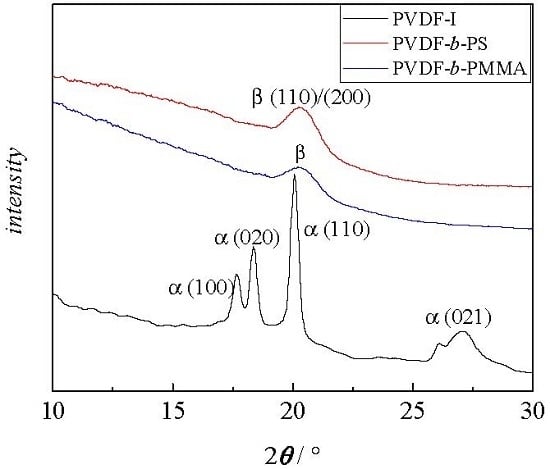
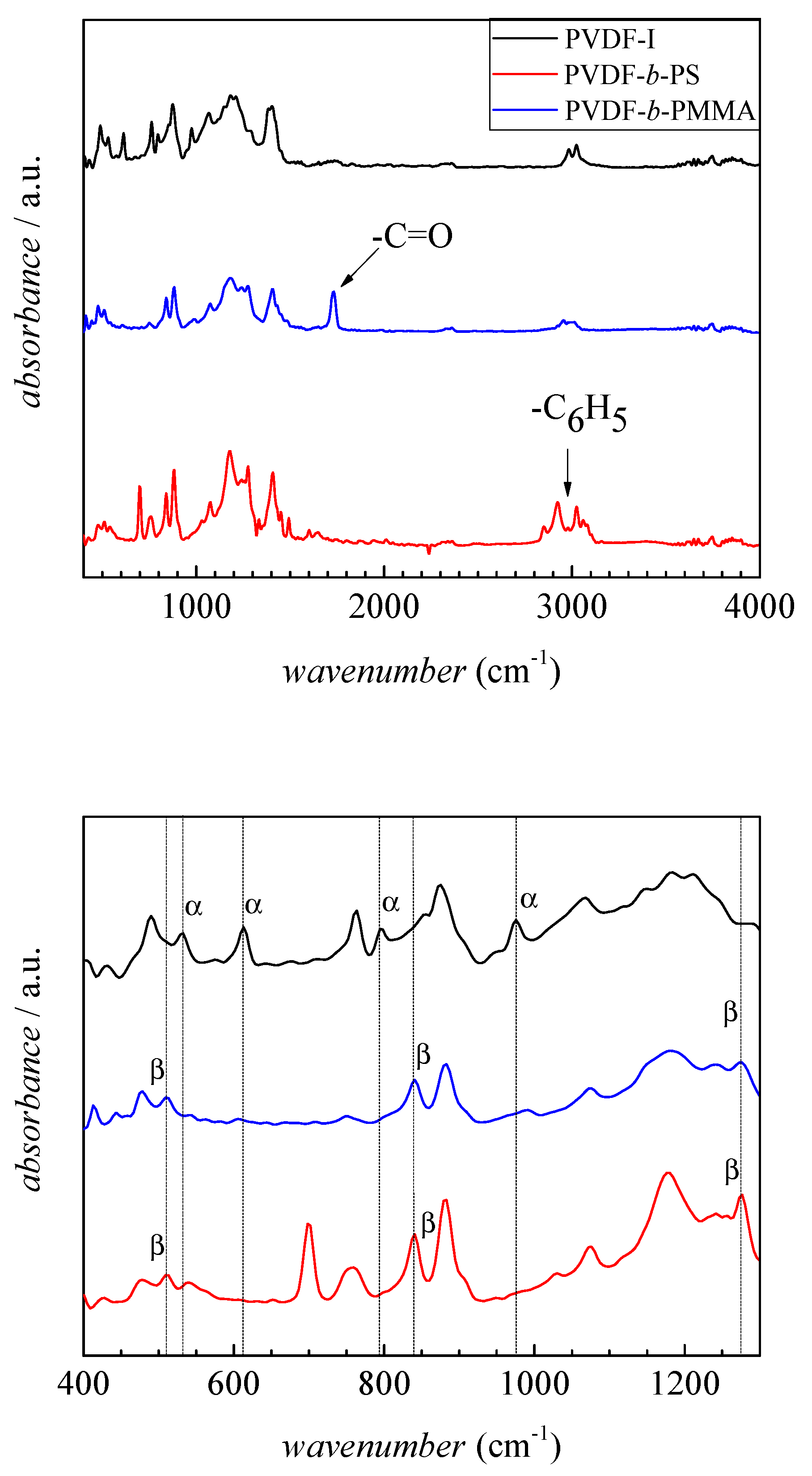
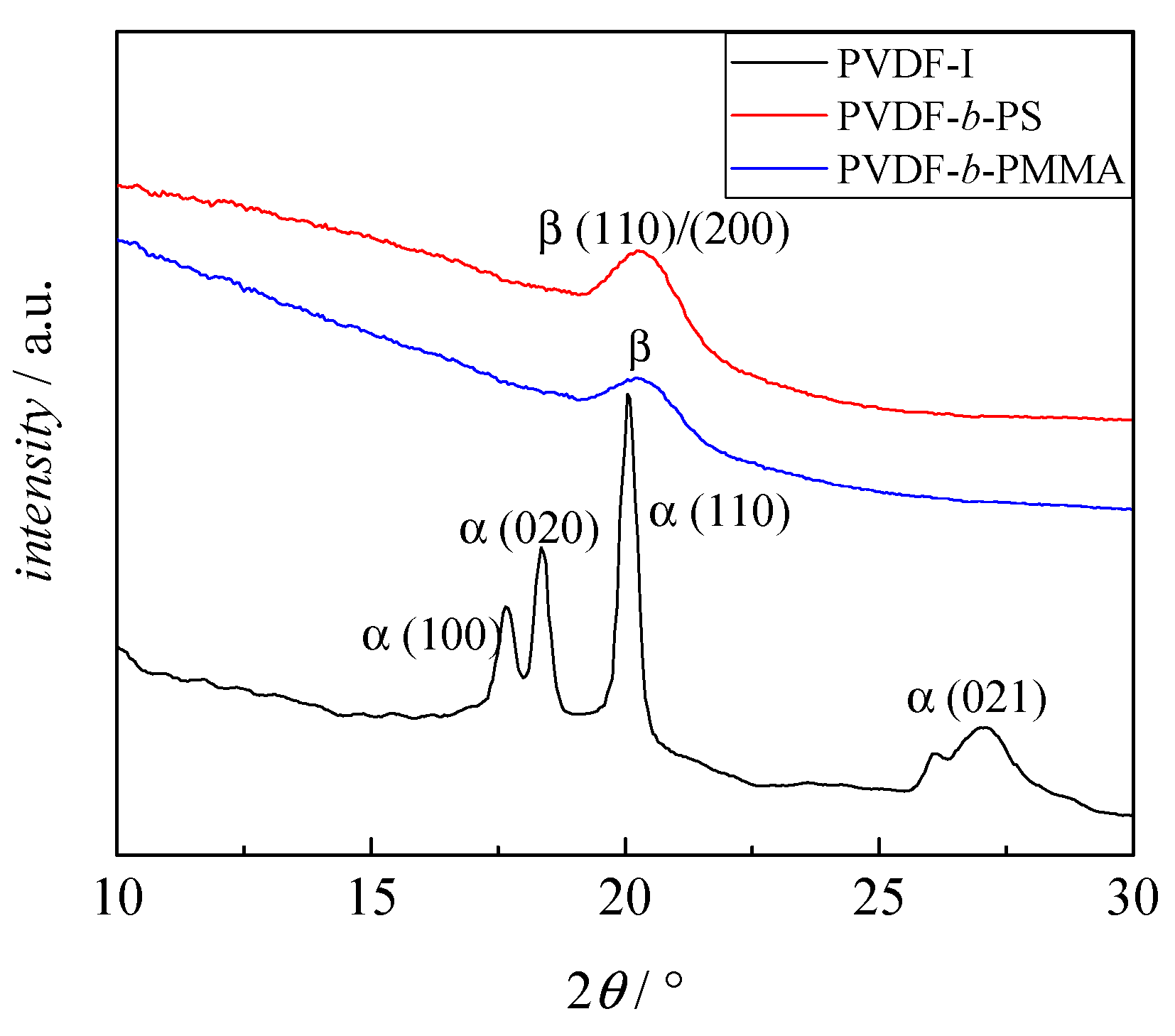
3.3. Crystallinity of the Block Copolymers
3.4. Phase Separation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Humphrey, J.S.; Amin-Sanayei, R. Vinylidene Fluoride Polymers. In Encyclopedia of Polymer Science and Technology, 3rd ed.; Wiley: New York, NY, USA, 2002; pp. 510–533. [Google Scholar]
- Scheirs, J. Modern Fluoropolymers: High Performance Polymers for Diverse Applications; Wiley: Chichester, UK; New York, NY, USA, 1997; ISBN 978-0-471-97055-2. [Google Scholar]
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [PubMed]
- Beuermann, S.; Imran-ul-haq, M. Homogeneous phase polymerization of vinylidene fluoride in supercritical CO2: Surfactant free synthesis and kinetics. Macromol. Symp. 2007, 259, 210–217. [Google Scholar] [CrossRef]
- Lu, F.J.; Hsu, S.L. Study of the crystallization behavior of poly(vinylidene fluoride) from melt under the effect of an electric field. Macromolecules 1986, 19, 326–329. [Google Scholar] [CrossRef]
- Kawai, H. The Piezoelectricity of Poly(vinylidene fluoride). Jpn. J. Appl. Phys. 1969, 8, 975–976. [Google Scholar] [CrossRef]
- Bergman, J.G., Jr.; Mcfee, J.H.; Crane, G.R. Pyroelectricity and optical second harmonic generation in poly(vinylidene fluoride) films. Appl. Phys. Lett. 1971, 18, 203. [Google Scholar] [CrossRef]
- Nakamura, K.; Wada, Y. Piezoelectricity, pyroelectricity, and the electrostriction constant of poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1971, 9, 161–173. [Google Scholar] [CrossRef]
- Lovinger, A.J. Molecular Mechanism for α (δ Transformation in Electrically Poled Poly(vinylidene fluoride)). Macromolecules 1981, 14, 225–227. [Google Scholar] [CrossRef]
- Kepler, R.G. Ferroelectric, Pyroelectric, and Piezoelectric Properties of Poly(vinylidene fluoride) in Ferroelectric Polymers Chemistry, in Physics and Applications; Nalwa, H.S., Ed.; CRC Press: New York, NY, USA, 1995; p. 183. ISBN 0-8247-9468-0. [Google Scholar]
- Hasegawa, R.; Kobayashi, M.; Tadokoro, H. Molecular conformation and packing of poly(vinylidene fluoride). Stability of three crystalline forms and the effect of high pressure. Polym. J. 1972, 3, 591–599. [Google Scholar] [CrossRef]
- Lovinger, A.; Reed, D. Inhomogeneous Thermal Degradation of Poly(vinylidene fluoride) Crystallized from the Melt. Macromolecules 1980, 13, 989–994. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Fukada, E. History and recent progress in piezoelectric polymers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F. Relaxor fluorinated polymers: Novel applications and recent developments. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 1106–1112. [Google Scholar] [CrossRef]
- Jungnickel, B.J. PVDF and Its Blends. In Ferroelectric Polymers Chemistry, in Physics and Applications; Nalwa, H.S., Ed.; CRC Press: New York, NY, USA, 1995; p. 183. ISBN 0-8247-9468-0. [Google Scholar]
- Valade, D.; Boyer, C.; Ameduri, B.; Boutevin, B. Poly(vinylidene fluoride)-b-poly(styrene) Block Copolymers by Iodine Transfer Polymerization (ITP): Synthesis, Characterization, and Kinetics of ITP. Macromolecules 2006, 39, 8639–8651. [Google Scholar] [CrossRef]
- Boutevin, B. From telomerization to living radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3235–3243. [Google Scholar] [CrossRef]
- David, G.; Boyer, C.; Tonnar, J.; Ameduri, B.; Lacroix-Desmazes, P.; Boutevin, B. Use of Iodocompounds in Radical Polymerization. Chem. Rev. 2006, 106, 3936–3962. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Valade, D.; Sauguet, L.; Ameduri, B.; Boutevin, B. Iodine Transfer Polymerization (ITP) of Vinylidene Fluoride (VDF). Influence of the defect of VDF chaining on the control of ITP. Macromolecules 2005, 38, 10353–10362. [Google Scholar] [CrossRef]
- Girard, E.; Marty, J.D.; Ameduri, B.; Destarac, M. Direct synthesis of Vinylidene Fluoride-Based Amphiphilic Diblock Copolymers by RAFT/MADIX Polymerization. ACS Macro Lett. 2012, 1, 270–274. [Google Scholar] [CrossRef]
- Guerre, M.; Campagne, B.; Gimello, O.; Parra, K.; Ameduri, B.; Ladmiral, V. Deeper Insight into the MADIX Polymerization of Vinylidene Fluoride. Macromolecules 2015, 48, 7810–7822. [Google Scholar] [CrossRef]
- Guerre, M.G.; Lopez, T.; Soulestin, C.; Totée, B.; Améduri, G.; Silly, V.; Ladmiral, A. Journey into the Microstructure of PVDF Made by RAFT. Macromol. Chem. Phys. 2016, 217, 2275–2285. [Google Scholar] [CrossRef]
- Monteiro, M.J.; Adamy, M.M.; Leeuwen, B.J.; van Herk, A.M.; Destarac, M. A “Living” Radical ab Initio Emulsion Polymerization of Styrene Using a Fluorinated Xanthate Agent. Macromolecules 2005, 38, 1538–1541. [Google Scholar] [CrossRef]
- Guerre, M.; Schmidt, J.; Talmon, Y.; Ameduri, B.; Ladmiral, V. An amphiphilic poly(vinylidene fluoride)-b-poly(vinyl alcohol) block copolymer: Synthesis and self-assembly in water. Polym. Chem. 2017, 8, 1125–1128. [Google Scholar] [CrossRef]
- Guerre, M.; Uchiyama, M.; Folgado, E.; Semsarilar, M.; Ameduri, B.; Satoh, K.; Kamigato, M.; Ladmiral, V. Combination of Cationic and Radical RAFT Polymerizations: A Versatile Route to Well-Defined Poly(ethyl vinyl ether)-block-poly(vinylidene fluoride) Block Copolymers. ACS Macro Lett. 2017, 6, 393–398. [Google Scholar] [CrossRef]
- Boyer, C.; Valade, D.; Lacroix-Desmazes, P.; Ameduri, B.; Boutevin, B. Kinetics of the Iodine Transfer Polymerization of Vinylidene Fluoride. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5763–5777. [Google Scholar] [CrossRef]
- Vukićević, R.; Vukovic, I.; Stoyanov, H.; Korwitz, A.; Pospiech, D.; Kofod, G.; Loos, K.; Brinke, G.; Beuermann, S. Poly(vinylidene fluoride)-functionalized single-walled carbon nanotubes for the preparation of composites with improved conductivity. Polym. Chem. 2012, 3, 2261–2265. [Google Scholar] [CrossRef]
- Vukićević, R.; Hirzenberger, P.; Hild, S.; Beuermann, S. Functionalization of carbon black nanoparticles with poly(vinylidene fluoride). J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4847–4854. [Google Scholar] [CrossRef]
- Vukićević, R.; Schwadtke, U.; Schmücker, S.; Schäfer, P.; Kuckling, D.; Beuermann, S. Alkyne-azide coupling of tailored poly(vinylidene fluoride) and polystyrene for the synthesis of block copolymers. Polym. Chem. 2012, 3, 409–414. [Google Scholar] [CrossRef]
- Asandei, A.D.; Adebolu, O.I.; Simpson, C.P. Mild-Temperature Mn2(CO)10-Photomediated Controlled Radical Polymerization of Vinylidene Fluoride and Synthesis of Well-Defined Poly(vinylidene fluoride) Block Copolymers. J. Am. Chem. Soc. 2012, 134, 6080–6083. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.P.; Adebolu, O.I.; Kim, J.-S.; Vasu, V.; Asandei, A.D. Metal and Ligand Effects of Photoactive Transition Metal Carbonyls in the Iodine Degenerative Transfer Controlled Radical Polymerization and Block Copolymerization of Vinylidene Fluoride. Macromolecules 2015, 48, 6404–6420. [Google Scholar] [CrossRef]
- Asandei, A.D. Photomediated Controlled Radical Polymerization and Block Copolymerization of Vinylidene Fluoride. Chem. Rev. 2016, 116, 2244–2247. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, G.J. Radicals in organic synthesis. Part 1. Tetrahedron 2009, 65, 8603–8655. [Google Scholar] [CrossRef]
- Goodman, J.L.; Peters, K.S.; Vaida, V. The Determination of the Mn-Mn Bond Strength in Mn2(CO)10 Using Pulsed Time Resolved Photoacoustic Calorimetry. Organometallics 1986, 5, 815–816. [Google Scholar] [CrossRef]
- Sarakha, M.; Ferraudi, G. Photophysical Features of the M2(CO)10, M = Mn and Re, Solution Photochemistry. Inorg. Chem. 1999, 38, 4605–4607. [Google Scholar] [CrossRef] [PubMed]
- Voet, V.S.D.; Hermida-Merino, D.; ten Brinke, G.; Loos, K. Block copolymer route towards poly(vinylidene fluoride)/poly(methacrylic acid)/nickel nanocomposites. RSC Adv. 2013, 3, 7938–7946. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Alberda van Ekenstein, G.O.R.; Meereboer, N.L.; Hofman, A.H.; ten Brinke, G.; Loos, K. Double-crystalline PLLA-b-PVDF-b-PLLA triblock copolymers: Preparation and crystallization. Polym. Chem. 2014, 5, 2219–2230. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Tichelaar, M.; Tanase, S.; Mittelmeijer-Hazeleger, M.C.; ten Brinke, G.; Loos, K. Poly(vinylidene fluoride)/nickel nanocomposites from semicrystalline block copolymer precursors. Nanoscale 2013, 5, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Beuermann, S.; Imran-ul-haq, M. Homogeneous Phase Polymerization of Vinylidene Fluoride in Supercritical Carbon Dioxide. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 5626–5635. [Google Scholar] [CrossRef]
- Möller, E.; Beuermann, S. Homogeneous Phase Copolymerizations of Vinylidene Fluoride and Hexafluoropropene in Supercritical Carbon Dioxide. Macromol. React. Eng. 2011, 5, 8–21. [Google Scholar] [CrossRef]
- Brandl, F.; Beuermann, S. Halb-kontinuierliche Emulsionspolymerisation von Vinylidenfluorid. Chem. Ing. Tech. submitted.
- Nick, L.; Kindermann, A.; Fuhrmann, J. Morphological studies of spin-coated films of poly(styrene-block-methyl methacrylate) copolymers by atomic force microscopy. Colloid Polym. Sci. 1994, 272, 367–371. [Google Scholar] [CrossRef]
- Gallot-Grubisic, Z.; Rempp, P.; Benoit, H.J. A universal calibration for gel permeation chromatography. Polym. Sci. Part B Polym. Lett. 1967, 5, 753–759. [Google Scholar] [CrossRef]
- Siegmann, R.; Drache, M.; Beuermann, S. Propagation rate coefficients for vinylidene fluoride homopolymerizations. Macromolecules 2013, 46, 9507–9514. [Google Scholar] [CrossRef]
- Guiot, J.; Ameduri, B.; Boutevin, B. Radical Homopolymerization of Vinylidene Fluoride Initiated by tert-Butyl Peroxypivalate. Investigation of the Microstructure by 19F and 1H NMR Spectroscopies and Mechanisms. Macromolecules 2002, 35, 8694–8707. [Google Scholar] [CrossRef]
- Balague, J.; Ameduri, B.; Boutevin, B.; Caporiccio, G. Controlled step-wise telomerization of vinylidene fluoride, hexafluoropropene and trifluoroethylene with iodofluorinated transfer agents. J. Fluor. Chem. 2000, 102, 253–268. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. Spektroskopische Methoden in der Organischen Chemie, 7th ed.; Thieme: Stuttgart, Germany, 2005. [Google Scholar]
- Paul, D.R.; Altamirano, J.O. Properties of compatible blends of poly(vinylidene fluoride) and poly(methyl methacrylate). Adv. Chem. Ser. 1975, 142, 371–385. [Google Scholar] [CrossRef]
- Noland, J.S.; Hsu, N.N.; Schmitt, J.M. Compatible high polymers: Poly(vinylidene fluoride) blends with homopolymers of methyl and ethyl methacrylate. Adv. Chem. Ser. 1971, 99, 15–28. [Google Scholar] [CrossRef]
- Yano, S. Dielectric relaxation and molecular motion in poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1970, 8, 1057–1072. [Google Scholar] [CrossRef]
- Kakutani, H. Dielectric absorption in oriented poly(vinylidene fluoride). J. Polym. Sci. Part B Polym. Phys. 1970, 8, 1177–1186. [Google Scholar] [CrossRef]
- Li, P.; Maier, J.M.; Vik, E.C.; Yehl, C.J.; Dial, B.E.; Rickher, A.E.; Smith, M.D.; Pellechia, P.J.; Shimizu, K.D. Stabilizing Fluorine—π Interactions. Angew. Chem. Int. Ed. 2017, 56, 7209–7212. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L. The C-F bond as a conformational tool in organic and biological chemistry. Beilstein J. Org. Chem. 2010, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C-F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Hof, F.; Scofield, D.M.; Schweizer, W.B.; Diederich, F. A weak attractive interaction between organic fluorine and an amide group. Angew. Chem. Int. Ed. 2004, 43, 5056–5059. [Google Scholar] [CrossRef] [PubMed]
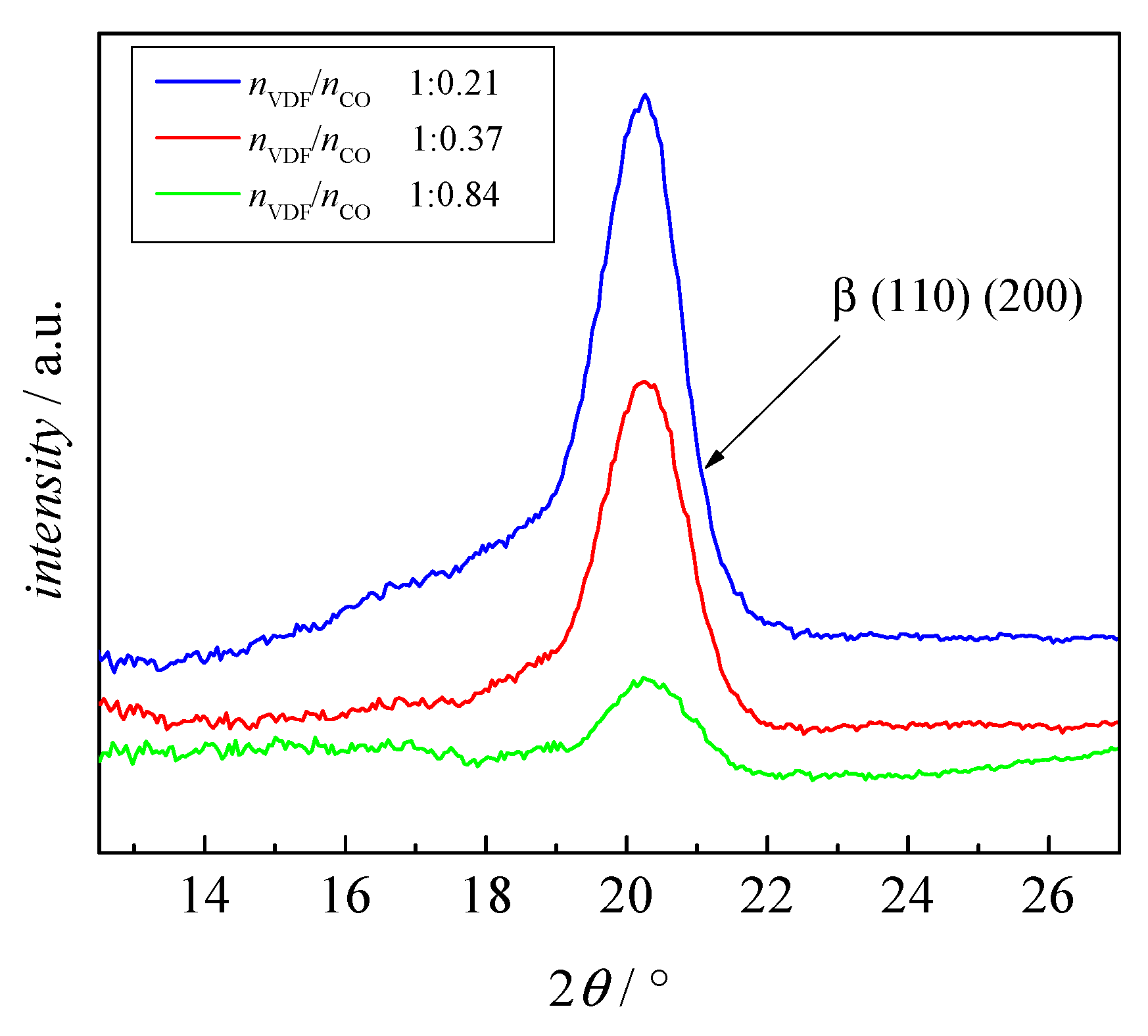

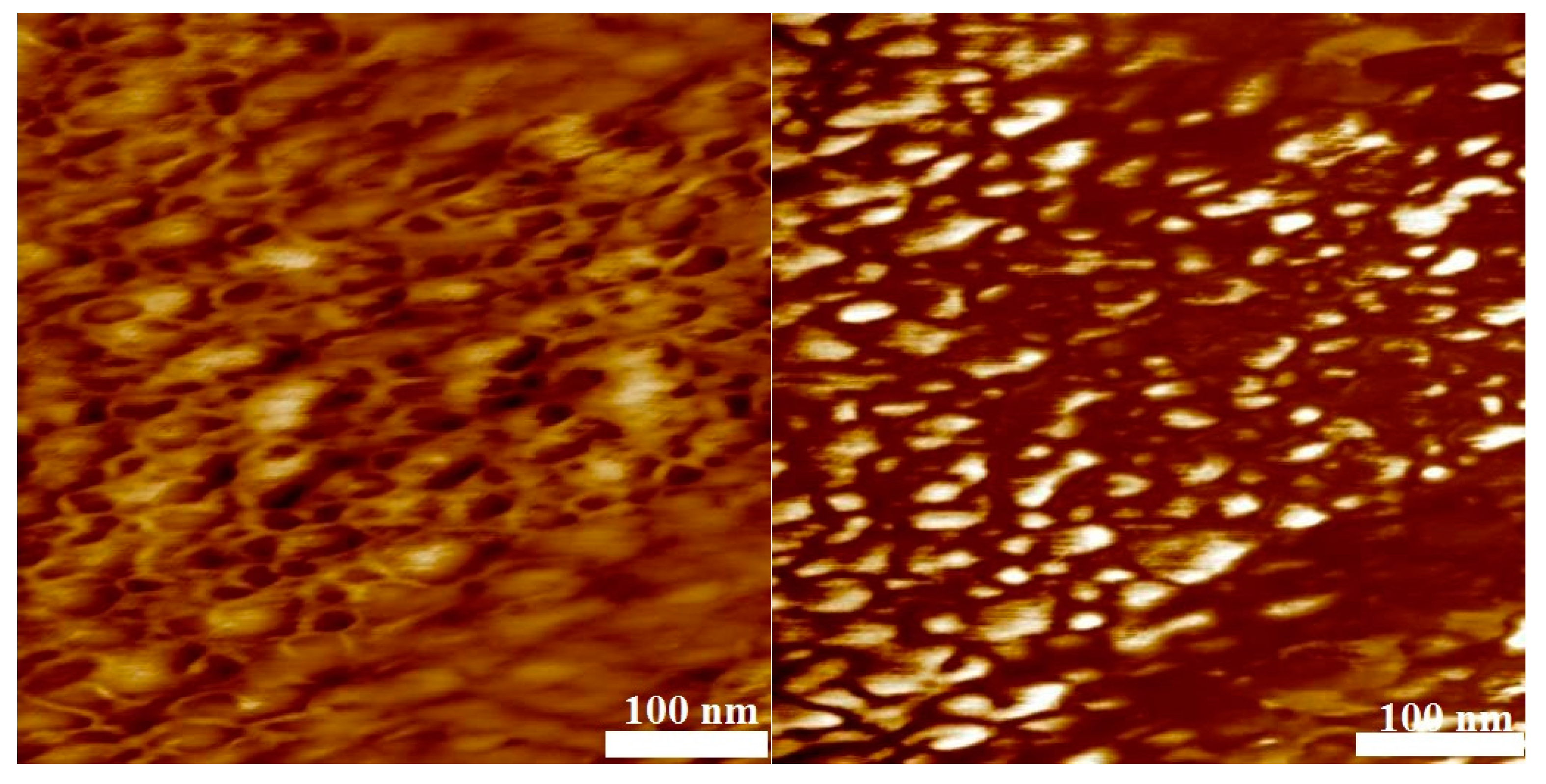
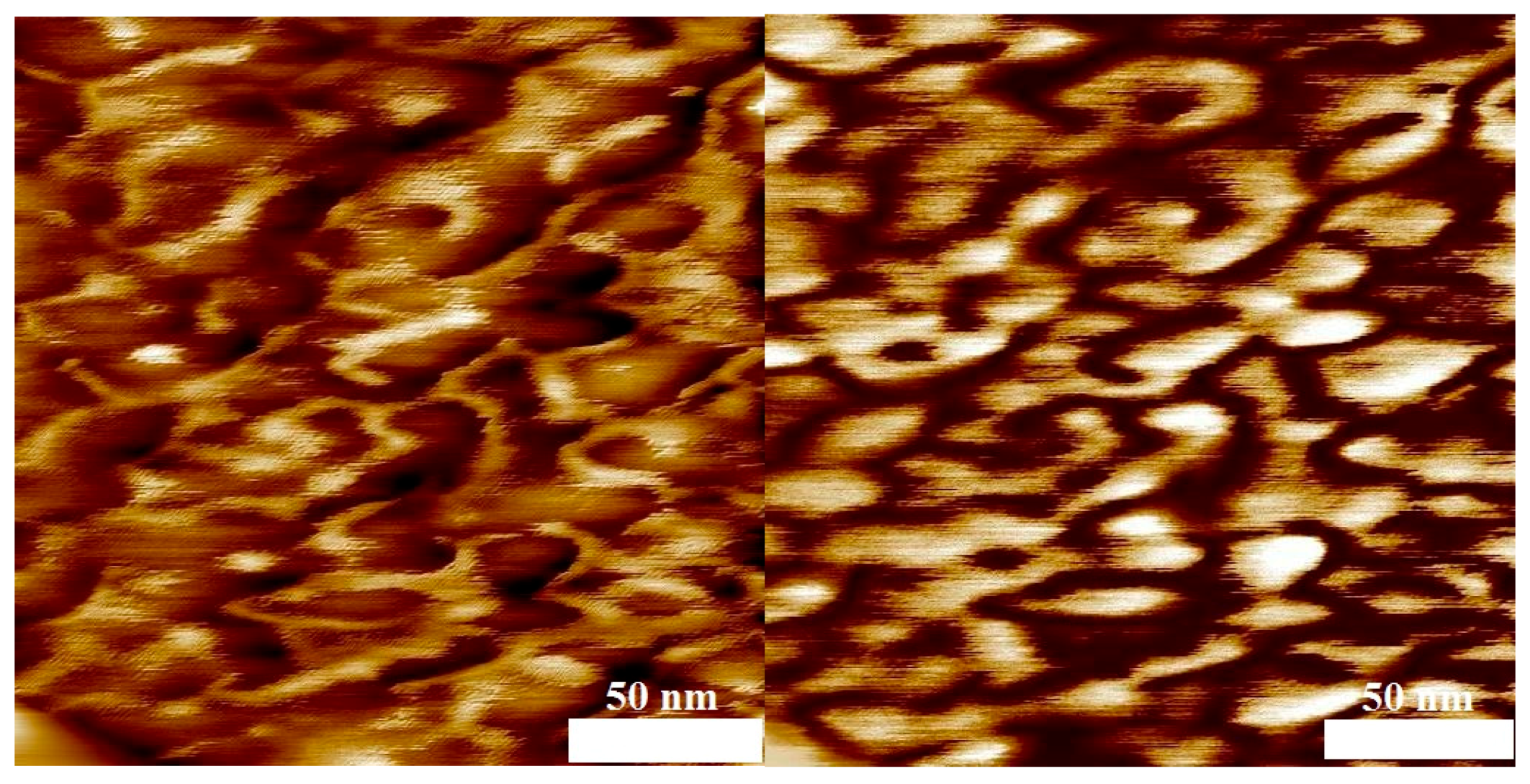
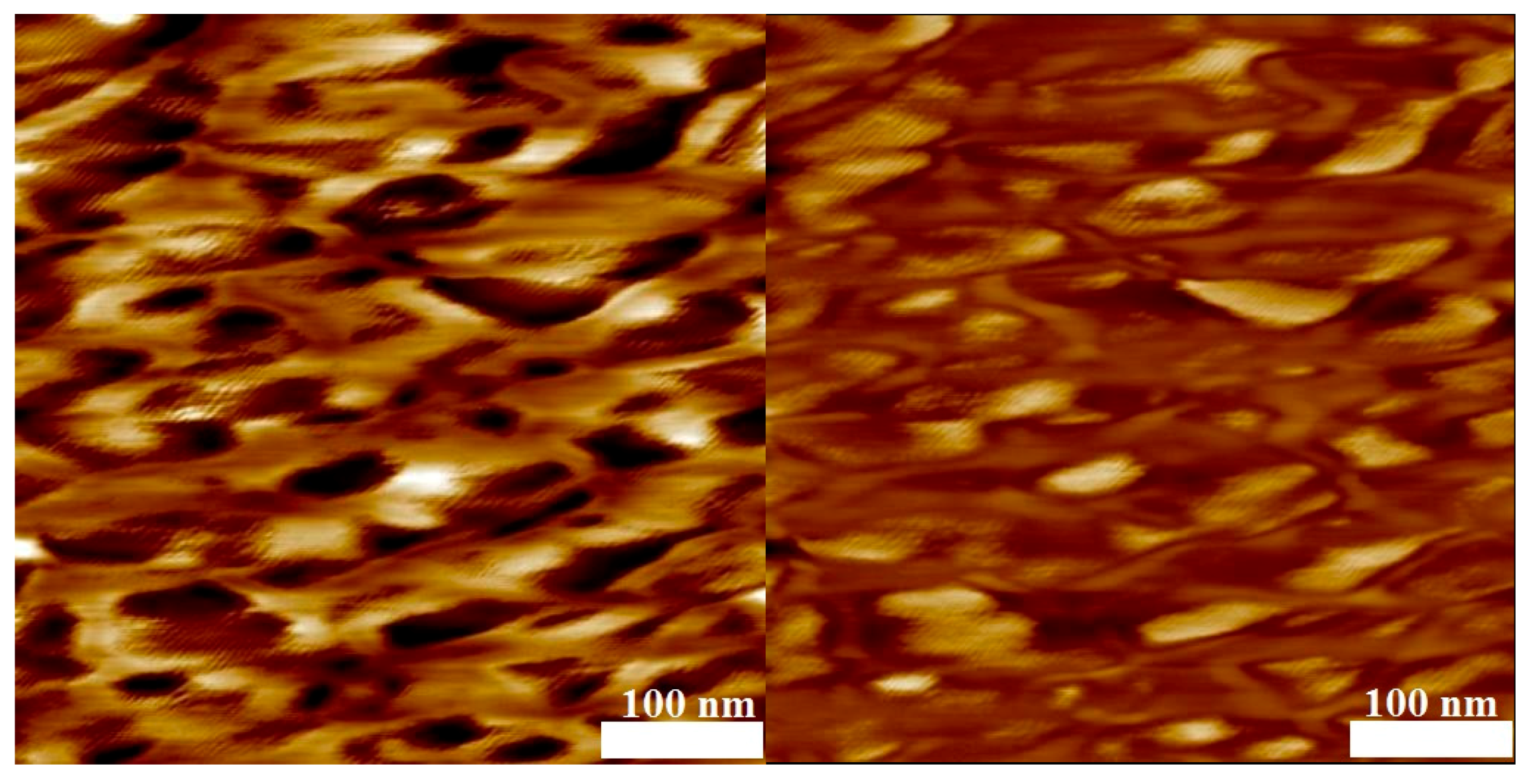
| No. | Mn,PVDF/ g·mol−1 | DPVDF | mPVDF/ mg | Comonomer | Vco/mL | Mn,block/ g·mol−1 | Dblock | nVDF/nco | nVDF | nco | ϕco |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 a | 2033 | 1.5 | 100 | MMA | 1 | 22,344 | 1.6 | 1/3 | 27 | 81 | 0.84 |
| 2 b | 4344 | 2.0 | 100 | MMA | 0.5 | 15,176 | 1.6 | 1/0.83 | 58 | 48 | 0.64 |
| 3 b | 4344 | 2.0 | 100 | MMA | 4 | 40,524 | 1.8 | 1/4 | 58 | 232 | 0.87 |
| 4 a | 4518 | 1.4 | 100 | MMA | 1 | 39,450 | 1.8 | 1/2.3 | 60 | 138 | 0.85 |
| 5 a | 11,500 | 1.4 | 100 | MMA | 1 | 50,520 | 1.7 | 1/0.67 | 153 | 102 | 0.58 |
| 6 a | 11,500 | 1.4 | 100 | MMA | 0.2 | 12,080 | 1.5 | 1/0.21 | 153 | 33 | 0.33 |
| 7 a | 11,500 | 1.4 | 100 | MMA | 0.5 | 36,170 | 1.3 | 1/0.37 | 153 | 57 | 0.46 |
| 8 a | 11,500 | 1.4 | 100 | MMA | 1.5 | 60,880 | 2.1 | 1/0.84 | 153 | 128 | 0.66 |
| 9 a | 2033 | 1.5 | 100 | S | 1 | 13,220 | 3.6 | 1/0.56 | 27 | 15 | 0.59 |
| 10 a | 11,500 | 1.4 | 100 | S | 3 | 33,040 | 1.3 | 1/0.91 | 153 | 140 | 0.58 |
| 11 a | 11,500 | 1.4 | 200 | S | 1 | 19,856 | 1.9 | 1/0.71 | 153 | 109 | 0.50 |
| 12 a | 11,500 | 1.4 | 200 | S | 2 | 21,669 | 1.7 | 1/0.77 | 153 | 118 | 0.54 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golzari, N.; Adams, J.; Beuermann, S. Inducing β Phase Crystallinity in Block Copolymers of Vinylidene Fluoride with Methyl Methacrylate or Styrene. Polymers 2017, 9, 306. https://doi.org/10.3390/polym9080306
Golzari N, Adams J, Beuermann S. Inducing β Phase Crystallinity in Block Copolymers of Vinylidene Fluoride with Methyl Methacrylate or Styrene. Polymers. 2017; 9(8):306. https://doi.org/10.3390/polym9080306
Chicago/Turabian StyleGolzari, Nahal, Jörg Adams, and Sabine Beuermann. 2017. "Inducing β Phase Crystallinity in Block Copolymers of Vinylidene Fluoride with Methyl Methacrylate or Styrene" Polymers 9, no. 8: 306. https://doi.org/10.3390/polym9080306