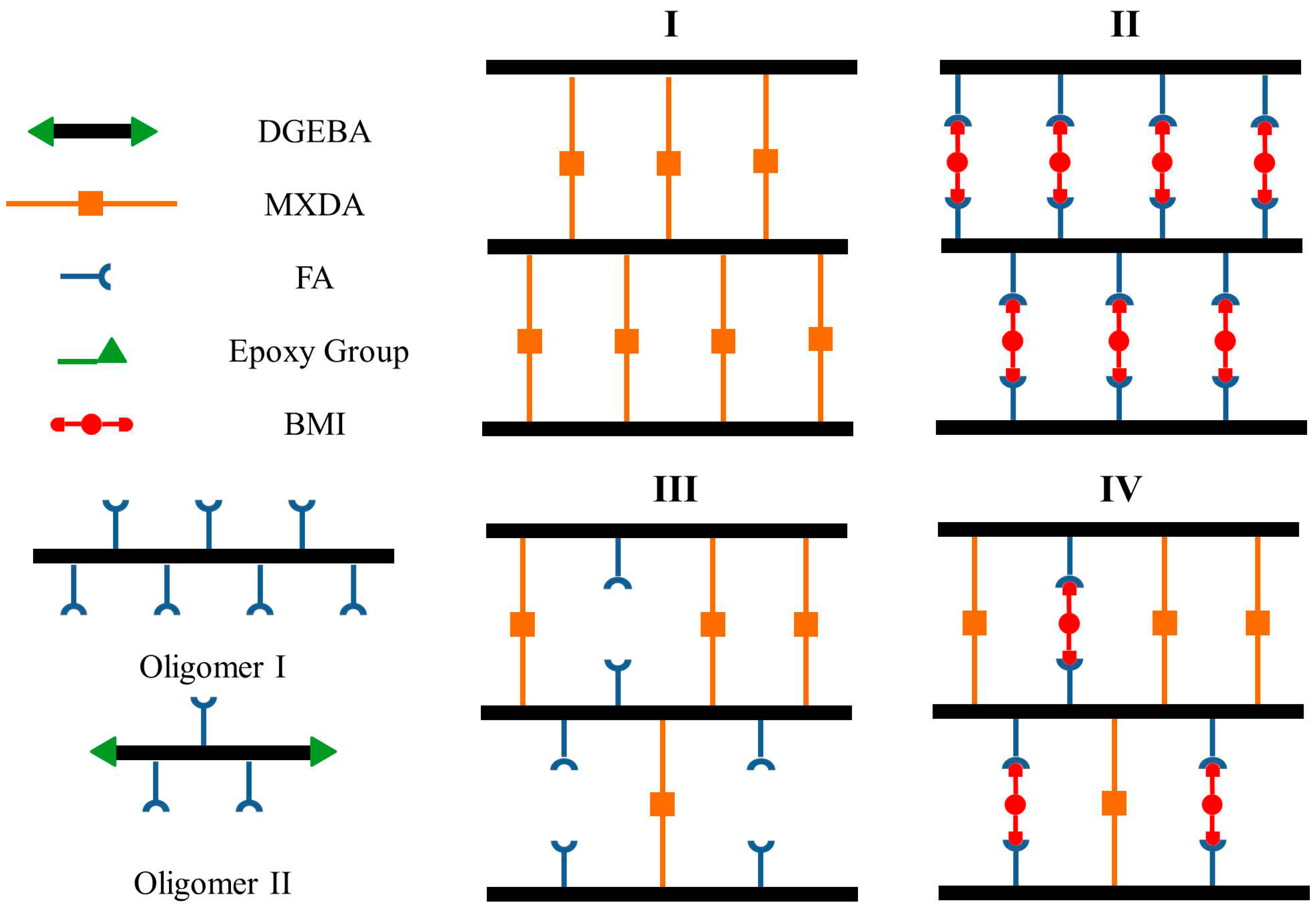
The dense irreversible epoxy Network I (
Scheme 2), which is usually involved in practical applications, was first prepared as the basic architecture based on the curing reaction of DGEBA and MXDA in a stoichiometric proportion. Because of its relatively fast kinetics and mild reaction conditions, the DA reversible reaction was utilized to create the dense reversible Network II as shown in
Scheme 2. DGEBA was reacted with FA in a stoichiometric proportion to synthesize Oligomer I first, and the reversible crosslinking was then achieved via the DA reaction among Oligomer I and BMI. As can be seen in the third architecture, a loose irreversible Network III was obtained via the curing reaction between MXDA and the remained epoxy groups in Oligomer II which was synthesized from DGEBA and FA in a non-stoichiometric proportion. The molar ratio of DGEBA to FA in Oligomer II was 1:0.6, i.e., the molar ratio of epoxy/amine groups was 3.333. The partially reversible and partially irreversible Network IV was finally generated. In addition to the irreversible connection resulting from MXDA, BMI was introduced to Oligomer II simultaneously to create the reversible coupling.
The chemical structures and thermal properties of Oligomer I and II were investigated using FTIR, 1H-NMR, GPC, and DSC. The four network architectures were examined subsequently using FTIR, DSC, TGA, and swelling experiments. How thermally-induced self-healing of the coatings with different architectures behaved was evaluated based on the observation of the closure and repair steps. Mechanical properties, UV aging resistance, and corrosion resistance of the four coatings were also characterized.
3.1. Structures and Properties of Oligomers
The reactivity of DGEBA with FA has been reported in previous reports, facilitating the determination of synthesis conditions for the oligomers [
17,
18,
27]. As reported, the reaction among DGEBA and FA initiated at 57 °C with a maximum exothermal peak at 117 °C [
27]. A significant portion of FA (b.p. 147 °C), however, volatilized at 105 °C. In addition, FA was considered to provide a reaction between labile hydrogens in furan rings with epoxy groups, contributing to an irreversible network [
27]. Besides, the polymer chains with moderate molecular weight and correspondingly good mobility are commonly required for healing [
10]. For these reasons above, the reaction temperature and time were chosen as 100 °C and 6 h in this case.
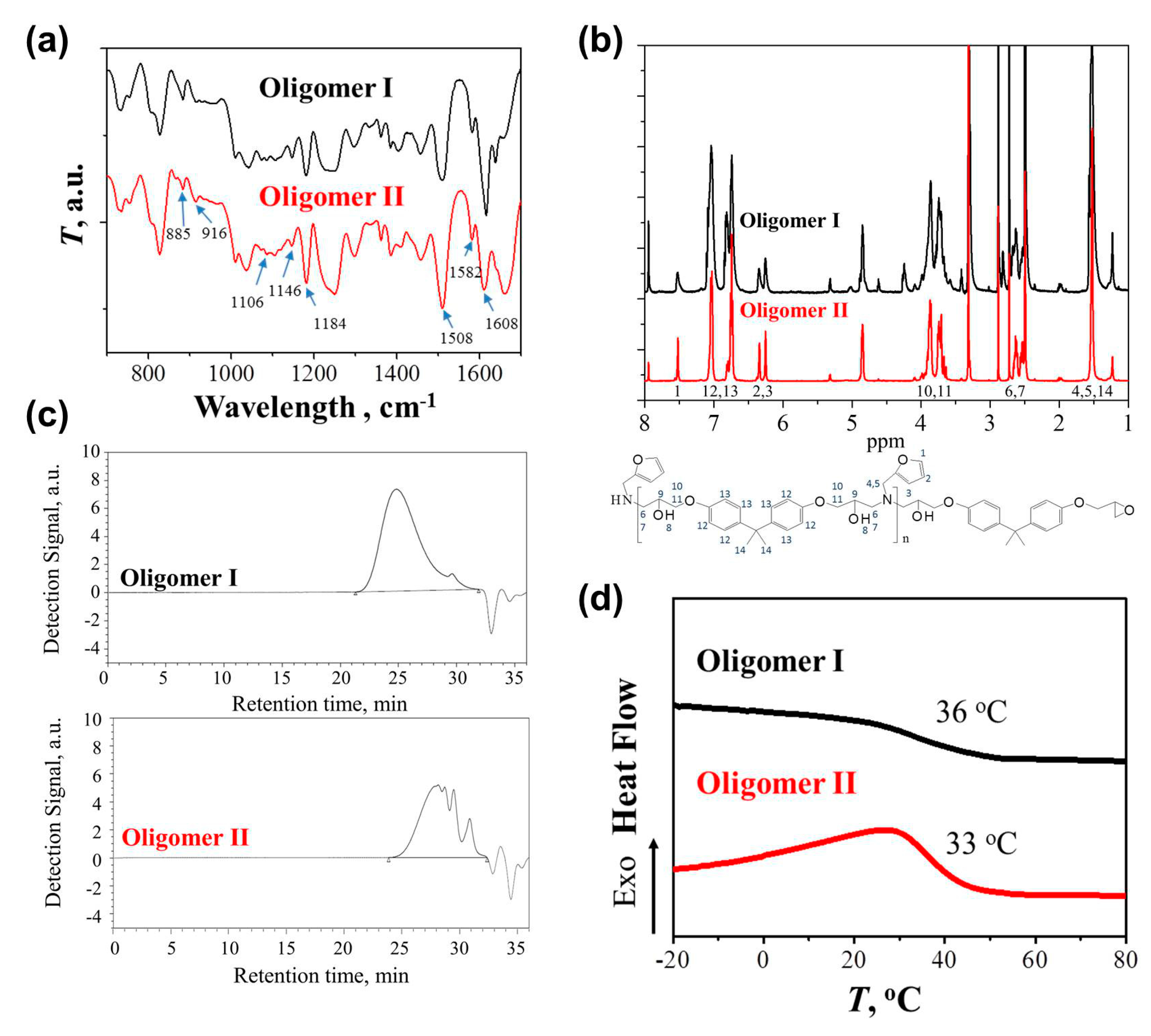
The chemical structures of the synthesized Oligomer I and II were characterized by FTIR first. As shown in
Figure 1a, the peaks at 1508, 1582, and 1608 cm
−1 were attributed to the C=C bonds of the 1,4 substituted benzene from DGEBA, while the peak at 1184 cm
−1 was assigned to the benzenic C-H bonds [
28]. Because a stoichiometric proportion of epoxy and amine was used in Oligomer I, neglected epoxy groups from DGEBA were determined from the peak at 916 cm
−1, which was related to the epoxide rings [
28,
29,
30,
31]. In comparison, unreacted epoxy groups were found in Oligomer II, providing the reactivity points for further curing. The incorporation of the FA moiety generated the peaks belonging to furan rings, including those at 885, 1106, and 1146 cm
−1 [
15,
16,
19,
20,
21]. The results from the
1H NMR spectra were also used to confirm the successful synthesis (
Figure 1b). The spectra of the two oligomers were the superposition of signals from DGEBA and FA [
17,
20,
22]. The peaks at 7.56, 6.35, and 6.26 ppm were contributed by protons 1, 2, 3 of furan rings resulting from FA, while the DGEBA moiety was responsible for the typical peaks at 6.74–7.11 ppm, corresponding to the aromatic protons 12, 13 [
17,
20,
22]. The peak at 1.6 ppm was contributed by methylene protons 4, 5 from FA as well as methyl protons 14 from DGEBA [
17,
22].
The molecular weight of the synthesized oligomers was measured by GPC (
Figure 1c). Oligomer I exhibited the
Mw and
Mn of 17.2 and 5.9 kDa, respectively, with a PDI of 2.9. Several overlapping peaks were observed in Oligomer II with the non-stoichiometric proportion, corresponding to
Mws of 4.4, 1.6, 1.0, and 0.5 kDa, respectively, while the PDI varied from 1.1 to 1.3. Several research groups have also reported the molecular weight of DGEBA-FA oligomers. Palmese et al. performed the reaction of the stoichiometric reagents in 15 wt % DMF at 90 °C for 30 h [
18]. The achieved oligomer presented a
Mn of 4.2 kDa. Turkenburg and coworkers synthesized the oligomer in a non-stoichiometric ratio (epoxy/amine = 2.5) at 125 °C for 2 h, which exhibited the
Mn and PDI of 1.3 kDa and 2.9 [
17]. Besides, Mignard et al. prepared the oligomers containing different amount of FA moieties via the condensation reaction among DGEBA, FA, and phenyl glycidyl ether as the end-capping reagent (epoxy/amine = 2) at 100 °C for 15 min [
16]. With the aid of catalyst, the
Mns for deca-, hexa-, and tetra-furan oligomers were 4.1, 2.5, and 1.6 kDa, respectively. The molecular weights of the oligomers synthesized in the present work were relatively higher in both stoichiometric and non-stoichiometric ratios, possibly because of the high reaction temperature or the long synthesis time. The glass transition temperatures (
Tg) of the oligomers were measured using DSC. As shown in
Figure 1d, the
Tgs of Oligomer I and II were 36 and 33 °C, respectively. As reported by Turkenburg and coworkers, the
Tg of the oligomer with a relatively small
Mn (1.3 kDa) was 41 °C [
17], while the deca-, hexa-, and tetra-furan oligomers, reported by Mignard et al., presented the
Tgs of 34, 34, and 20 °C, respectively [
16]. These results suggest that the dependence of
Tg on oligomer
Mn was not strong. It might be PDI that played a more dominant role.
3.2. Structures and Thermal Properties of the Four Networks
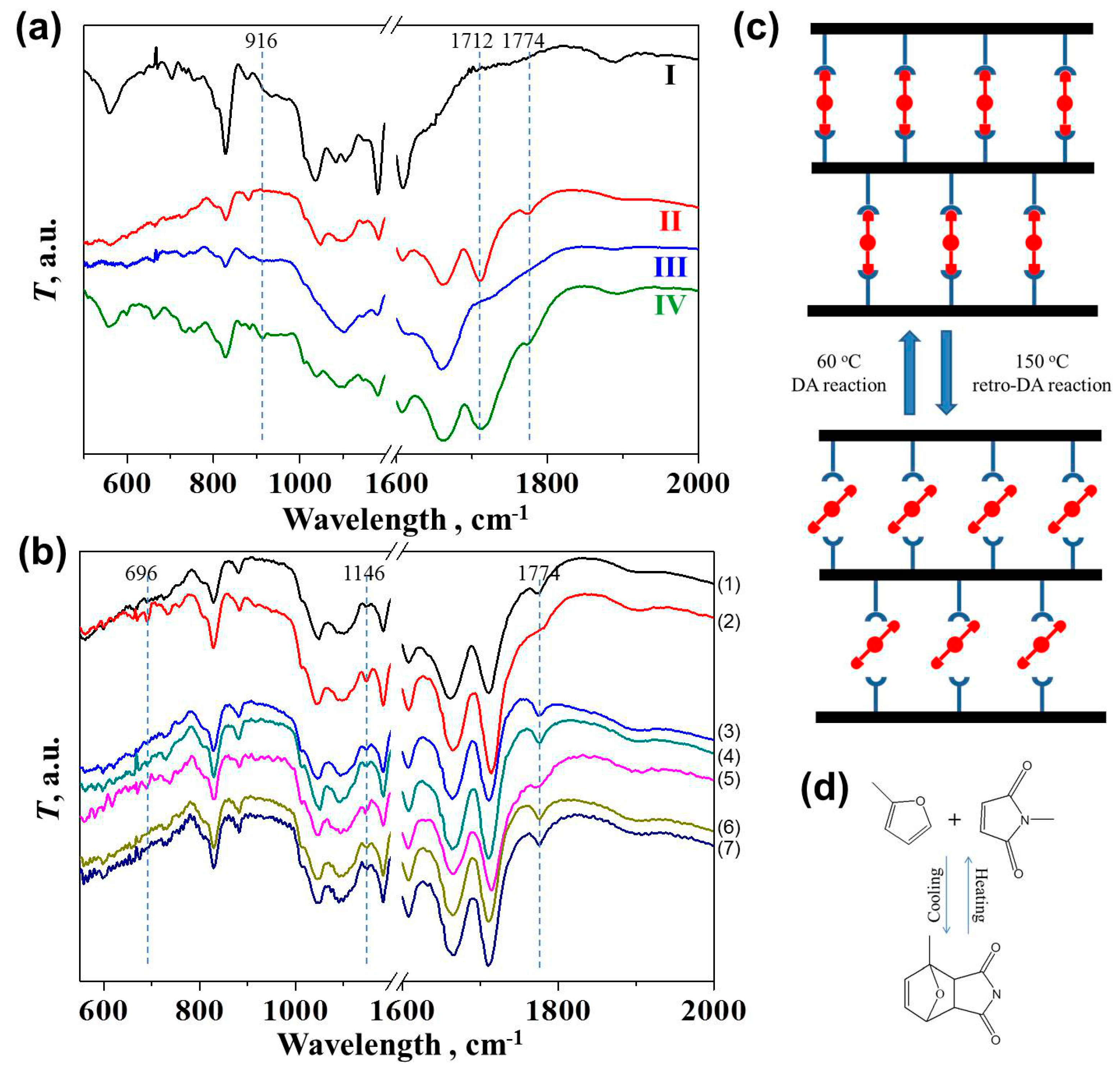
After synthesis of the oligomers, four epoxy materials with different networks were created according to
Scheme 1. As shown in the FTIR spectra (
Figure 2a), no evident peak was observed at 916 cm
−1 for dense irreversible Network I and loose irreversible Network III, indicating the complete curing of unreacted epoxy groups with the amine groups from MXDA [
28,
29,
30,
31]. The curing conditions for the dense irreversible Network I were 60 °C for 2 h, 100 °C for 3 h, and 140 °C for another hour, as presented previously [
9,
32]. We have shown in a previous report that a sufficient conversion of epoxy groups over 93% occurred within the first hour of the reaction between DGEBA and MXDA at 60 °C [
32]. Therefore, the synthesis condition for Network III here (60 °C for 12 h) was also considered to ensure the complete curing reaction. A tiny peak was observed in the partially reversible and partially irreversible Network IV. Under the same curing condition as Networks II and III, some epoxy groups remained in Network IV possibly because the DA reaction retarded the curing reaction via increasing the viscosity. Due to the involvement of the DA reaction, the curing temperature cannot be set over 60 °C. With BMI participation, two evidently new peaks at 1712 and 1774 cm
−1 were found in Network II and Network IV [
21,
33,
34]. The peak at 1712 cm
−1 presented the C=O bond in maleimides, while the peak at 1774 cm
−1 typically presented the existence of DA adducts, suggesting successful DA reactions [
21,
33,
34].
An off-site FTIR experiment (
Figure 2b) was performed to examine the reversibility of the crosslinking bonds (
Figure 2c) resulting from the DA-rDA reaction (
Figure 2d) in Network II. Such variation in the spectra of Network IV cannot be distinguished clearly because less BMI was introduced. The heating and cooling conditions were selected based on the previous full investigation on the thermodynamic and kinetic factors in the DA reaction to ensure the decoupling and recoupling of the fractured polymeric parts [
15,
21,
22]. After heating the sample at 150 °C for 10 min, the distinguishing peak at 1774 cm
−1 became less evident, indicating the disappearance of DA adducts and the occurrence of the retro-DA reaction [
22]. The DA reaction took place again during the subsequent treatment at 60 °C for 1 and 2 h, leading to the peak’s reappearance. Such variation repeated in the second heating and cooling cycle. In addition, the peak at 696 cm
−1 attributed to the maleimide characteristic band showed up at the high temperature, before it vanished again at 60 °C [
21]. Similar repeated change in peak intensity can also be observed for the peak at 1146 cm
−1, corresponding to furan rings which were consumed by BMI at 60 °C but reappeared at 150 °C [
22].
As shown in
Figure 3a and
Table 1, Network I presented a
Tg of 118 °C, close to a previous report [
35]. Network III showed a
Tg of 33 °C, similar to that of Oligomer II, suggesting that the introduction of a loose crosslinking network through the reaction of the remaining epoxy groups in Oligomer II with MXDA caused negligible influence on
Tg. The thermal reversibility of Network II and IV can also be demonstrated via DSC analysis. Network II exhibited a
Tg of 69 °C because the sufficiently generated reversible DA adducts contributed to the dense crosslinking network and the limitation of chain mobility in Oligomer I (
Tg = 37 °C). A following endothermic peak from 80 to 130 °C was assigned to the retro-DA reaction with an enthalpy of 1.59 J g
−1 [
20,
21]. The DA reaction occurred again during the subsequent cooling step, leading to the regeneration of DA adducts and correspondingly another endothermic peak with a smaller enthalpy of 1.04 J g
−1 in the second heating treatment. The reduction in the enthalpy suggested that the relatively fast cooling procedure did not allow the complete DA reaction. The
Tg, thus, decreased to 55 °C, while the unconnected BMI may act as plasticizer. Network IV also exhibited an endothermic peak of 1.01 J g
−1 since less DA adducts contributed to the network. Interestingly, the ratio of rDA reaction enthalpies in Network II and IV was 1.58, close to the theoretical ratio (1.67) of the incorporated BMI amount. During the second heating, no evident endothermic peak was observed. We argue that the regeneration of DA adducts became negligible, possibly because of the existence of irreversible crosslinking bonds. The unreacted BMI thus reduced the
Tg as the plasticizer to 25 °C, lower than Oligomer II and Network III.
The swelling degree and crosslinking density of the four networks were determined subsequently using swelling experiments (
Figure 3b). As expected, Network I and III presented the gel content (
G) of 99% and 81% as well as the swelling ratio of 124% and 361%, respectively. Network II exhibited the
G and
Q of 87% and 221%, suggesting a relatively looser network than the dense irreversible Network I. As reported [
19], at 60 °C, the conversion between furfuryl alcohol and BMI increased slowly towards 65% within the first 5 h, at which the maximum conversion was almost reached. Due to the steric hindrance of polymer chains, the conversion between furan-ring-terminated oligomers and BMI was even lower [
19]. Therefore, in our case, the amount of reversible crosslinks (Network II) based on DA reactions cannot be comparable to the irreversible crosslinks (Network I), generating a lower crosslinking density. Besides, it is interesting to observe that Network IV possessed a lower G of 71% than Network III (81%), indicating that the DA reaction may retard the reaction among epoxy groups and amines as well as the formation of a dense network. The thermo-reversibility of the DA reaction was confirmed by the complete dissolution of the Network II in DMF at 100 °C for 10 min. The other three networks which contained the irreversible network remained insoluble.
The thermal resistance of the four networks was evaluated using TGA (
Figure 3c). After dehydrogenation, the decomposition of epoxy resins consists of two steps: decomposition into stable carbonaceous char and volatiles as well as the degradation of carbonaceous char into volatiles [
36]. As shown in
Table 1, Network I presented the best thermal resistance during the first step, i.e., the temperature at 5% weight loss was 305 °C, while Networks II, III, and IV presented the specific temperature values of 164, 144, and 160 °C, respectively. The loss in crosslink density may contribute to such reduction. The introduction of FA and BMI into epoxy networks increased the degradation temperature within the second step, i.e., the temperatures at the 50% weight loss of Network II and IV were 462 and 400 °C, higher than those of Network I (429 °C) and III (383 °C), respectively.
3.3. Thermally Induced Self-Healing Behaviors of the Four Coatings
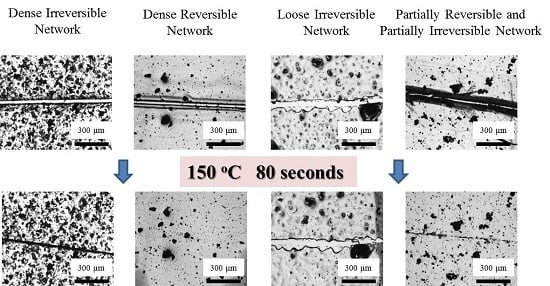
After the preparation of four epoxy coatings with different network architectures, their thermally-induced self-healing behaviors were investigated to clarify how the close and repair steps occurred. Carbon black (0.5 phr) was introduced as the trace substance to ensure the comparison of the same area before and after thermal treatment, while a similar crack width of 20–30 μm was created on all the four coatings.
The four coatings were first treated through a stepwise heating strategy (
Figure 4 and
Figure 5). The crack width in Network I decreased from 30 to 0 μm at 60 and 150 °C, indicating a reversible plasticity-driven gap closure (
Figure 4) [
23,
24]. The force damaging the coating caused the localized plastic deformation and temporary chain orientation in the areas close to crack surfaces. Upon heating, with the aid of irreversible netpoints, the oriented chains rebounded to their relaxed status and resulted in the observable gap closure. Since physical diffusion and re-entanglement of polymer chains was not allowed for such dense network, the crack was still visible at a high temperature. The second repair step, thus, did not occur efficiently. Also driven by reversible plasticity, Network II presented a gap closure from 20 to 5 μm with increasing the temperature from room temperature to 90 °C (
Figure 4). However, the gap became wider again from 5 to 30 μm with further increasing the temperature from 100 to 150 °C. Initiating from 80 °C, the rDA reaction resulted in the de-crosslinking of the reversible network, eliminating the reversible plasticity and correspondingly preventing the sufficient contact of crack surfaces. The “released” fractured Oligomer I with free mobility (
T >
Tg of 37 °C) may dewet the underlying glass substrate, leading to an even wider gap than the previous one.
As far as Network III was concerned, the gap did not vary evidently with temperature (
Figure 5). A low crosslink density possibly may impair the reversible plasticity, i.e., upon heating, the temporary chain orientation relaxed locally instead of “rebounding back”. Besides, since the DGEBA-FA oligomer tended to de-wet the underlying substrate, the gap closure was not favored by surface tension driven viscoelastic flow on glass plates either. Meanwhile, the presence of the irreversible network may prevent the occurrence of dewetting, anchoring the coating towards the substrate with increasing temperature. Finally, the crack in Network IV closed till 90 °C but varied negligibly later. Even though Network IV exhibited a lower crosslinking density than Network III, the gap closure still took place. We crudely speculate that the reversible plasticity was dominated by both crosslinking density and orientation level. The lower crosslinking density in Network IV may result in a high orientation during deformation and correspondingly the high tendency of rebounding, since crosslinking limited the chain mobility and accordingly the degree of molecular orientation during deformation [
37]. After the disappearance of the reversible network at a high temperature, the irreversible coupling may also retard the coating dewetting. Therefore, the gap maintained its narrow status, instead of becoming wider again as behaved by Network II.
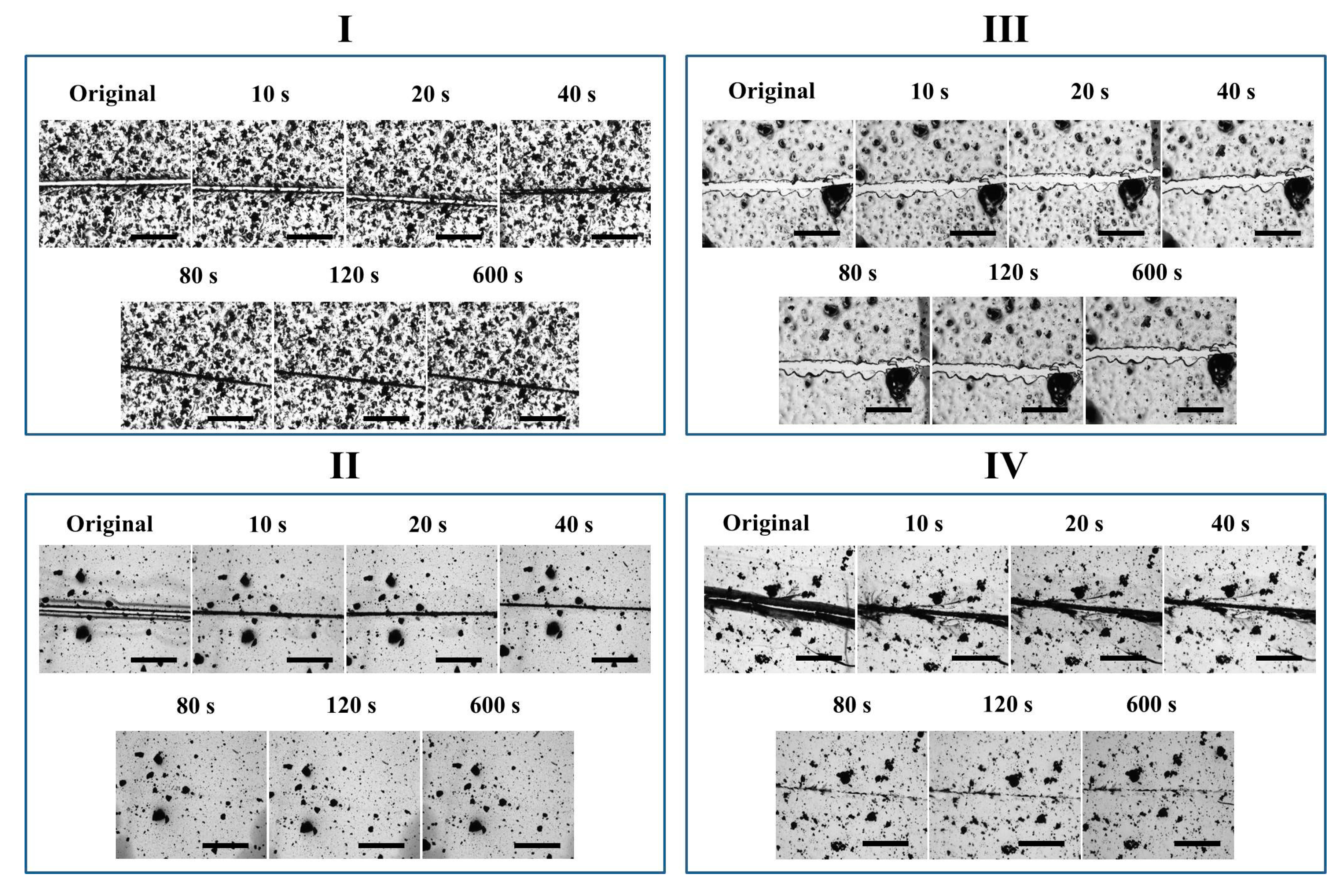
The four coatings were also directly treated at 150 °C to observe their self-healing performances. As shown in
Figure 6, the crack on Network I narrowed down very quickly within 40 s driven by reversible plasticity, while the crack was observed all the time. Network III did not exhibit evident variation in crack width, which is similar to the case in stepwise heating. In comparison, upon direct heating at 150 °C, the self-healing behaviors of Networks II and IV with reversible bonds were different. Network II presented a complete gap closure in 40 s, facilitating the second repair step and causing a crack disappearance after only 80 s. Direct heating at a high temperature triggered an immediate gap closure by reverse plasticity, while the tight contact of crack surfaces allowed Oligomer I resulting from rDA reaction to diffuse into the opposite crack surface, preventing the occurrence of dewetting and ensuring the complete crack repair. Based on an image analysis method as described previously [
32], the healing efficiency was calculated to be 91 ± 6%, suggesting a sufficient crack repair. Similarly, Network IV also exhibited a gap closure in 40 s, while the crack to some extent disappeared after 80 s. Since less reversible bonds were incorporated, the complete crack repair was not achieved. In addition, the post-curing may exist in Network IV treated at 150 °C. After the de-coupling of the reversible crosslinking points, the unreacted epoxy groups could be further cured to limit the healing procedures.
Based on the results above, the coating with Network II presented the best thermally-induced self-healing capability on glass plates only in the case of direct heating at 150 °C. The influence of network architecture on self-healing behaviors can, to some extent, be discussed. The crack closure can be contributed by reversible plasticity [
23,
24] or surface-tension-driven flow [
12], while the repair can be achieved by physical molecular re-entanglement [
9], reversible non-covalent reactions [
38], or reversible covalent reactions in this case. Dense networks (I and II) with a high crosslinking density usually facilitate gap closure driven by reversible plasticity. The disadvantages of the dense irreversible network in complete crack repair has been improved by mixing electrospun thermoplastic poly(
ε-caprolactone) fibers into epoxy coatings to ensure further gap filling [
24]. The dense reversible network enabled not only the gap closure, but complete crack repair as well, leading to complete crack disappearance. The loose network or linear architecture is required for surface-tension-driven flow. We reported another two epoxy coatings with the loose irreversible network, which presented the gap closure and crack disappearance due to chain diffusion and re-entanglement [
9,
32]. However, in the case here, the surface tension did not drive the gap closure but triggered the dewetting, leading to the failure of self-healing. Therefore, the loose irreversible network cannot guarantee good self-healing. Furthermore, sufficient reversible plasticity-driven gap closure must be triggered before the de-crosslinked oligomers in reversible networks which finally contributed to the chain diffusion and further repair due to regeneration of reversible crosslinks.
3.4. Properties and Anti-Corrosion Behaviors of the Four Coatings
In addition to the investigations on structures, thermo-reversibility, and self-healing performance, there is a need to evaluate the practical properties of the coating with Network II. Therefore, the basic properties and anti-corrosion performance of Network II were investigated, in addition to the other three networks, to obtain a global view. The film thickness of the four coatings was 26 ± 1 μm.
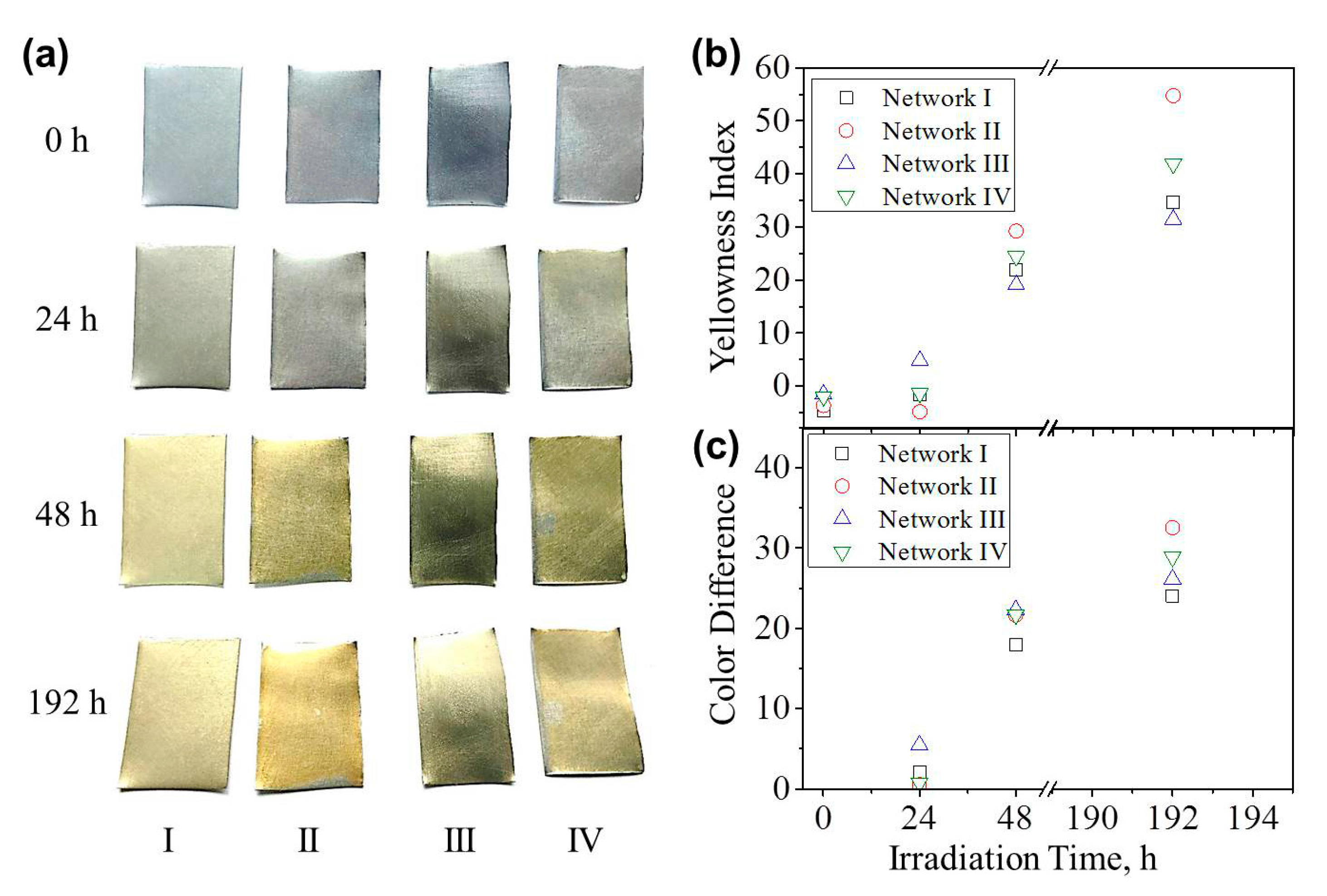
As shown in
Figure 7a, an evident color variation in all four coatings occurred after UV illumination for 48 h. Further increasing the irradiation time deepened the yellow color more slowly. The formed chromophoric impurities generated a protective layer on the coating surface and prevented further degradation. The corresponding yellowness index and the color difference from the as-prepared coating were calculated and are shown in
Figure 7b,c. It has been reported that the amine structures were dominant in color development [
39]. In our case, Network II presented the largest yellowness index and color variation, suggesting the worst UV aging resistance.
Surface hardness, adhesion, and flexibility are three significant mechanical properties for polymer coatings. Hardness can be used to evaluate the capability of withstanding a certain amount of physical damage, and sufficient hardness obviously reduces the demand of self-healing. The degree of adhesion determines the interaction force between a coating and the underlying substrate, and good adhesion ensures that the damage cannot result in secondary fracture, and facilitates healing. Flexibility is a critical property to assess whether the coating maintains its steady shape when the underlying substrate is bent. As shown in
Table 1, Network I exhibited the highest pencil hardness of 3H. Because of the reduced
Tg and crosslinking density, the coating hardness of Network II decreased to 1H–2H, which further reduced to HB–1H for Network IV. It is a conflict that self-healing and surface hardness have inverse requirements for chain mobility. In comparison with the self-healing mechanism based on chain diffusion and re-entanglement, the reversible crosslinking network retarded hardness deterioration. Besides, the adhesion level was improved to some extent from Grade 2–3 in Network I to Grade 1–2 in Networks II, III, and IV, while the flexibility did not vary with the coating networks. In comparison with the conventional epoxy coating with a dense irreversible network, the coating with the self-healing capability based on a reversible DA reaction did not show the evident deterioration in the mechanical properties.
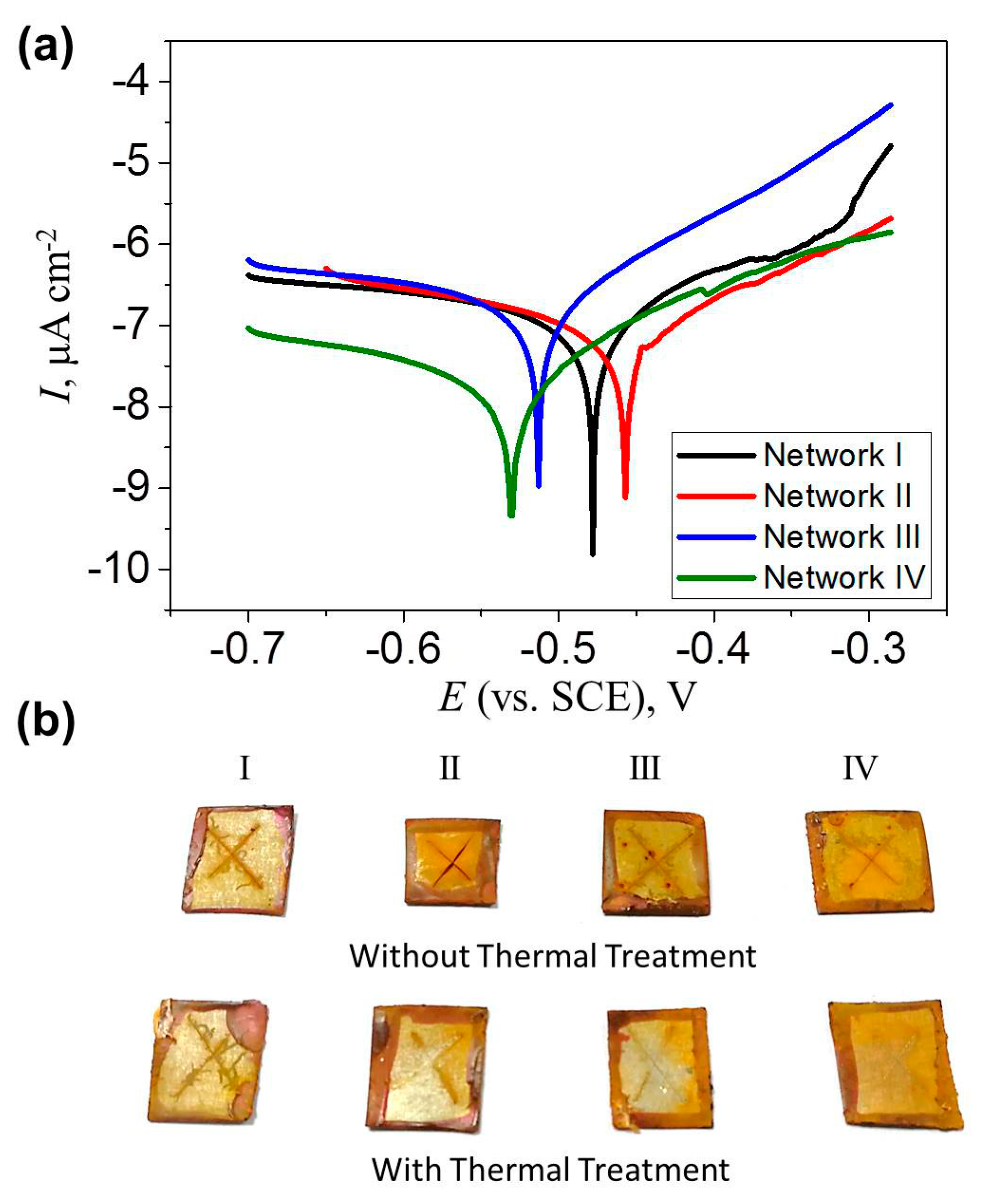
Figure 8a shows the Tafel plot of epoxy coatings with four different network architectures to evaluate their electrochemical corrosion properties. The hyperbolic plot of Network I shifted towards a more positive corrosion potential (
Ecorr) as reversible Network II was involved. The corrosion current (
Icorr), which was obtain from the Tafel plot via extending the straight lines along the linear portions of anodic and cathodic plots as well as extrapolating it to the
Ecorr axis, decreased slightly. Polarization resistance (
Rp) is given using the Stern–Geary equation, [
40]
where,
βa and
βc are the anodic and cathodic Tafel constants. As shown in
Table 1, the
Rp increased from 5.66 to 10.89 MΩ cm
−2 when the irreversible network was replaced by the reversible network. The increased
Ecorr and
Rp, as well as the reduced
Icorr indicated that Network II offered slightly better corrosion protection on tinplates. In comparison, Networks III and IV exhibited weakened corrosion protection, as shown in
Figure 8a and
Table 1. The corrosion process in a neutral chloride solution consists of an anodic process where Fe becomes Fe
2+, and a cathodic process where OH– is generated by O
2 and H
2O. Fe
2O
3 finally generates on a metal surface via chemical oxidation processes. The existence of a polymer coating retards the diffusion of H
2O and O
2 onto the metal surface and inhibits metal oxidation. Here, due to its high crosslinking density, Network I presented a good anti-corrosion property, which was slightly improved in Network II with a lower crosslinking density. The reason was possibly related to the solvent difference in evaluating the crosslinking level (DMF) and the anti-corrosion property (water). Therefore, the water uptake ratio of the four networks was measured as the mass increase ratio of the sample immersed in water for 7 days, i.e., the swelling ratio in water. As shown in
Table 1, since the water uptake ratio of Network II can be comparable with that of Network I, both of them effectively retarded the corrosive media to pass through the coatings. The slight reduction in adhesion might lead to the easy generation of a metal oxidation layer on Network I. Because of the high water uptake ratios, the anti-corrosion property was weakened in Networks III and IV. In addition, the four networks exhibited the similar hydrophilicity, as determined by the close contact angle values in
Table 1, which influenced negligibly the anti-corrosion property.
Finally, the self-healing behaviors of the four coatings were assessed by their corrosion protection performance. The damaged coatings were heated at 150 °C for 10 min before immersing in 3.5 wt % NaCl solution. It can be seen in
Figure 8b that after 120 h immersion, severe corrosion occurred in the four cracked coatings without any healing procedures. After the thermal treatment, the coating with a dense irreversible Network I exhibited a similar rust situation as the one with the fresh crack. On the contrary, the other three healed coatings presented less rust on the surfaces. The undamaged coating prevents the contact between the NaCl solution and the underlying steel plates. Therefore, the gap closure presented by Network I still allowed the penetration of the corrosive medium onto the substrate, while the improvement in the corrosion resistance indicated the efficient self-healing of the cracks on the coatings with Networks II–IV. Interestingly, the coating with Network III presented good healing efficiency as well, indicating that the surface tension on steel plates may drive the viscoelastic flow of the materials before the ultimate healing of the cracks.