Silane-Treated Basalt Fiber–Reinforced Poly(butylene succinate) Biocomposites: Interfacial Crystallization and Tensile Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
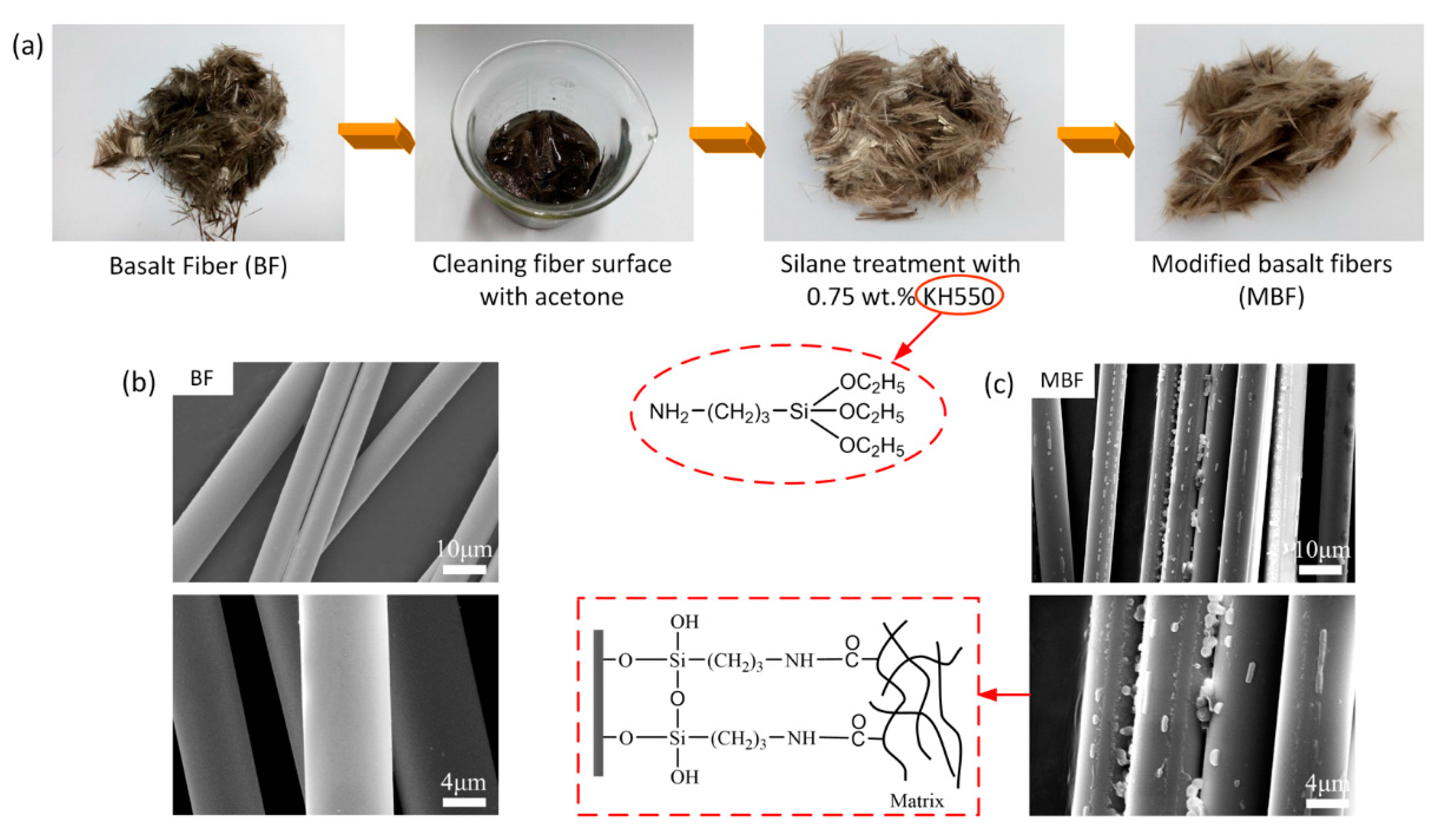
2.2. The Silane Treatment of Bbasalt Fiber (BF)
2.3. Preparation of PBS/MBF Biocomposites
2.4. Characterization
2.4.1. Scanning Electron Microscope (SEM)
2.4.2. X-ray Photoelectron Spectrometer (XPS)
2.4.3. Polarized Optical Microscope (POM)
2.4.4. Differential Scanning Calorimetry (DSC)
2.4.5. Tensile Properties
3. Results
3.1. Modification of Basalt Fiber
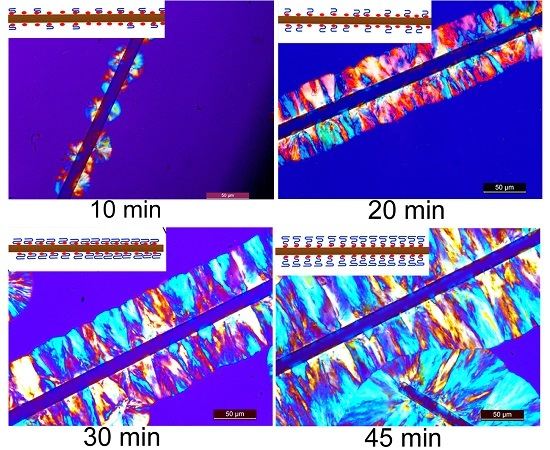
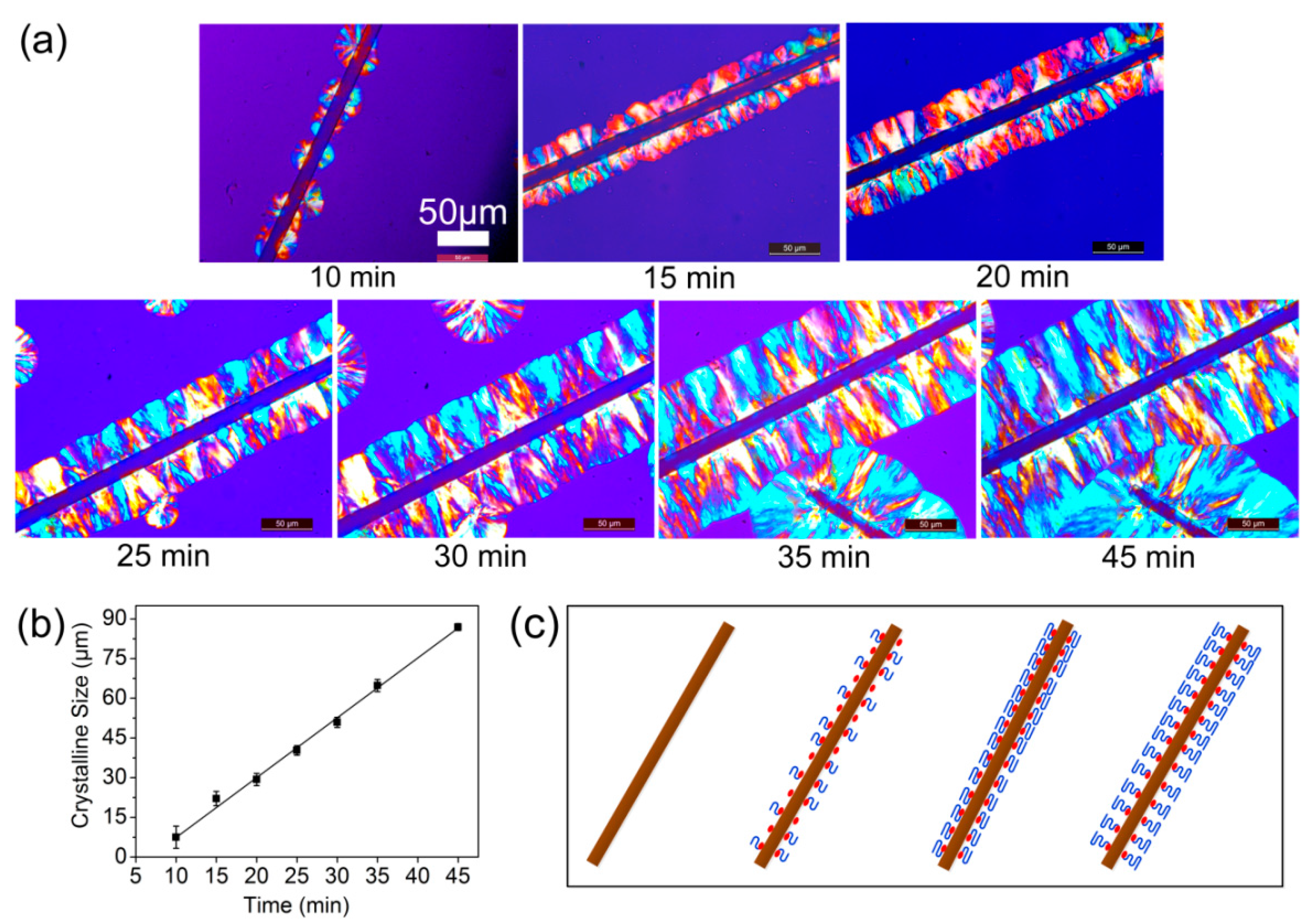
3.2. MBF-Induced Transcrystallization (TC) of PBS
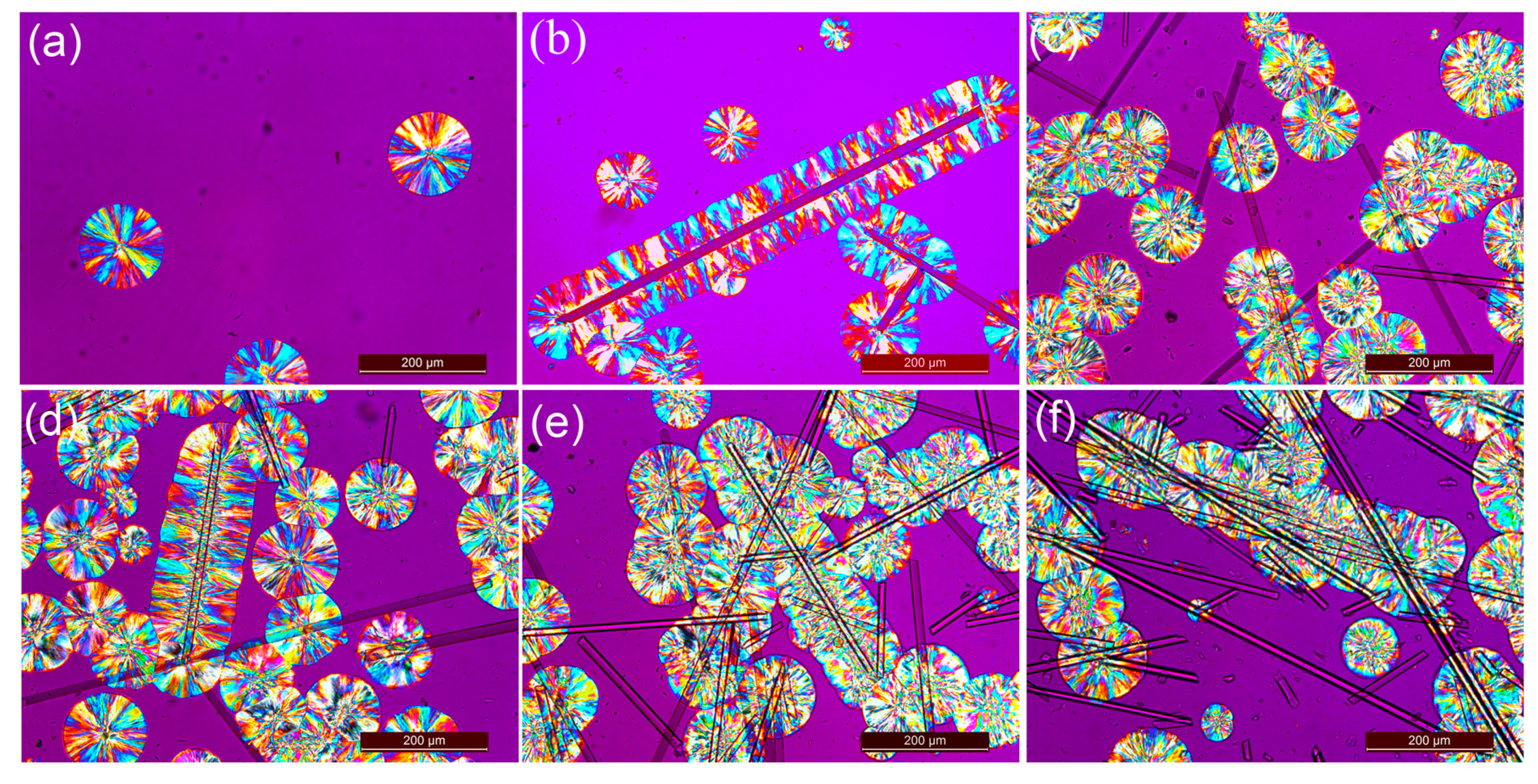
3.3. Isothermal Crystallization Behaviors
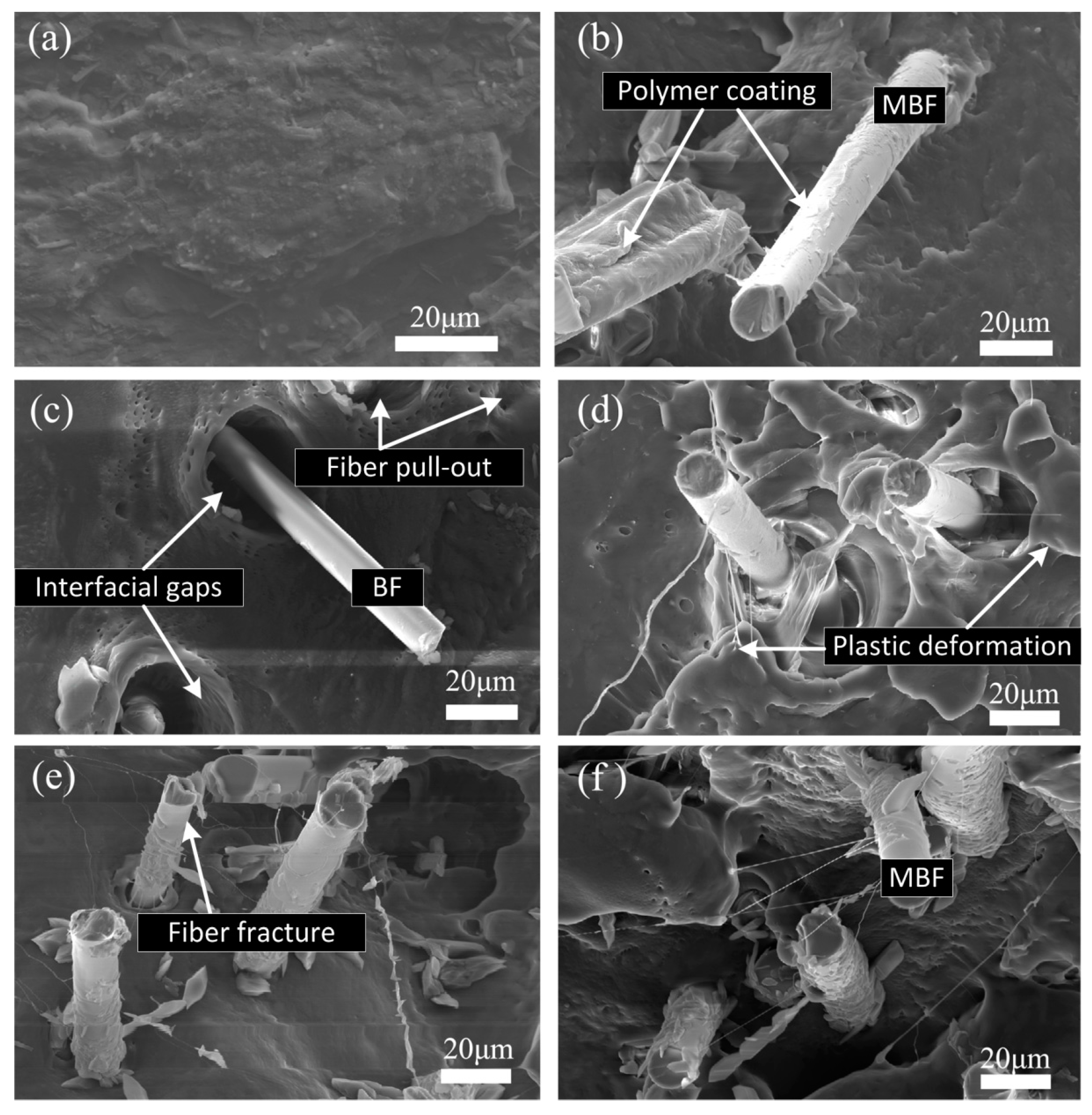
3.4. Tensile Properties of PBS/MBF Biocomposites
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lopresto, V.; Leone, C.; De Iorio, I. Mechanical characterization of basalt fibre reinforced plastic. Compos. Part B Eng. 2011, 42, 717–723. [Google Scholar] [CrossRef]
- Yu, A.; Kadykova, A. Structural polymeric composite material reinforced with basalt fiber. Russ. J. Appl. Chem. 2012, 85, 1434–1438. [Google Scholar]
- Guo, J.; Mu, S.Y.; Yu, C.F.; Hu, C.N.; Guan, F.C.; Zhang, H.; Gong, Y.M. Mechanical and thermal properties of polypropylene/modified basalt fibric composites. J. Appl. Polym. Sci. 2015, 132, 42504. [Google Scholar] [CrossRef]
- Liu, Q.; Shaw, M.T.; Parnas, R.S.; McDonnel, A.M. Investigation of basalt fibre composite aging behaviour for applications in transportation. Polym. Compos. 2006, 27, 475–483. [Google Scholar] [CrossRef]
- Dhand, V.; Mittal, G.; Rhee, K.Y.; Park, S.J.; Hui, D. A short review on basalt fiber reinforced polymer composites. Compos. Part B Eng. 2015, 73, 166–180. [Google Scholar] [CrossRef]
- Fiore, V.; Scalici, T.; Di Bella, G.; Valenza, A. A review on basalt fibre and its composites. Compos. Part B Eng. 2015, 74, 74–94. [Google Scholar] [CrossRef]
- Zeng, J.B.; Jiao, L.; Li, Y.D.; Srinivasan, M.; Li, T.; Wang, Y.Z. Bio-based blends of starch and poly(butylene succinate) with improved miscibility, mechanical properties, and reduced water absorption. Carbohydr. Polym. 2011, 83, 762–768. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Cheng, L.; Yang, X. Biodegradability of poly(butylene succinate) (PBS) composite reinforced with jute fibre. Polym. Degrad. Stab. 2009, 94, 90–94. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.H.; He, C.; Jin, T.X.; Wang, K.; Fu, Q. Interfacial crystallization enhanced interfacial interaction of poly(butylene succinate)/ramie fiber biocomposites using dopamine as a modifier. Compos. Sci. Technol. 2014, 91, 22–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Chu, P.K.; Lv, F.; Zhang, C.; Ji, J.; Zhang, R.; Wang, H. Mechanical and thermal properties of basalt fiber reinforced poly(butylene succinate) composites. Mater. Chem. Phys. 2012, 133, 845–849. [Google Scholar] [CrossRef]
- Haque, M.M.; Ali, M.E.; Hasan, M.; Islam, M.N.; Kim, H. Chemical treatment of coir fiber reinforced polypropylene composites. Ind. Eng. Chem. Res. 2012, 51, 3958–3966. [Google Scholar] [CrossRef]
- España, J.M.; Samper, M.D.; Fages, E.; Sánchez-Nácher, L.; Balart, R. Investigation of the effect of different silane coupling agents on mechanical performance of basalt fibre composite laminates with biobased epoxy matrices. Polym. Compos. 2013, 34, 376–381. [Google Scholar] [CrossRef]
- Lee, S.O.; Rhee, K.Y.; Park, S.J. Influence of chemical surface treatment of basalt fibers on interlaminar shear strength and fracture toughness of epoxy-based composites. J. Ind. End. Chem. 2015, 32, 153–156. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Zeng, H.; Xiong, X. Effect of transcrystallinity on tensile behavior of discontinuous carbon fibre reinforced semicrystalline thermoplastic composites. Polymer 1996, 37, 5151–5158. [Google Scholar] [CrossRef]
- Sukhannova, T.E.; Lednicky, F.; Urban, J.; Baklagina, Y.G.; Mikhailov, G.M.; Kudryavtsev, V.V. Morphology of melt crystallized polypropylene in the presence of polyimide fibres. J. Mater. Sci. 1995, 30, 2201–2214. [Google Scholar] [CrossRef]
- Wang, C.; Liu, C.R. Transcrystallization of PTFE fiber/PP composites-III effect of fibre pulling on the crystallization kinetics. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1361–1370. [Google Scholar] [CrossRef]
- Quan, H.; Li, Z.M.; Yang, M.B.; Huang, R. On transcrystallinity in semi-crystalline polymer composites. Compos. Sci. Technol. 2005, 65, 999–1021. [Google Scholar] [CrossRef]
- Han, S.J.; Ren, K.; Geng, C.Z.; Wang, K.; Zhang, Q.; Chen, F.; Fu, Q. Enhanced interfacial adhesion via interfacial crystallization between sisal fiber and isotactic polypropylene: Direct evidence from single-fiber fragmentation testing. Polym. Int. 2014, 63, 646–651. [Google Scholar] [CrossRef]
- Teishev, A.; Marom, G. The effect of transcrystallinity on the transverse mechanical properties of single-polymer polyethylene composites. J. Appl. Polym. Sci. 1995, 56, 959–966. [Google Scholar] [CrossRef]
- Loos, J.; Schimanski, T.; Hofman, J.; Peijs, T.; Lemstra, P.J. Morphological investigations of polypropylene single-fibre reinforced polypropylene model composites. Polymer 2001, 42, 3827–3834. [Google Scholar] [CrossRef]
- Chen, M.Y.; Liu, Z.X. A comparative study of the load transfer mechanisms of the carbon nanotube reinforced polymer composites with interfacial crystallization. Compos. Part B Eng. 2015, 79, 114–123. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, J.J.; Li, Y.H.; Geng, C.Z.; He, C.; Wang, K.; Fu, Q. Interfacial strength and mechanical properties of biocomposites based on ramie fibers and poly(butylene succinate). RSC. Adv. 2013, 3, 26418–26426. [Google Scholar] [CrossRef]
- Abdou, J.P.; Reynolds, K.J.; Pfau, M.R.; van Staden, J.; Braggin, G.A.; Tajaddod, N.; Minus, M.; Reguero, V.; Vilatela, J.J.; Zhang, S.J. Interfacial crystallization of isotactic polypropylene surrounding macroscopic carbon nanotube and graphene fibers. Polymer 2016, 91, 136–145. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.F.; Wang, Y.; Zhang, B.M. Effects of thermal histories on carbon fiber/polyamide 6 microcomposites load transfer efficiency: Crystallization, modulus, and measurement. Polym. Compos. 2016. [Google Scholar] [CrossRef]
- Wang, Y.M.; Tong, B.B.; Hou, S.J.; Li, M.; Shen, C.Y. Transcrystallization behavior at the poly(lactic acid)/sisal fibre biocomposite interface. Compos. Part A Appl. Sci. Manuf. 2011, 42, 66–74. [Google Scholar] [CrossRef]
- Sang, L.; Wang, Y.K.; Chen, G.Y.; Liang, J.C.; Wei, Z.Y. A comparative study of the crystalline structure and mechanical properties of carbon fiber/polyamide 6 composites enhanced with/ without silane treatment. RSC Adv. 2016, 6, 107739–107747. [Google Scholar] [CrossRef]
- Orue, A.; Jauregi, A.; Unsuain, U.; Labidi, J.; Eceiza, A.; Arbelaiz, A. The effect of alkaline and silane treatments on mechanical properties and breakage of sisal fibers and poly(lactic acid)/sisal fiber composites. Compos. Part A Appl. Sci. Manuf. 2016, 84, 186–195. [Google Scholar] [CrossRef]
- Cui, H.Y.; Kessler, M.R. Pultruded glass fiber/biobased polymer: Interface tailoring with silane treatment. Compos. Part A Appl. Sci. Manuf. 2014, 65, 83–90. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers—The Scienta ESCA 300 Data Base; John Wiley & Sons, Ltd.: Chichester, UK, 1992; p. 295. [Google Scholar]
- Lin, C.W.; Lai, Y.C.; Liu, S.S. Effect of the surface roughness of sulfuric acid-anodized aluminum mold on the interfacial crystallization behavior of isotactic polypropylene. J. Adhes. Sci. Technol. 2001, 15, 929–944. [Google Scholar] [CrossRef]
- Jose, E.T.; Joseph, A.; Skrifvars, M.; Thomas, S.; Joseph, K. Thermal and crystallization behavior of cotton-Polypropylene commingled composite systems. Polym. Compos. 2010, 31, 1487–1494. [Google Scholar]
- Avrami, M. Kinetics of phase change. II. Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I. General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Liu, T.; Yu, X.J.; Yu, F.M.; Zhao, X.L.; Lu, A.; Wang, J.H.; Wang, X.Z.; Liu, T.L. Isothermal crystallization kinetics of fiber/polylactic acid composites and morphology. Polym. Plast. Technol. 2012, 51, 597–604. [Google Scholar] [CrossRef]
- Hermida, E.B.; Mega, V.I. Transcrystallization kinetics at the poly(3-hydroxybutyrate-co-hydroxyvalerate)/hemp fibre interface. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1387–1394. [Google Scholar] [CrossRef]
- Kelly, A.; Tyson, W.R. Tensile properties of fibre-reinforced metals: Copper/tungsten and copper/molybdenum. J. Mech. Phys. Solids 1965, 13, 329–338. [Google Scholar] [CrossRef]
- Faghihi, M.; Shojaei, A.; Bagheri, R. Characterization of polyamide 6/carbon nanotube composites prepared by melt mixing-effect of matrix molecular weight and structure. Compos. Part B Eng. 2015, 78, 50–64. [Google Scholar] [CrossRef]
- Thomason, J.L. The influence of fibre length and concentration on the properties of glass fibre reinforced polypropylene: 7. Interface strength and fibre strain in injection moulded long fibre PP at high fibre. Compos. Part A Appl. Sci. Manuf. 2007, 38, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Shojaei, A.; Nourbakhsh, P.; Faghihi, M. An investigation on the structural characteristics and reinforcement of melt processed polyamide 66/multiwalled carbon nanotube composites. Polym. Adv. Technol. 2014, 25, 406–417. [Google Scholar] [CrossRef]

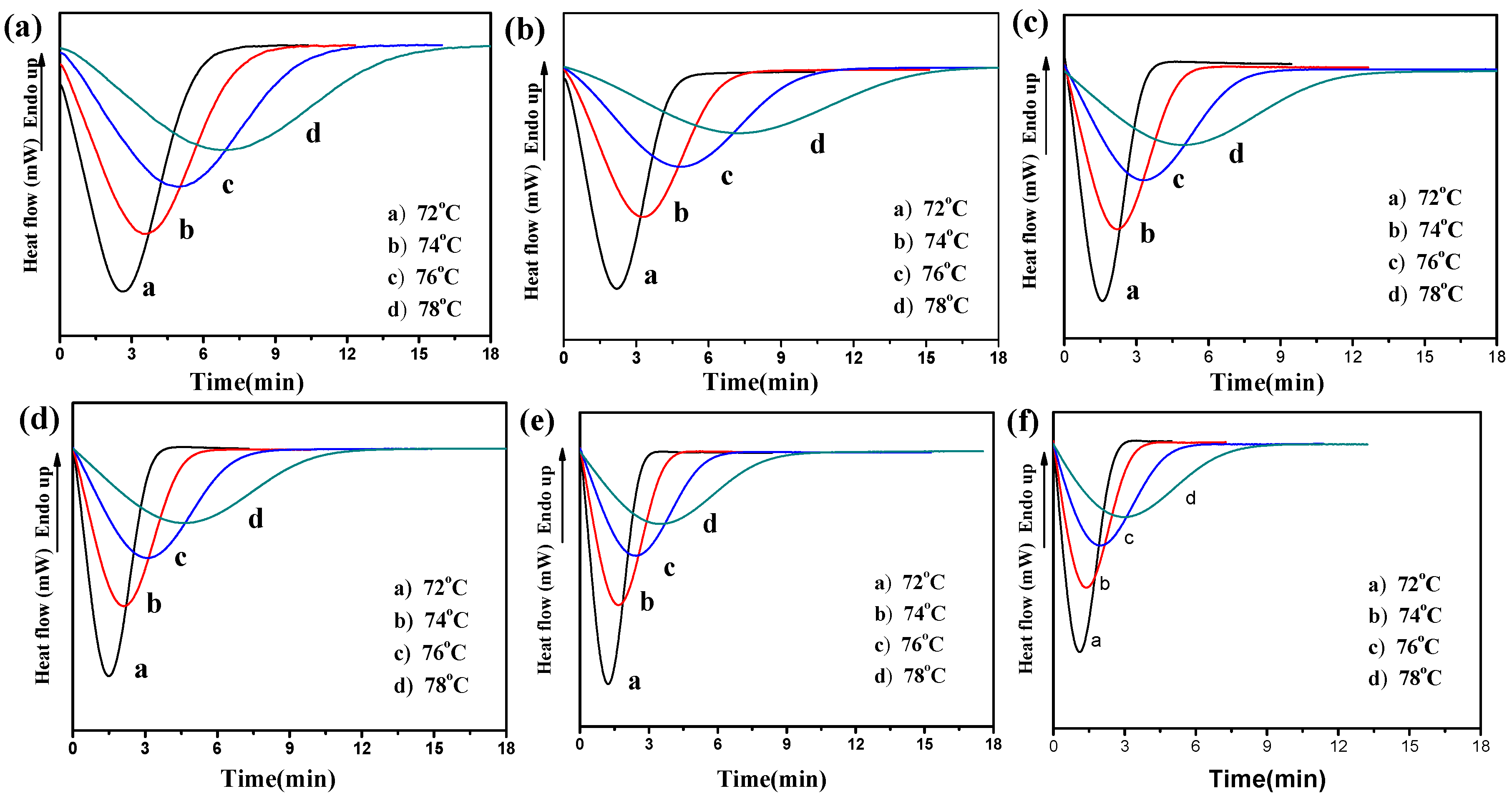
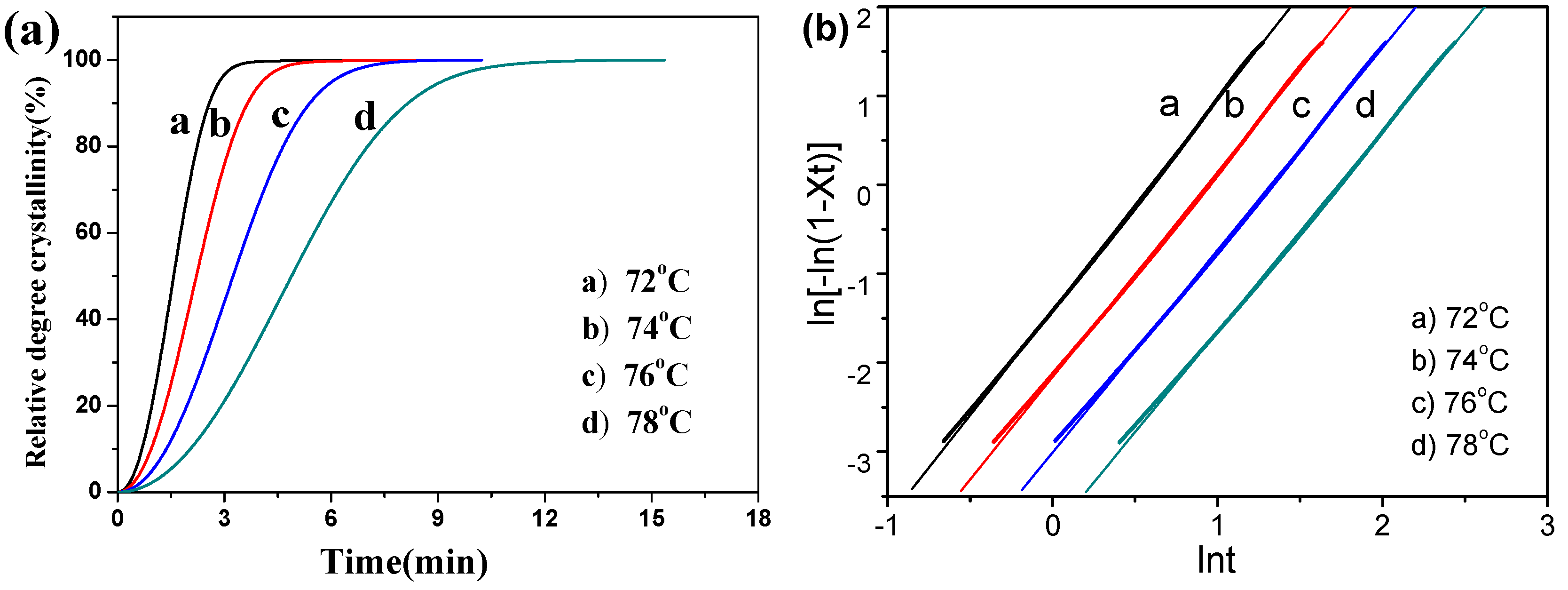
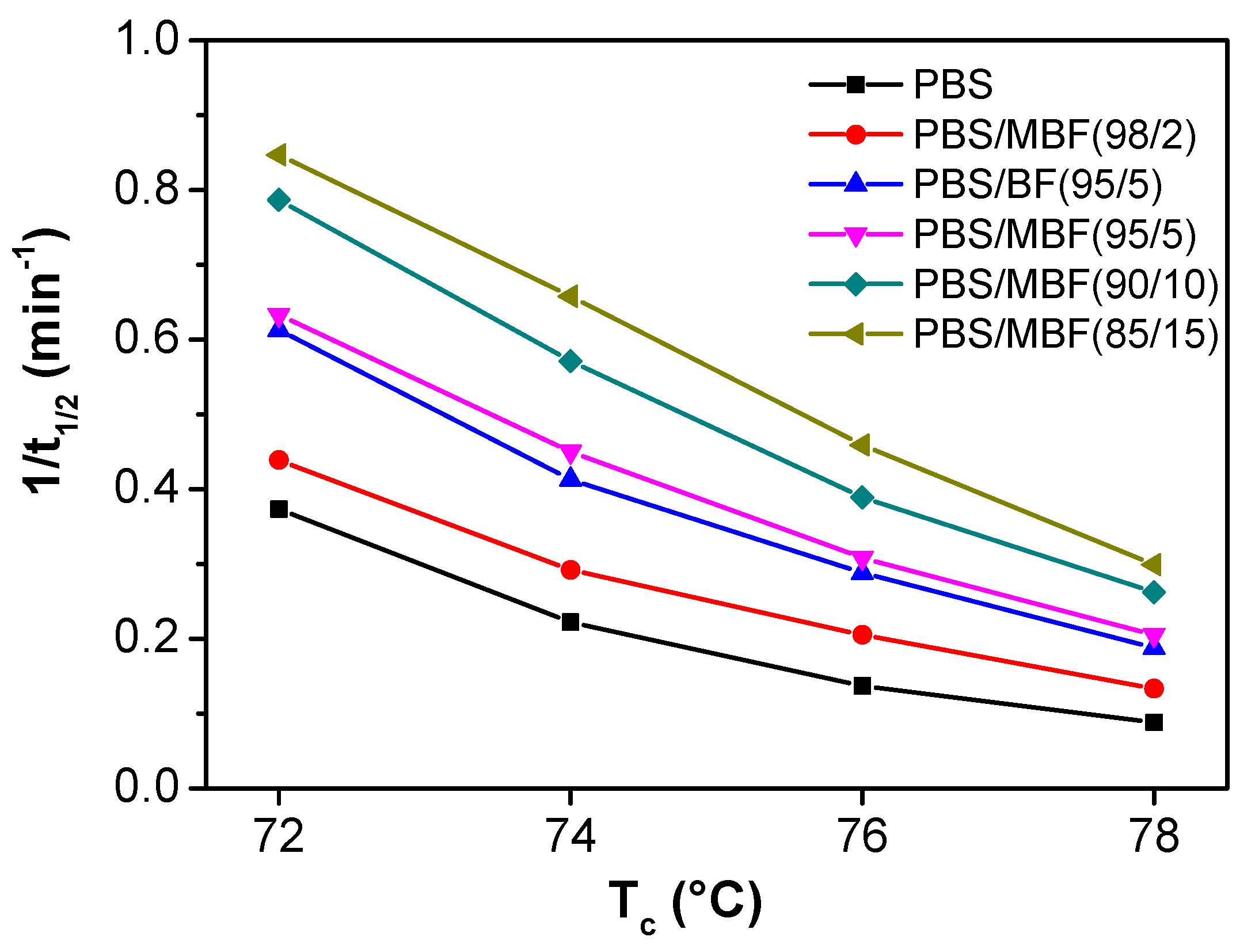
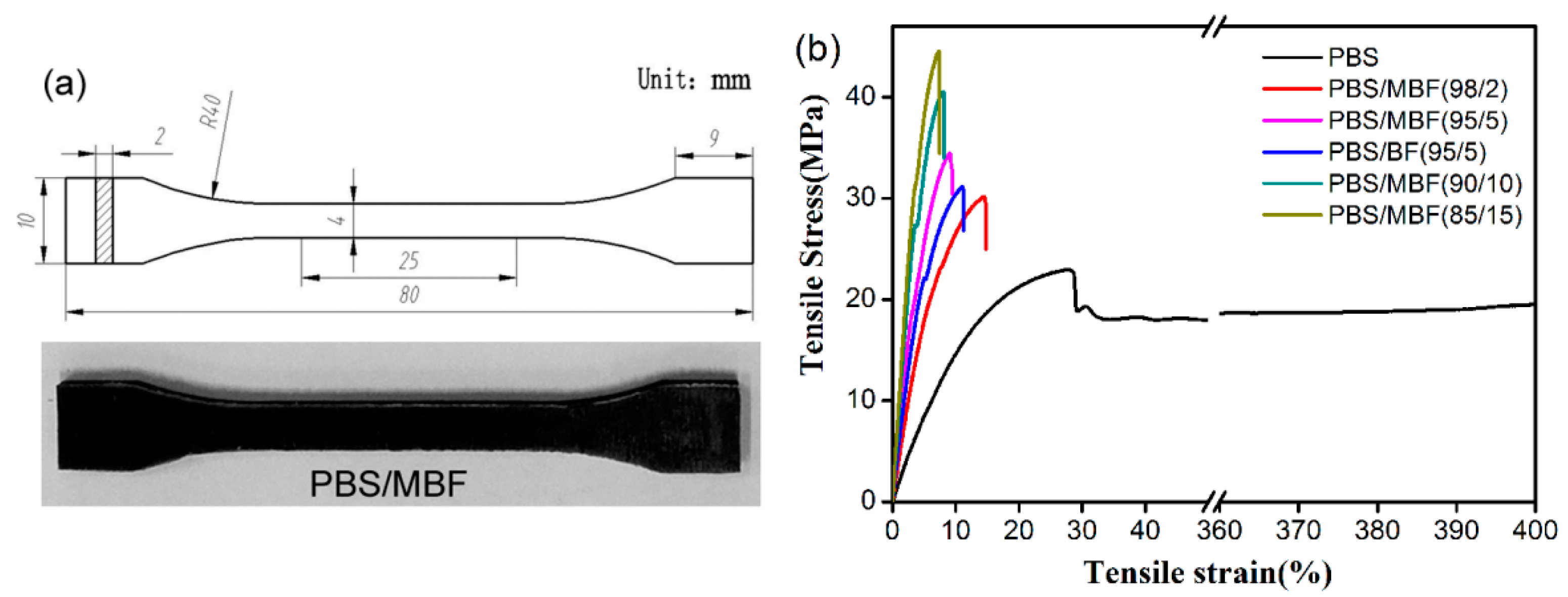
| Sample | Tc (°C) | t1/2 (min) | 1/t1/2 (min−1) | k·(min−n) | n | Average (n) |
|---|---|---|---|---|---|---|
| PBS | 72 | 2.68 | 0.373 | 0.054 | 2.58 | 2.47 |
| 74 | 4.50 | 0.222 | 0.017 | 2.47 | ||
| 76 | 7.30 | 0.137 | 0.006 | 2.43 | ||
| 78 | 11.4 | 0.088 | 0.002 | 2.40 | ||
| PBS/MBF (98/2) | 72 | 2.28 | 0.439 | 0.080 | 2.67 | 2.59 |
| 74 | 3.42 | 0.292 | 0.031 | 2.55 | ||
| 76 | 4.88 | 0.205 | 0.013 | 2.55 | ||
| 78 | 7.53 | 0.133 | 0.004 | 2.58 | ||
| PBS/BF (95/5) | 72 | 1.63 | 0.613 | 0.232 | 2.34 | 2.26 |
| 74 | 2.32 | 0.413 | 0.102 | 2.33 | ||
| 76 | 3.47 | 0.288 | 0.046 | 2.20 | ||
| 78 | 5.33 | 0.188 | 0.020 | 2.17 | ||
| PBS/MBF (95/5) | 72 | 1.58 | 0.633 | 0.245 | 2.36 | 2.30 |
| 74 | 2.22 | 0.45 | 0.115 | 2.30 | ||
| 76 | 3.25 | 0.308 | 0.049 | 2.27 | ||
| 78 | 4.88 | 0.205 | 0.020 | 2.25 | ||
| PBS/MBF (90/10) | 72 | 1.27 | 0.787 | 0.420 | 2.36 | 2.26 |
| 74 | 1.75 | 0.571 | 0.201 | 2.28 | ||
| 76 | 2.57 | 0.389 | 0.088 | 2.23 | ||
| 78 | 3.82 | 0.262 | 0.039 | 2.16 | ||
| PBS/MBF (85/15) | 72 | 1.18 | 0.847 | 0.499 | 2.26 | 2.16 |
| 74 | 1.52 | 0.658 | 0.293 | 2.16 | ||
| 76 | 2.18 | 0.459 | 0.137 | 2.12 | ||
| 78 | 3.34 | 0.299 | 0.058 | 2.09 |
| Sample | σm (MPa) | σct (MPa) | E (MPa) | Wf (%) | Vf (%) | L (μm) | τi (MPa) |
|---|---|---|---|---|---|---|---|
| PBS/MBF (98/2) | 23.2 | 29.8 ± 1.1 | 163.2 ± 5.6 | 2 | 1 | 317.3 | 100.1 |
| PBS/BF (95/5) | 23.2 | 30.5 ± 0.5 | 267.4 ± 32.5 | 5 | 2.3 | 315.5 | 50.2 |
| PBS/MBF (95/5) | 23.2 | 34.5 ± 0.3 | 290.0 ± 19.6 | 5 | 2.3 | 314.2 | 76.1 |
| PBS/MBF (90/10) | 23.2 | 39.6 ± 0.7 | 373.9 ± 22.3 | 10 | 4.8 | 307.3 | 55.2 |
| PBS/MBF (85/15) | 23.2 | 45.8 ± 1.0 | 442.3 ± 16.8 | 15 | 7.3 | 303.2 | 51.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, L.; Zhao, M.; Liang, Q.; Wei, Z. Silane-Treated Basalt Fiber–Reinforced Poly(butylene succinate) Biocomposites: Interfacial Crystallization and Tensile Properties. Polymers 2017, 9, 351. https://doi.org/10.3390/polym9080351
Sang L, Zhao M, Liang Q, Wei Z. Silane-Treated Basalt Fiber–Reinforced Poly(butylene succinate) Biocomposites: Interfacial Crystallization and Tensile Properties. Polymers. 2017; 9(8):351. https://doi.org/10.3390/polym9080351
Chicago/Turabian StyleSang, Lin, Mingyuan Zhao, Qiushi Liang, and Zhiyong Wei. 2017. "Silane-Treated Basalt Fiber–Reinforced Poly(butylene succinate) Biocomposites: Interfacial Crystallization and Tensile Properties" Polymers 9, no. 8: 351. https://doi.org/10.3390/polym9080351
APA StyleSang, L., Zhao, M., Liang, Q., & Wei, Z. (2017). Silane-Treated Basalt Fiber–Reinforced Poly(butylene succinate) Biocomposites: Interfacial Crystallization and Tensile Properties. Polymers, 9(8), 351. https://doi.org/10.3390/polym9080351