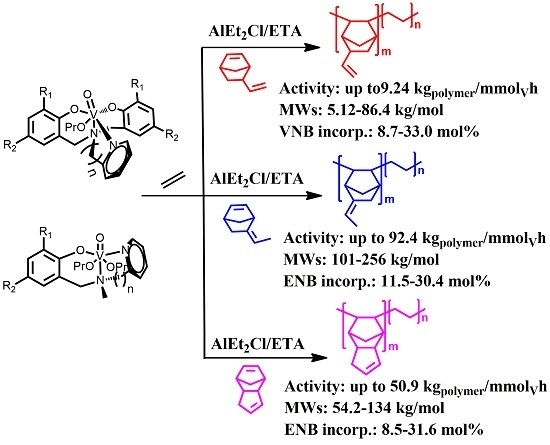
Facile, Efficient Copolymerization of Ethylene with Norbornene-Containing Dienes Promoted by Single Site Non-Metallocene Oxovanadium(V) Catalytic System
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Procedures and Materials
2.2. Characterization
2.3. General Procedure for Copolymerization
3. Results and Discussion
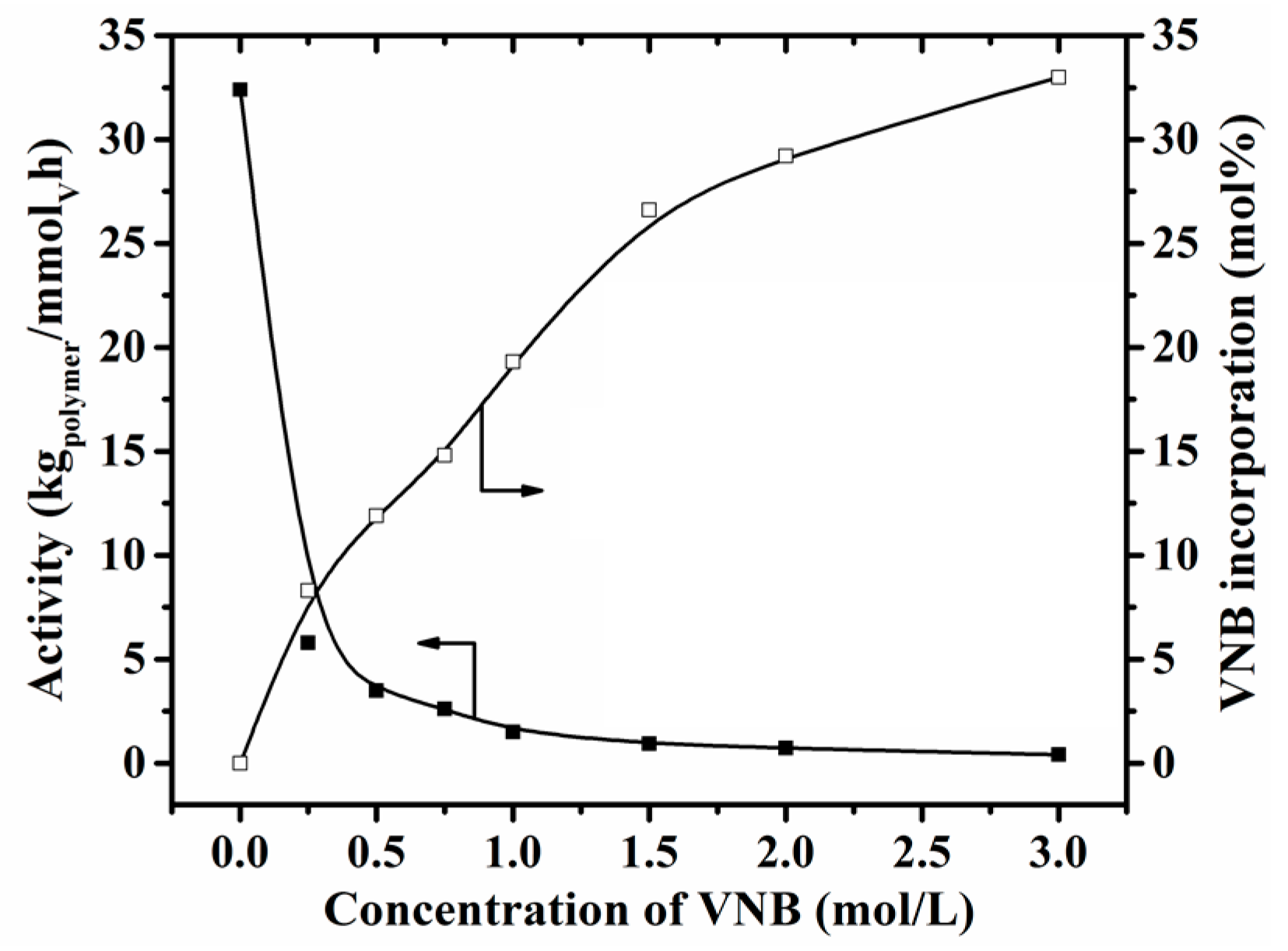
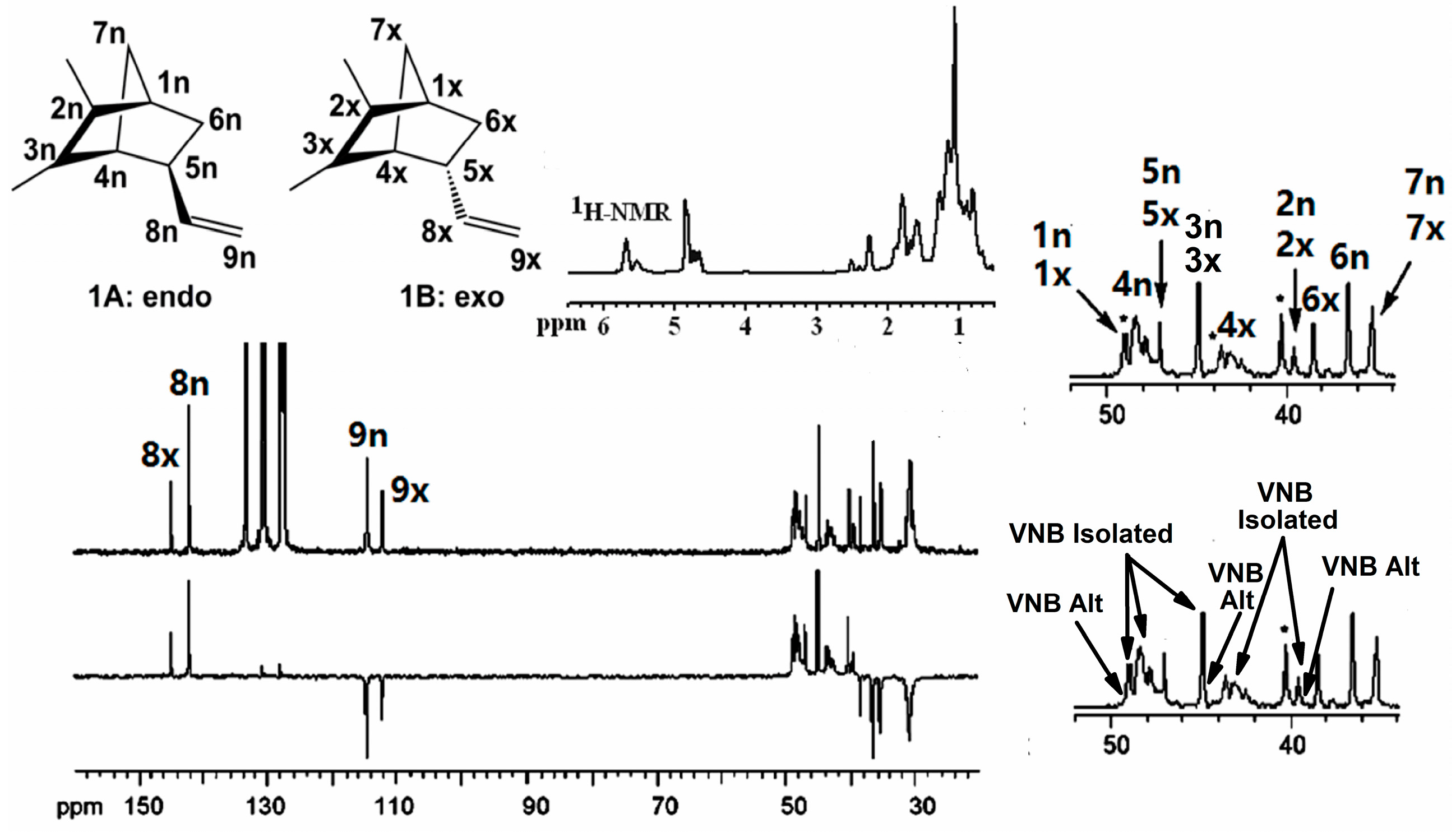
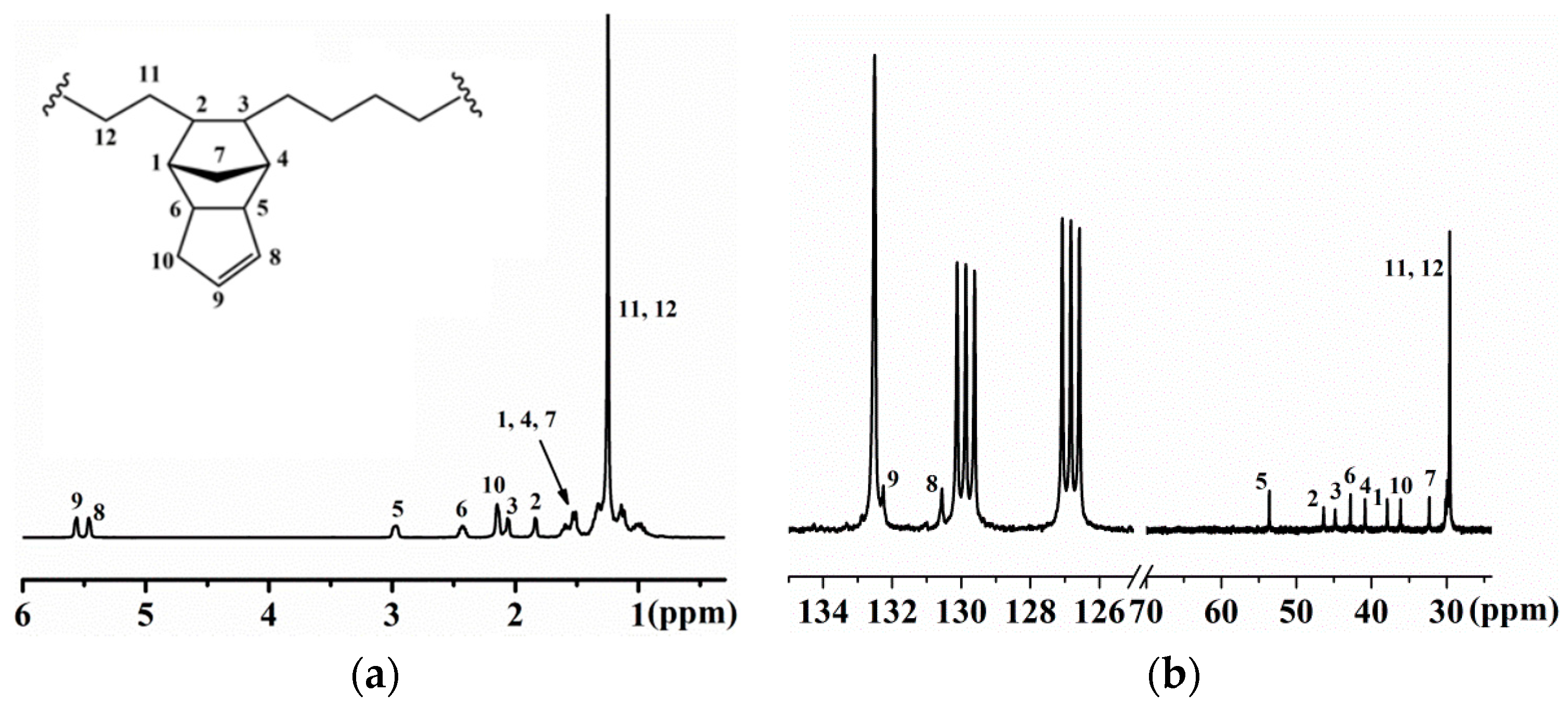
3.1. Copolymerization of Ethylene with VNB
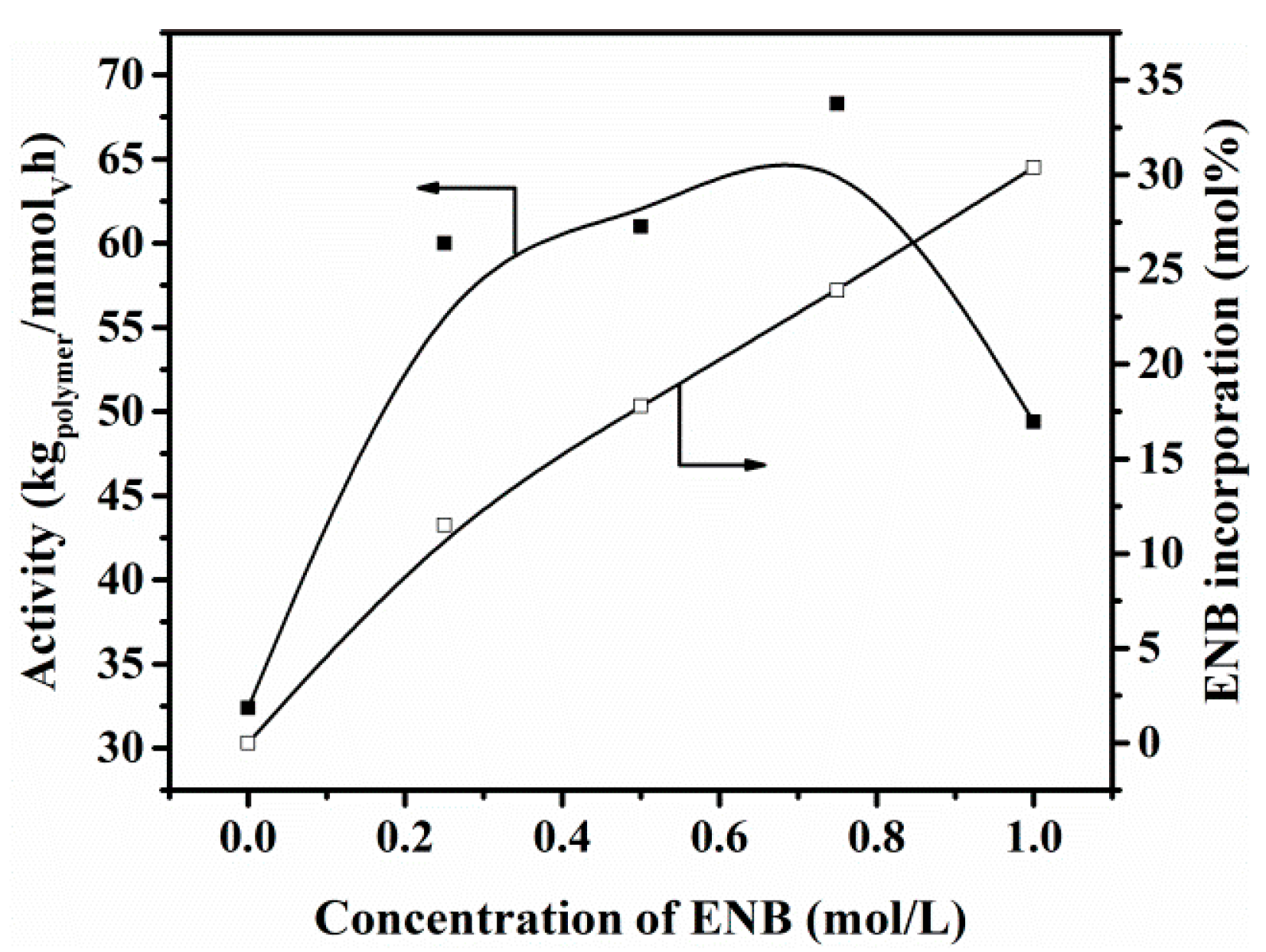
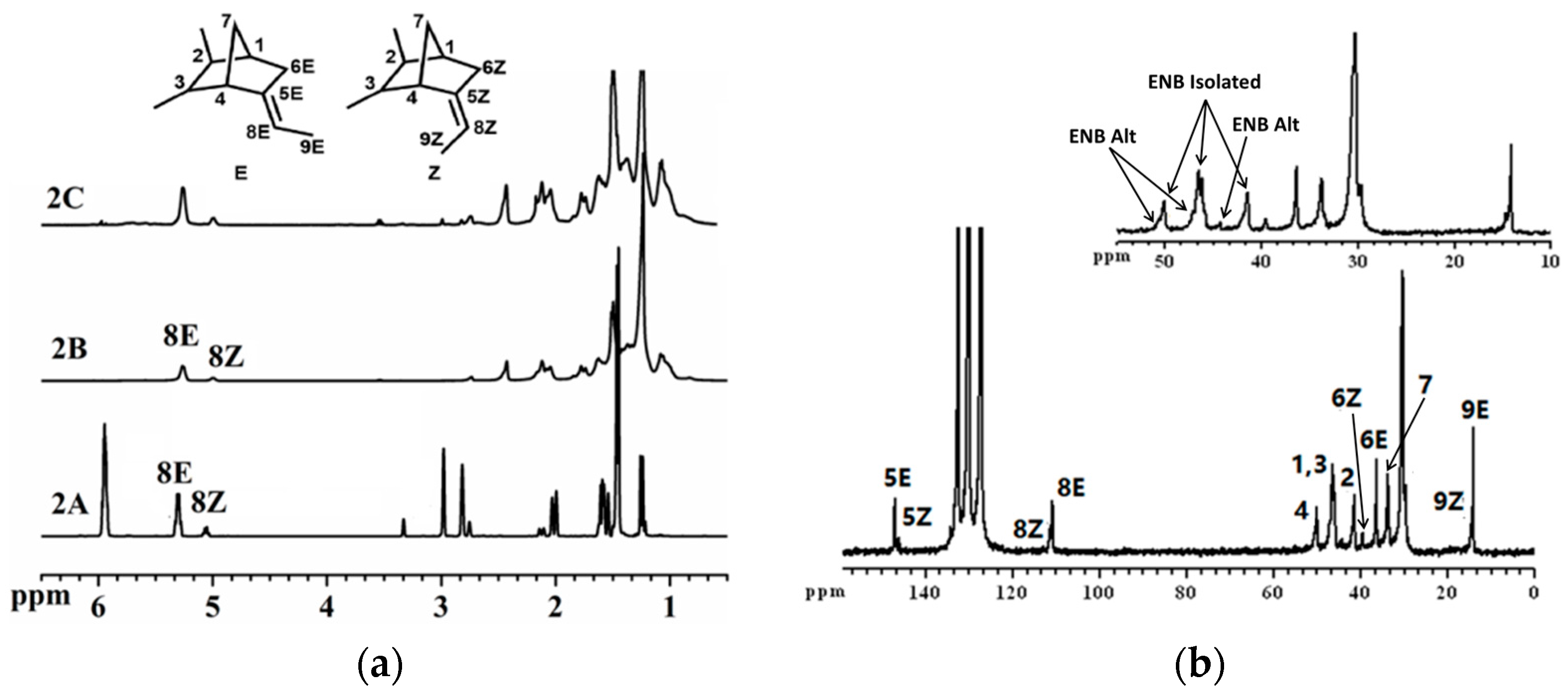
3.2. Copolymerization of Ethylene with ENB
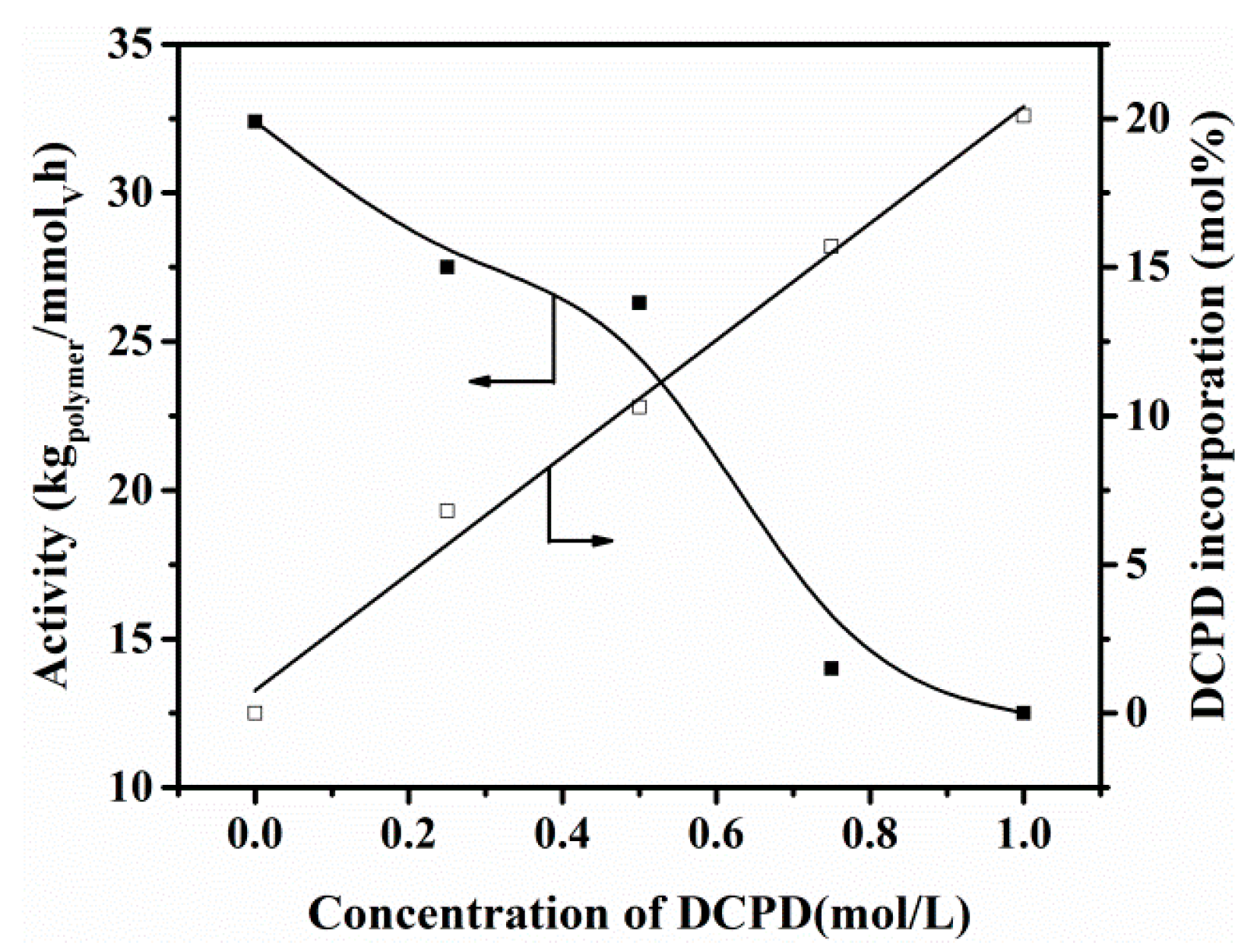
3.3. Copolymerization of Ethylene with DCPD
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chung, T.C.; Lu, H.L.; Li, C.L. Synthesis and functionalization of unsaturated polyethylene: Poly(ethylene-co-1,4-hexadiene). Macromolecules 1994, 27, 7533–7537. [Google Scholar] [CrossRef]
- Chung, T.C.; Dong, J.Y. Synthesis of linear ethylene/divinylbenzene copolymers by metallocene catalysis. Macromolecules 2002, 35, 2868–2870. [Google Scholar] [CrossRef]
- Chung, T.C. Synthesis of functional polyolefin copolymers with graft and block structures. Prog. Polym. Sci. 2002, 27, 39–85. [Google Scholar] [CrossRef]
- Hustad, P.D.; Coates, G.W. Insertion/isomerization polymerization of 1,5-hexadiene: Synthesis of functional propylene copolymers and block copolymers. J. Am. Chem. Soc. 2002, 124, 11578–11579. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Takemoto, A.; Hatanaka, Y.; Okumura, H.; Fujiki, M.; Hasegawa, K. Polymerization of 1,5-hexadiene by half-titanocenes-MAO catalyst systems: Factors affecting the selectivity for the favored repeated 1,2-insertion. Macromolecules 2006, 39, 4009–4017. [Google Scholar] [CrossRef]
- Nomura, K.; Hatanaka, Y.; Okumura, H.; Fujiki, M.; Hasegawa, K. Polymerization of 1,5-hexadiene by the nonbridged half-titanocene complex-MAO catalyst system: Remarkable difference in the selectivity of repeated 1,2-insertion. Macromolecules 2004, 37, 1693–1695. [Google Scholar] [CrossRef]
- Kobayashi, S.; Lu, C.; Hoye, T.R.; Hillmyer, M.A. Controlled polymerization of a cyclic diene prepared from the ring-closing metathesis of a naturally occurring monoterpene. J. Am. Chem. Soc. 2009, 131, 7960–7961. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Baldamus, J.; Nishiura, M.; Tardif, O.; Hou, Z. Cationic rare-earth polyhydrido complexes: Synthesis, structure, and catalytic activity for the cis-1,4-selective polymerization of 1,3-cyclohexadiene. Angew. Chem. Int. Ed. 2006, 45, 8184–8188. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Liu, J.; Fujiki, M.; Takemoto, A. Facile, Efficient functionalization of polyolefins via controlled incorporation of terminal olefins by repeated 1,7-octadiene insertion. J. Am. Chem. Soc. 2007, 129, 14170–14171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wang, Y.X.; Li, Y.S.; Pan, L. Convenient Syntheses and Versatile Functionalizations of Isotactic Polypropylene Containing Plentiful Pendant Styrene Groups with High Efficiency. Macromolecules 2015, 48, 1991–1998. [Google Scholar] [CrossRef]
- Mulhaupt, R.; Ovenall, D.W.; Ittel, S.D. Control of composition in ethylene copolymerizations using magnesium mhloride supported Ziegler–Natta catalysts. J. Polym. Sci. Part A Polym. Chem. 1988, 26, 2487–2500. [Google Scholar] [CrossRef]
- Tsujino, T.; Saegusa, T.; Furukawa, J. Polymerization of norbornene by modified Ziegler-catalysts. Die Makromol. Chem. 2003, 85, 71–79. [Google Scholar] [CrossRef]
- Pan, L.; Ye, W.P.; Liu, J.Y.; Hong, M.; Li, Y.S. Efficient, regioselective copolymerization of ethylene with cyclopentadiene by the titanium complexes bearing two β-enaminoketonato ligands. Macromolecules 2008, 41, 2981–2983. [Google Scholar] [CrossRef]
- Pan, L.; Hong, M.; Liu, J.Y.; Ye, W.P.; Li, Y.S. Living copolymerization of ethylene with dicyclopentadiene using titanium catalyst: Formation of well-defined polyethylene-block-poly(ethylene-co-dicyclopentadiene)s and their transformation into novel polyolefin-block-(functional polyolefin)s. Macromolecules 2009, 42, 4391–4393. [Google Scholar] [CrossRef]
- Lasarov, H.; Pakkanen, T.T. Copolymerization of ethylene with 5-vinyl-2-norbornene in the presence of the Ph2C(Flu)(Cp)ZrCl2/MAO catalyst. Macromol. Chem. Phys. 2000, 201, 1780–1786. [Google Scholar] [CrossRef]
- Liu, J.Y.; Liu, S.R.; Pan, L.; Li, Y.S. Facile, efficient copolymerization of ethylene with bicyclic, non-conjugated dienes by titanium complexes bearing bis(β-enaminoketonato) ligands. Adv. Synth. Catal. 2009, 351, 1505–1511. [Google Scholar] [CrossRef]
- Hou, Z.M.; Luo, Y.J.; Li, X.F. Cationic rare earth metal alkyls as novel catalysts for olefin polymerization and copolymerization. J. Organomet. Chem. 2006, 691, 3114–3121. [Google Scholar] [CrossRef]
- Li, X.F.; Nishiura, M.; Mori, K.; Mashiko, T.; Hou, Z.M. Cationic scandium aminobenzyl complexes. Synthesis, structure and unprecedented catalysis of copolymerization of 1-hexene and dicyclopentadiene. Chem. Commun. 2007, 40, 4137–4139. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Hou, Z.M. Organometallic catalysts for copolymerization of cyclic olefins. Coord. Chem. Rev. 2008, 252, 1842–1869. [Google Scholar] [CrossRef]
- Li, X.F.; Hou, Z.M. Scandium-catalyzed regio- and stereospecific cis-1,4-polymerization of 1,3-cyclohexadiene and copolymerization with ethylene. Macromolecules 2010, 43, 8904–8909. [Google Scholar] [CrossRef]
- Hasan, T.; Ikeda, T.; Shiono, T. Homo- and copolymerization of norbornene derivatives with ethene by ansa-fluorenylamidodimethyltitanium activated with methylaluminoxane. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 4581–4587. [Google Scholar] [CrossRef]
- Nishii, K.; Hayano, S.; Tsunogae, Y.; Cai, Z.G.; Nakayama, Y.; Shiono, T. Highly Active Copolymerization of Ethylene and Dicyclopentadiene with [(η1-t-BuN)SiMe2(η1-C29H36)]TiMe2(THF) Complex. Chem. Lett. 2008, 37, 590–591. [Google Scholar] [CrossRef]
- Christman, D.L.; Keim, G.I. Reactivities of nonconjugated dienes used in preparation of terpolymers in homogeneous systems. Macromolecules 1968, 1, 358–363. [Google Scholar] [CrossRef]
- Carrick, W.L.; Kluiber, R.W.; Bonner, E.F.; Wartman, L.H.; Rugg, F.M.; Smith, J.J. Transition Metal Catalysts. I. Ethylene polymerization with a soluble catalyst formed from an aluminum halide, tetraphenyltin and a vanadium halide. J. Am. Chem. Soc. 1960, 82, 3883–3887. [Google Scholar] [CrossRef]
- Carrick, W.L. Mechanism of ethylene polymerization with vanadium catalysts. J. Am. Chem. Soc. 2002, 80, 6455–6456. [Google Scholar] [CrossRef]
- Doi, Y.; Ueki, S.; Keii, T. “Living” Coordination polymerization of propene initiated by the soluble V(acac)3-Al(C2H5)2Cl System. Macromolecules 1979, 12, 814–819. [Google Scholar] [CrossRef]
- Adisson, E.; Deffieux, A.; Fontanille, M. Polymerization of ethylene at high temperature by vanadium-based heterogeneous Ziegler–Natta catalysts. I. study of the deactivation process. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 831–839. [Google Scholar] [CrossRef]
- Wu, J.Q.; Li, Y.S. Well-defined vanadium complexes as the catalysts for olefin polymerization. Coord. Chem. Rev. 2011, 255, 2303–2314. [Google Scholar] [CrossRef]
- Gambarotta, S. Vanadium-based Ziegler–Natta: Challenges, promises, problems. Coord. Chem. Rev. 2003, 237, 229–243. [Google Scholar] [CrossRef]
- Hagen, H.; Boersma, J.; van Koten, G. Homogeneous vanadium-based catalysts for the Ziegler–Natta polymerization of α-Olefins. Chem. Soc. Rev. 2002, 31, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, C. Vanadium procatalysts bearing chelating aryloxides: Structure-activity trends in ethylene polymerisation. Dalton Trans. 2010, 39, 5595–5604. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Zhang, S. Design of vanadium complex catalysts for precise olefin polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.T.; Wang, Y.X.; Wang, B.; Li, Y.G.; Li, Y.S. Introduction of constrained cyclic skeleton into β-enaminoketonato vanadium complexs: A strategy for stabilization of active center of vanadium catalyst for ethylene polymerization. J. Polym. Sci. 2017, 35, 1110–1121. [Google Scholar]
- Tomov, A.K.; Gibson, V.C.; Zaher, D.; Elsegood, M.R.; Dale, S.H. Bis(benzimidazole)amine vanadium catalysts for olefin polymerisation and co-polymerisation: Thermally robust, single-site catalysts activated by simple alkylaluminium reagents. Chem. Commun. 2004, 17, 1956–1957. [Google Scholar] [CrossRef] [PubMed]
- Redshaw, C.; Rowan, M.A.; Homden, D.M.; Dale, S.H.; Elsegood, M.R.; Matsui, S.; Matsuura, S. Vanadyl C and N-capped tris(phenolate) complexes: Influence of pro-catalyst geometry on catalytic activity. Chem. Commun. 2006, 31, 3329–3331. [Google Scholar] [CrossRef] [PubMed]
- Homden, D.M.; Redshaw, C.; Hughes, D.L. Vanadium complexes possessing N2O2S2-based ligands: Highly active procatalysts for the homopolymerization of ethylene and copolymerization of ethylene/1-hexene. Inorg. Chem. 2007, 46, 10827–10839. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Lu, L.P.; Liu, J.Y.; Li, Y.S. [ONN]-type amine pyridine(s) phenolate-based oxovanadium(V) catalysts for ethylene homo- and copolymerization. Dalton Trans. 2014, 43, 12926–12934. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Lu, L.P.; Liu, J.Y.; Mu, H.L.; Li, Y.S. [ONNO]-type oxovanadium(V) complexes containing amine pyridine bis(Phenolate) ligands: Synthesis, characterization and catalytic behavior for ethylene (co)polymerization. J. Mol. Catal. A Chem. 2015, 398, 289–296. [Google Scholar] [CrossRef]
- Li, Y.L.; Yang, J.X.; Wang, B.; Li, Y.S. Efficient copolymerization of ethylene with norbornene or its derivatives using half-metallocene zirconium(IV) catalysts. RSC Adv. 2016, 6, 59590–59599. [Google Scholar] [CrossRef]
- Chien, J.C.W.; Nozaki, T. Ethylene-hexene copolymerization by heterogeneous and homogeneous Ziegler–Natta catalysts and the “comonomer” effect. J. Polym. Sci. A 1993, 31, 227–237. [Google Scholar] [CrossRef]
- Koivumiiki, J.; Seppälä, J.V. Observations on the rate enhancement effect with MgC12/TiCl4 and Cp2ZrCl2 catalyst systems upon 1-hexene addition. Macromolecules 1994, 27, 2008–2012. [Google Scholar] [CrossRef]
- Suzukamo, G.; Fukao, M.; Minobe, M. Preparation of new solid super base and its catalytic activity. Chem. Lett. 1987, 16, 585–588. [Google Scholar] [CrossRef]
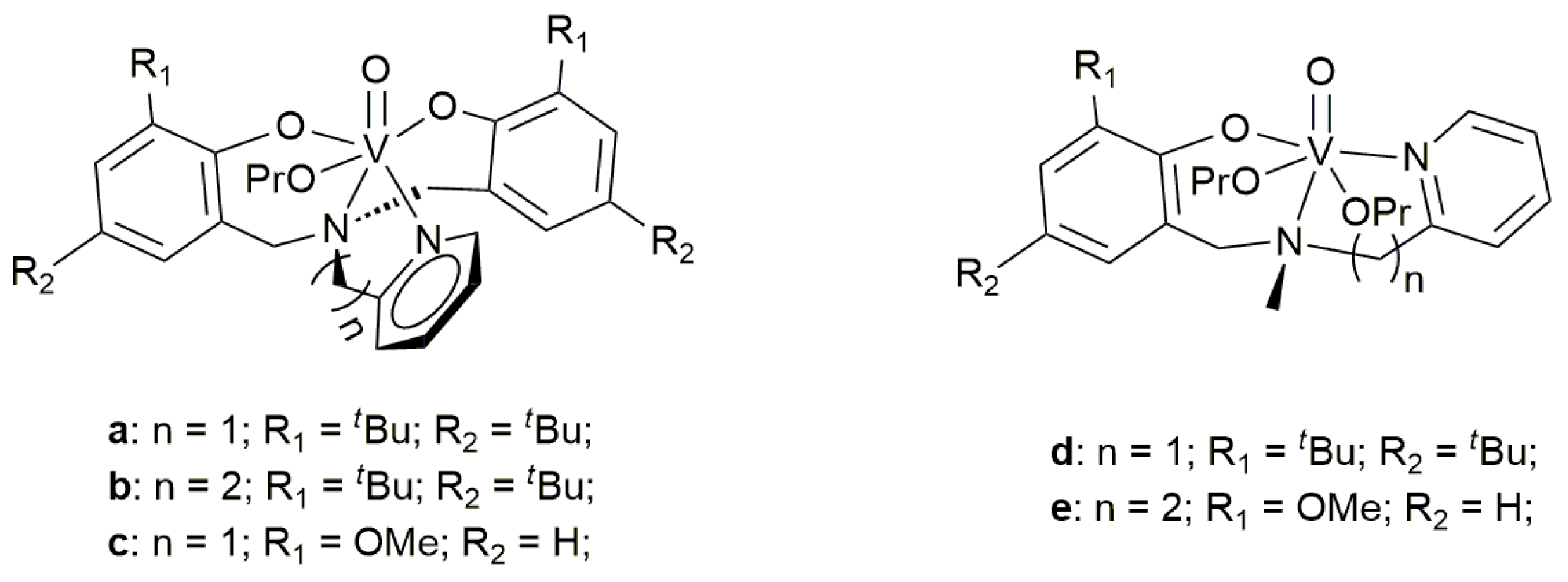
| Entry | Cat. (μmol) | Al/V (Molar Ratio) | VNB (mol/L) | Activity (kg/mmolV h) | VNB Incorp. a | Mw b (kDa) | PDI b | Tg c (°C) | Npolym/Ncat d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | a (2.0) | 4000 | 0 | 3.36 | - | 113 | 1.9 | - | 5.00 |
| 2 | a (2.0) | 4000 | 0.50 | 0.54 | 8.9 | 86.4 | 2.0 | 52 | 1.00 |
| 3 | b (2.0) | 4000 | 0 | 32.4 | - | 193 | 2.1 | - | 28.0 |
| 4 | b (2.0) | 4000 | 0.50 | 3.48 | 11.9 | 59.1 | 2.2 | 54 | 9.80 |
| 5 | c (2.0) | 4000 | 0 | 6.00 | - | 135 | 1.8 | - | 7.40 |
| 6 | c (2.0) | 4000 | 0.50 | 1.86 | 9.1 | 33.6 | 2.0 | 52 | 9.20 |
| 7 | b (2.0) | 2000 | 0.50 | 2.82 | 12.5 | 62.1 | 2.1 | 55 | 7.60 |
| 8 | b (2.0) | 6000 | 0.50 | 3.81 | 11.6 | 42.8 | 2.0 | 53 | 14.0 |
| 9 | b (2.0) | 4000 | 0.25 | 5.79 | 8.3 | 67.6 | 2.0 | 51 | 14.0 |
| 10 | b (2.0) | 4000 | 0.75 | 2.61 | 14.8 | 35.9 | 1.9 | 56 | 12.0 |
| 11 | b (2.0) | 4000 | 1.00 | 1.50 | 19.3 | 25.7 | 1.9 | 74 | 9.70 |
| 12 | b (2.0) | 4000 | 1.50 | 0.93 | 26.6 | 10.9 | 1.8 | 82 | 14.0 |
| 13 | b (2.0) | 4000 | 2.00 | 0.72 | 29.2 | 6.42 | 1.8 | 88 | 1.90 |
| 14 | b (2.0) | 4000 | 3.00 | 0.41 | 33.0 | 5.12 | 1.9 | 91 | 1.40 |
| 15 | d (1.0) | 4000 | 0 | 17.8 | - | 220 | 3.3 | - | 13.0 |
| 16 | d (1.0) | 4000 | 0.50 | 8.22 | 8.7 | 30.4 | 2.1 | 51 | 45.0 |
| 17 | e (1.0) | 4000 | 0 | 58.3 | - | 62.4 | 2.3 | - | 156 |
| 18 | e (1.0) | 4000 | 0.50 | 9.24 | 10.4 | 34.6 | 2.2 | 53 | 45.0 |
| 19 | e (1.0) | 4000 | 1.00 | 5.16 | 17.9 | 31.0 | 1.9 | 73 | 28.0 |
| Entry | Cat. (μmol) | Al/V (Molar Ratio) | VNB (mol/L) | Activity (kg/mmolV h) | ENB Incorp. a | Mw b (kDa) | PDI b | Tg c (°C) | npolym/ncat d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | b (0.25) | 4000 | 0.50 | 61.0 | 17.8 | 168 | 2.0 | 73 | 3.00 |
| 2 | c (0.25) | 4000 | 0.50 | 8.16 | 18.1 | 239 | 2.1 | 73 | 0.30 |
| 3 | b (0.25) | 2000 | 0.50 | 25.9 | 17.2 | 221 | 2.1 | 70 | 1.00 |
| 4 | b (0.25) | 6000 | 0.50 | 64.3 | 18.5 | 149 | 1.9 | 75 | 3.60 |
| 5 e | b (0.25) | 4000 | 0.50 | 48.5 | 15.3 | 256 | 2.2 | 63 | 1.60 |
| 6 f | b (0.25) | 4000 | 0.50 | 49.9 | 16.7 | 195 | 2.1 | 69 | 4.30 |
| 7 | b (0.25) | 4000 | 0.25 | 60.0 | 11.5 | 177 | 2.0 | 54 | 2.80 |
| 8 | b (0.25) | 4000 | 0.75 | 68.3 | 23.9 | 169 | 2.1 | 80 | 3.40 |
| 9 | b (0.25) | 4000 | 1.00 | 49.4 | 30.4 | 153 | 2.3 | 87 | 2.70 |
| 10 | d (0.10) | 4000 | 0.50 | 92.4 | 16.9 | 138 | 2.0 | 71 | 5.60 |
| 11 | e (0.10) | 4000 | 0.50 | 122 | 18.8 | 101 | 2.0 | 77 | 10.0 |
| Entry | Cat. (μmol) | Al/V (Molar Ratio) | DCPD (mol/L) | Activity (kg/mmolV h) | DCPD Incorp. a | Mw b (kDa) | PDI b | Tg c (°C) | Npolym/Ncat d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | b (0.50) | 4000 | 0.50 | 23.3 | 10.6 | 103 | 2.1 | 55 | 38.0 |
| 2 | c (0.50) | 4000 | 0.50 | 1.32 | 8.5 | 54.2 | 1.7 | 52 | 4.00 |
| 3 | b (0.50) | 2000 | 0.50 | 29.8 | 11.1 | 134 | 2.3 | 56 | 37.0 |
| 4 | b (0.50) | 6000 | 0.50 | 26.3 | 10.3 | 87.4 | 2.1 | 55 | 50.0 |
| 5 e | b (0.50) | 4000 | 0.50 | 24.7 | 9.7 | 96.8 | 2.0 | 52 | 21.0 |
| 6 | b (0.50) | 4000 | 0.25 | 27.5 | 6.8 | 131 | 1.9 | 51 | 35.0 |
| 7 | b (0.50) | 4000 | 0.75 | 14.0 | 15.7 | 89.4 | 1.9 | 65 | 26.0 |
| 8 | b (0.50) | 4000 | 1.00 | 12.5 | 20.1 | 63.1 | 1.6 | 77 | 33.0 |
| 9 | d (0.25) | 4000 | 0.50 | 39.4 | 19.8 | 80.9 | 2.0 | 76 | 81.0 |
| 10 | e (0.25) | 4000 | 0.50 | 50.9 | 21.4 | 71.4 | 2.2 | 79 | 119 |
| 11 | e (0.25) | 4000 | 1.00 | 32.9 | 31.6 | 59.7 | 2.1 | 98 | 92.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Wang, J.; Li, Y.; Pan, L.; Li, Y. Facile, Efficient Copolymerization of Ethylene with Norbornene-Containing Dienes Promoted by Single Site Non-Metallocene Oxovanadium(V) Catalytic System. Polymers 2017, 9, 353. https://doi.org/10.3390/polym9080353
Wang K, Wang J, Li Y, Pan L, Li Y. Facile, Efficient Copolymerization of Ethylene with Norbornene-Containing Dienes Promoted by Single Site Non-Metallocene Oxovanadium(V) Catalytic System. Polymers. 2017; 9(8):353. https://doi.org/10.3390/polym9080353
Chicago/Turabian StyleWang, Kaiti, Jiabao Wang, Yanguo Li, Li Pan, and Yuesheng Li. 2017. "Facile, Efficient Copolymerization of Ethylene with Norbornene-Containing Dienes Promoted by Single Site Non-Metallocene Oxovanadium(V) Catalytic System" Polymers 9, no. 8: 353. https://doi.org/10.3390/polym9080353