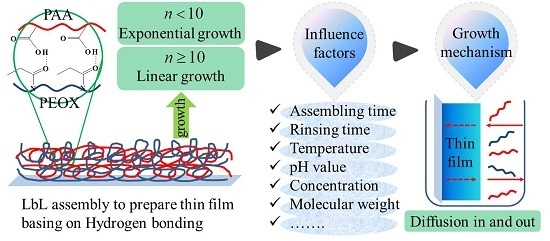
Hydrogen-Bonded Polymer Complex Thin Film of Poly(2-oxazoline) and Poly(acrylic acid)
Abstract
:1. Introduction
2. Experimental Section
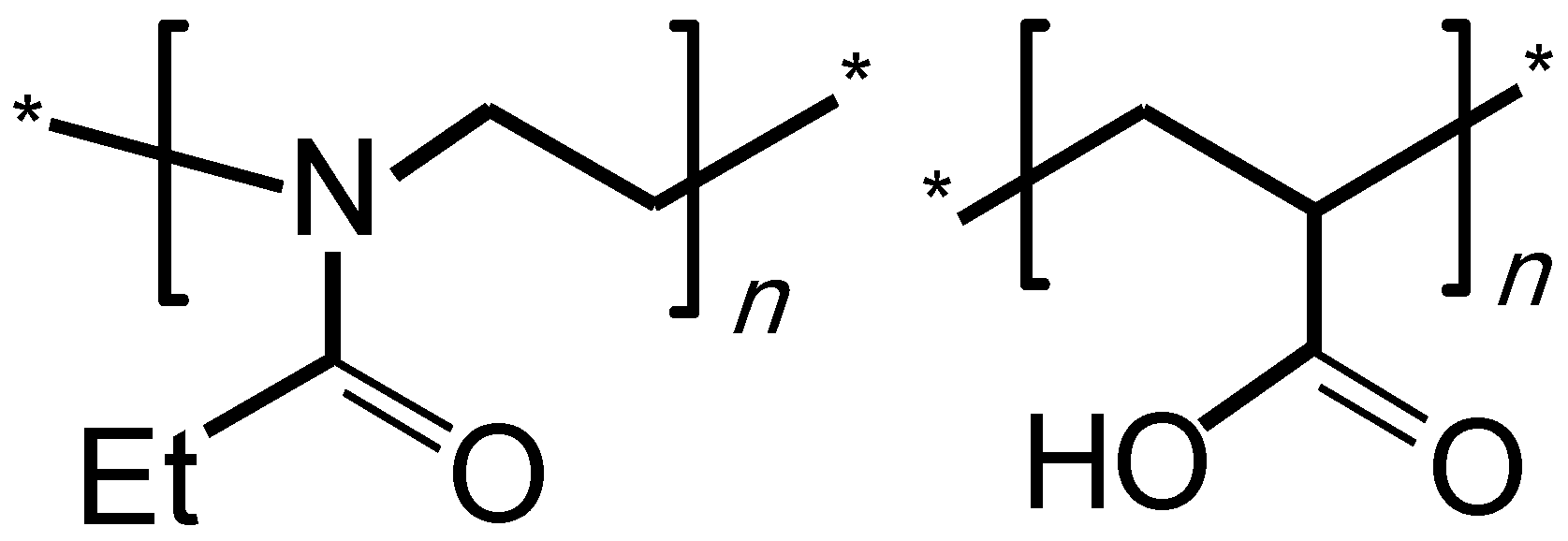
2.1. Materials
2.2. Film Fabrication
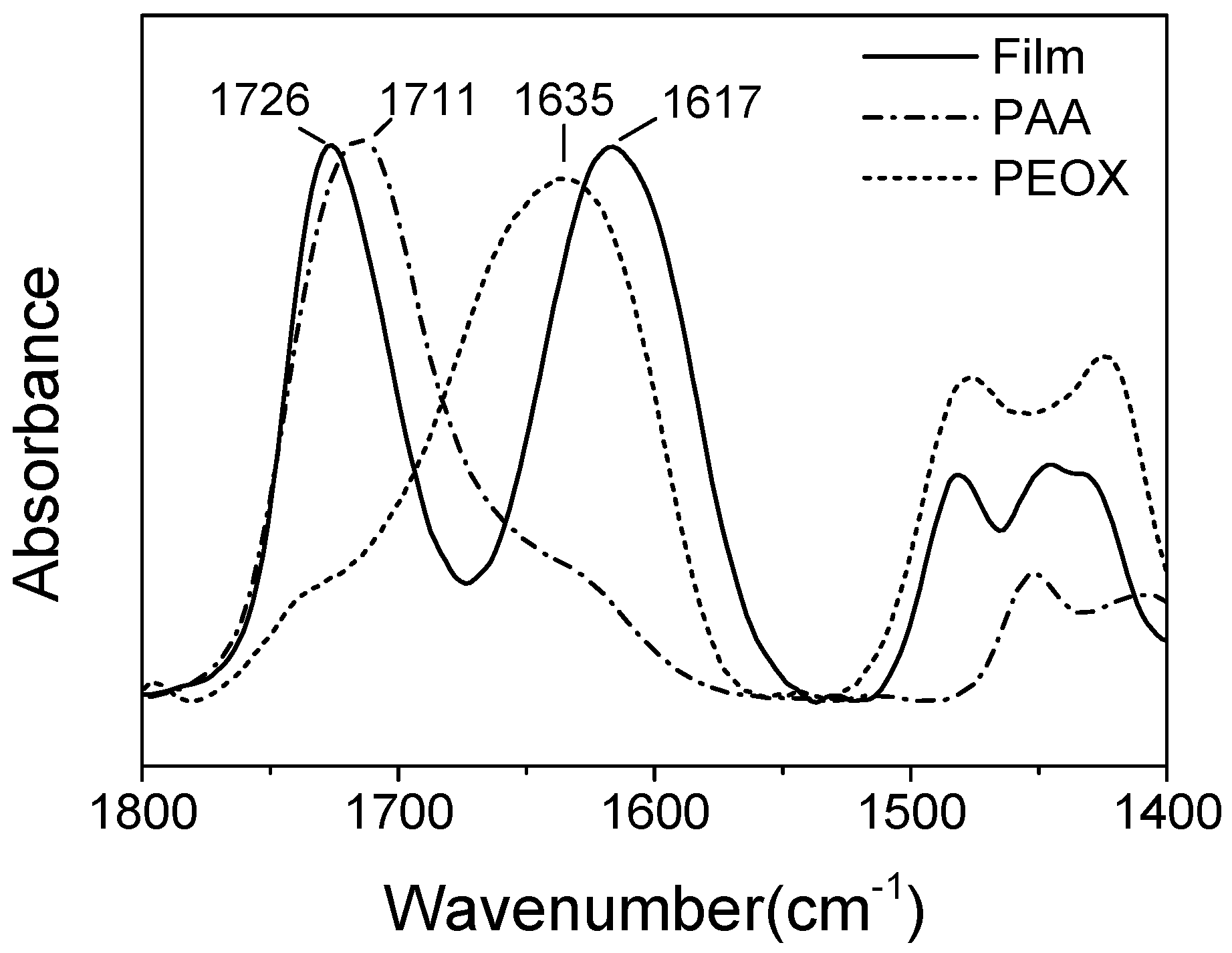
2.3. Characterization
3. Results and Discussion
3.1. Film Growth Mode
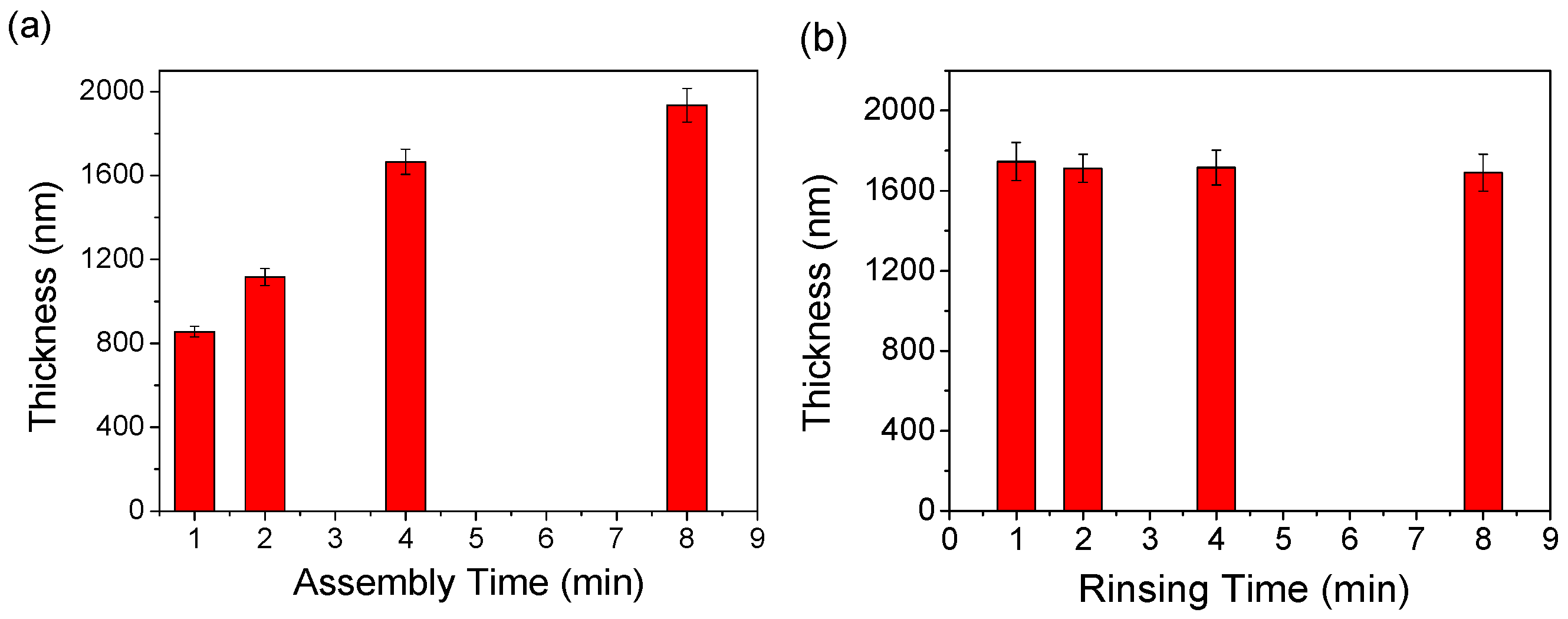
3.2. Assembling Time and Rinsing Time
3.3. Temperature
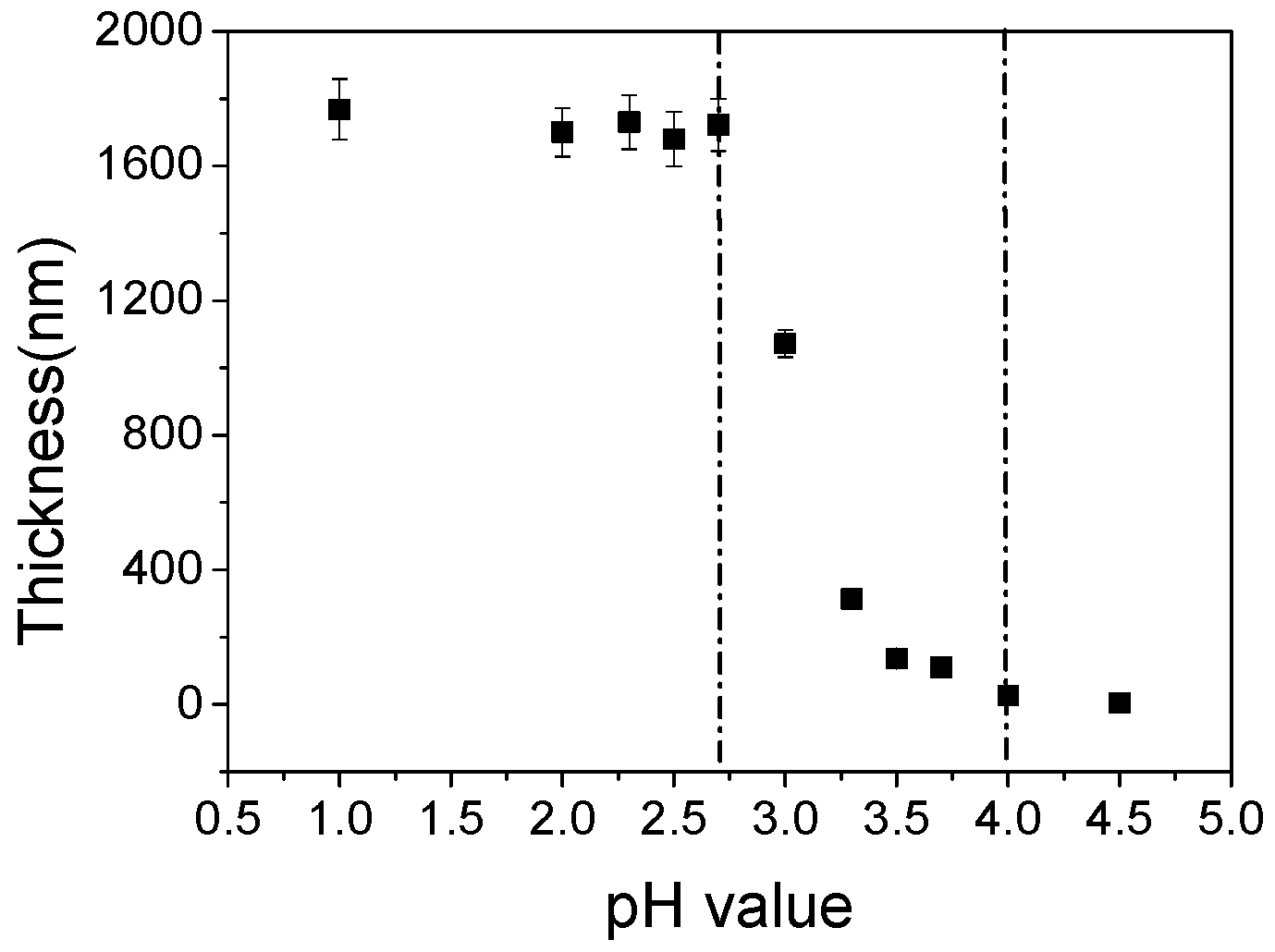
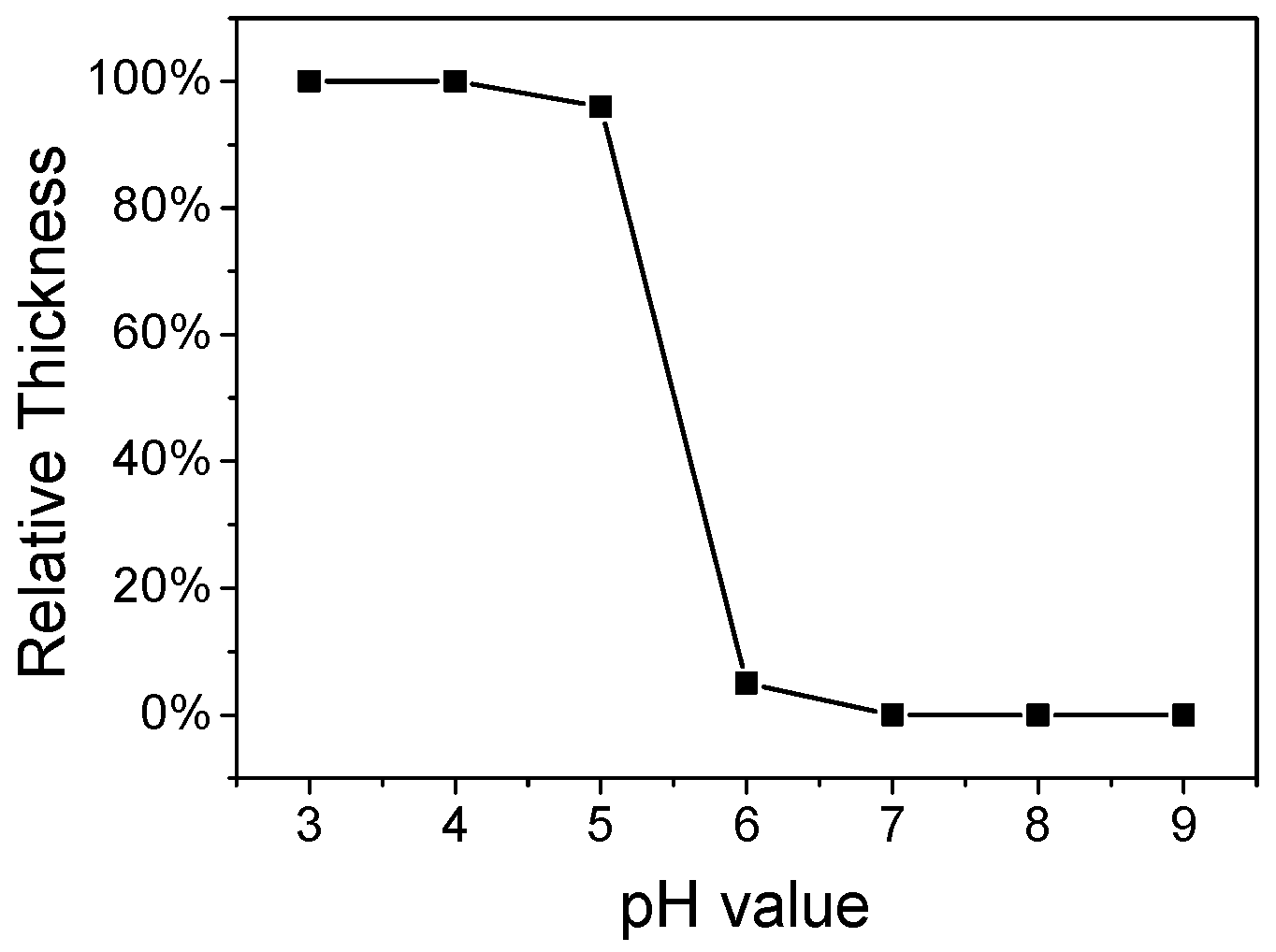
3.4. pH Value
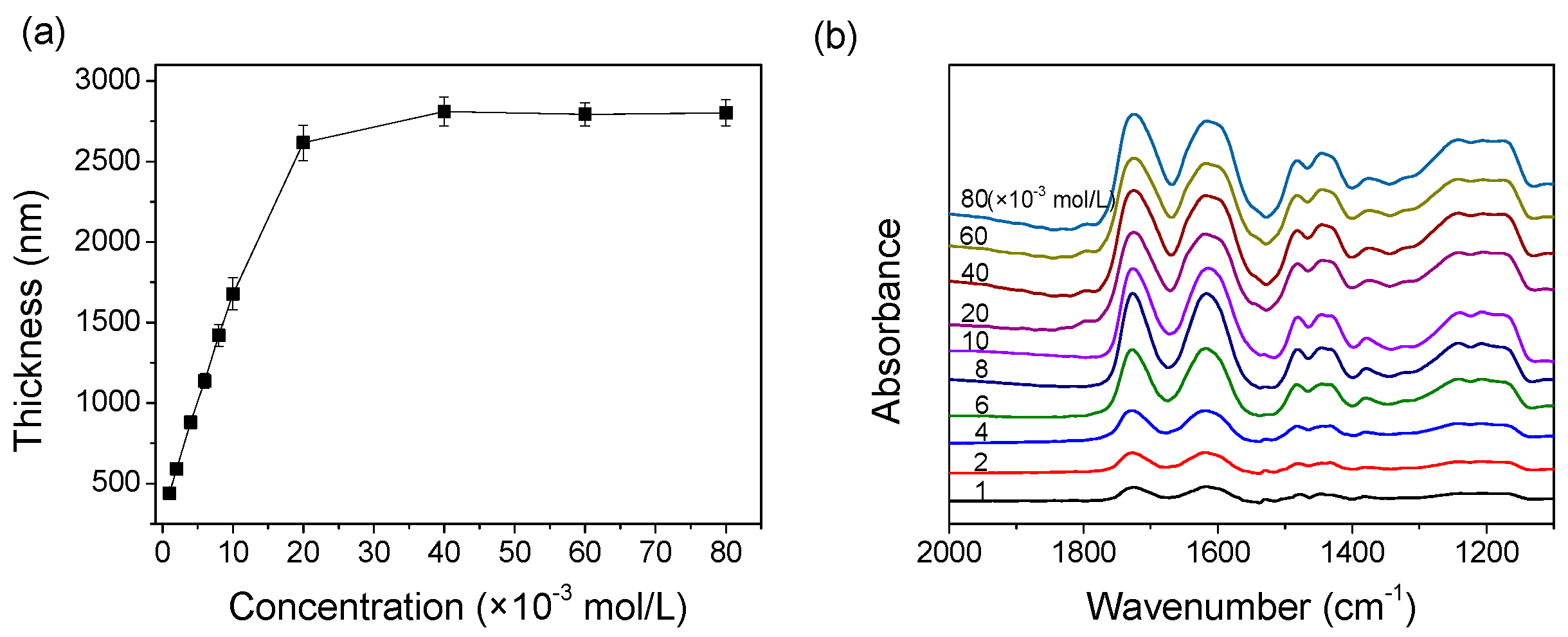
3.5. Concentration
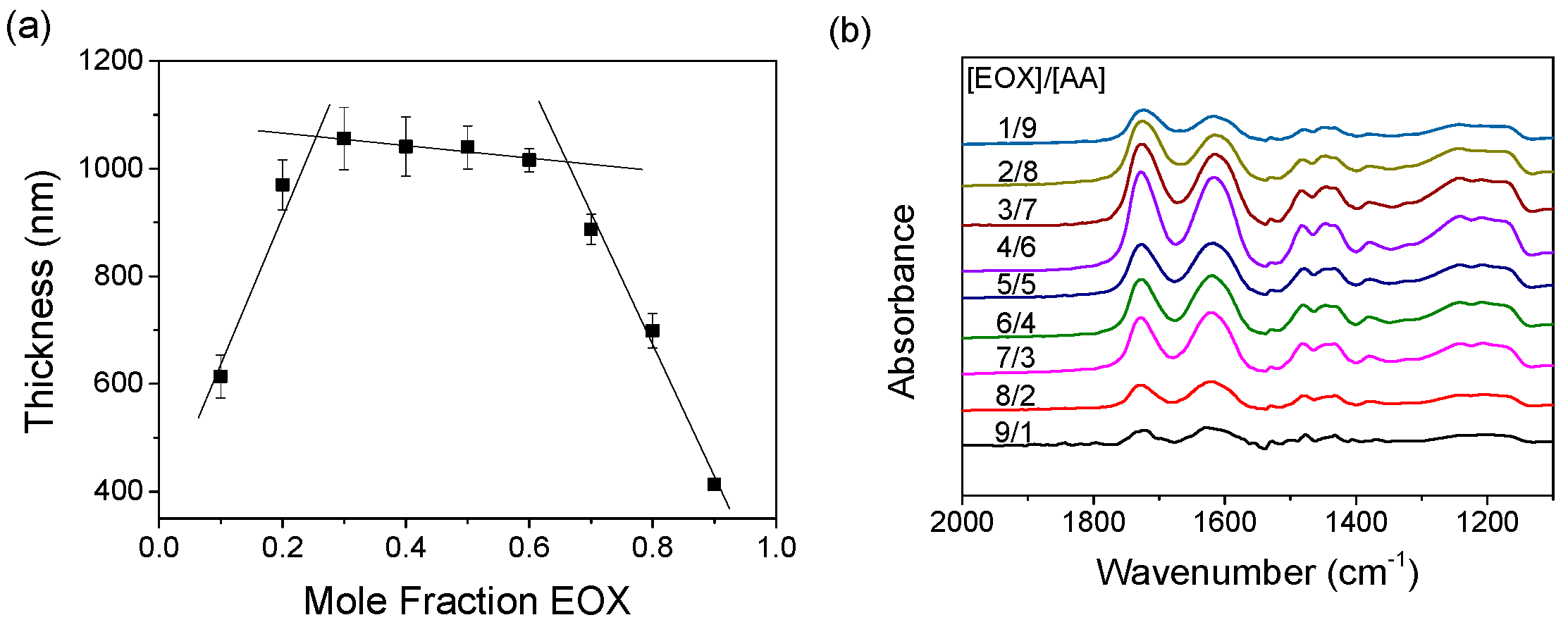
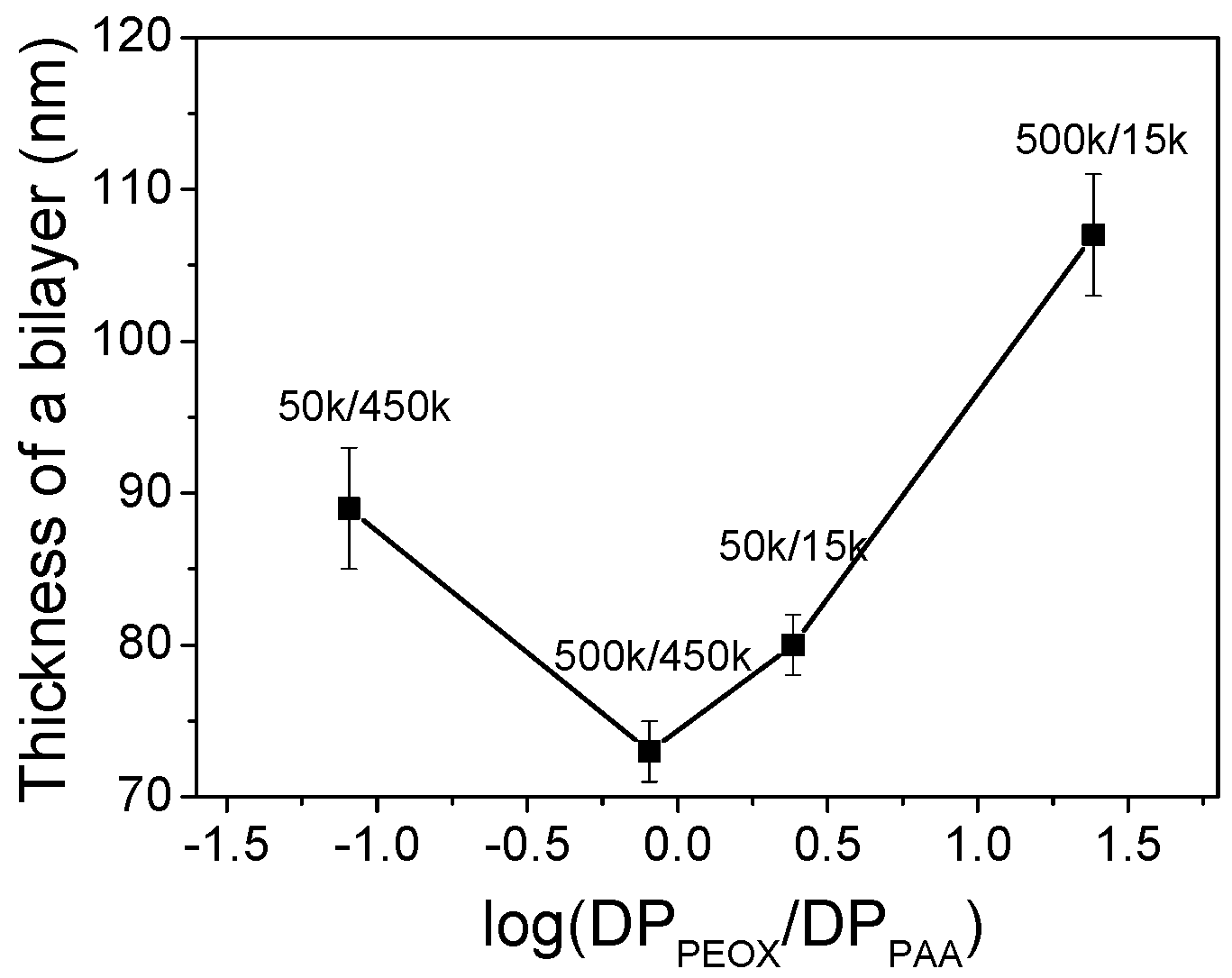
3.6. Molecular Weight
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomalia, D.A.; Sheetz, D.P. Homopolymerization of 2-alkyl- and 2-aryl-2-oxazolines. J. Polym. Sci. Part A 1966, 4, 2253–2265. [Google Scholar] [CrossRef]
- Seeliger, W.; Aufderhaar, E.; Diepers, W.; Feinauer, R.; Nehring, R.; Their, W.; Hellmann, H. Recent syntheses and reactions of cyclic imidic esters. Angew. Chem. Int. Ed. 1966, 5, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Kagiya, T.; Narisawa, S.; Maeda, T.; Fukui, K. Ring-opening polymerization of 2-substituted 2-oxazolines. J. Polym. Sci. Part B 1966, 4, 441–445. [Google Scholar] [CrossRef]
- Levy, A.; Litt, M. Polymerization of cyclic imino ethers. I. Oxazolines. J. Polym. Sci. Part B 1967, 5, 871–879. [Google Scholar]
- Gaertner, F.C.; Luxenhofer, R.; Blechert, B.; Jordan, R.; Essler, M. Synthesis, biodistribution and excretion of radiolabeled poly(2-alkyl-2-oxazoline)s. J. Control. Release 2007, 119, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Popelka, A.; Kronek, J.; Novák, I.; Kleinová, A.; Micusík, M.; Spírková, M.; Omastová, M. Surface modification of low-density polyethylene with poly(2-ethyl-2-oxazoline) using a low-pressure plasma treatment. Vacuum 2014, 100, 53–56. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Chiang, H.Z.; Wang, C.H.; Juang, T.M. Nonviral gene carriers based on diblock copolymers of poly(2-ethyl-2-oxazoline) and linear polyethylenimine. Bioconjugate Chem. 2006, 17, 781–786. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, V.R.; Tempelaar, S.; Dubois, P.; Hoogenboom, R.; Mespouille, L. Poly(2-ethyl-2-oxazoline)-block-polycarbonate block copolymers: From improved end-group control in poly(2-oxazoline)s to chain extension with aliphatic polycarbonate through a fully metal-free ring-opening polymerization process. Polym. Chem. 2016, 7, 1559–1568. [Google Scholar] [CrossRef]
- Christova, D.; Velichkova, R.; Loos, W.; Goethals, E.J.; Prez, F.D. New thermo-responsive polymer materials based on poly(2-ethyl-2-oxazoline) segments. Polymer 2003, 44, 2255–2261. [Google Scholar] [CrossRef]
- Chapman, R.; Bouten, P.J.M.; Hoogenboom, R.; Jolliffe, K.A.; Perrier, S. Thermoresponsive cyclic peptide-poly(2-ethyl-2-oxazoline) conjugate nanotubes. Chem. Commun. 2013, 49, 6522–6524. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Clash, C.; Pearce, E.M.; Kwei, T.K. Solubility and miscibility of poly(ethyl oxazoline). J. Phys. Chem. B 1988, 26, 603–619. [Google Scholar] [CrossRef]
- Park, C.; McAlvin, J.E.; Fraser, C.L.; Thomas, E.L. Iron cluster and microstructure formation in metal-centered star block copolymers: Amphiphilic iron tris(bipyridine)-centered polyoxazolines. Chem. Mater. 2002, 14, 1225–1230. [Google Scholar] [CrossRef]
- Mees, M.A.; Hoogenboom, R. Functional poly(2-oxazoline)s by direct amidation of methyl ester side chains. Macromolecules 2015, 48, 3531–3538. [Google Scholar] [CrossRef] [Green Version]
- Lava, K.; Verbraeken, B.; Hoogenboom, R. Poly(2-oxazoline)s and click chemistry: A versatile toolbox toward multi-functional polymers. Eur. Polym. J. 2015, 65, 98–111. [Google Scholar] [CrossRef] [Green Version]
- De la Rosa, V.R.; Zhang, Z.Y.; de Geest, B.G.; Hoogenboom, R. Colorimetric logic gates based on poly(2-alkyl-2-oxazoline) coated gold nanoparticles. Adv. Funct. Mater. 2015, 25, 2511–2519. [Google Scholar] [CrossRef]
- Aoi, K.; Okada, M. Polymerization of oxazolines. Prog. Polym. Sci. 1996, 21, 151–208. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Hoogenboom, R. Poly(2-oxazoline)s: A polymer class with numerous potential applications. Angew. Chem. 2009, 48, 7978–7994. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.C.; Bae, Y.H.; Kim, S.W. Electrically erodible polymer gel for controlled release of drugs. Nature 1991, 354, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process. I. consecutive adsorption of anionic and cationic bipolar amphiphiles charged surfaces, Makromol. Chem. Macromol. Symp. 1991, 46, 321–327. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D. Buildup of ultrathin multilayer films by a self-assembly process: II. Consecutive adsorption of anionic and cationic bipolar amphiphiles and polyelectrolytes on charged surfaces. Ber. Bunsenges. Phys. Chem. 1991, 95, 1430–1434. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process: III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210, 831–835. [Google Scholar] [CrossRef]
- Stockton, W.B.; Rubner, M.F. Molecular-level processing of conjugated polymers. 4. layer-by-layer manipulation of polyaniline via hydrogen-bonding interactions. Macromolecules 1997, 30, 2717–2725. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wang, Z.Q.; Zhang, X.; Shen, J.C.; Chi, L.F.; Fuchs, H. A new approach for the fabrication of an alternating multilayer film of poly(4-vinylpyridine) and poly(acrylic acid) based on hydrogen bonding. Macromol. Rapid Commun. 1997, 18, 509–514. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Lavalle, P.; Voegel, J.C.; Vautier, D.; Senger, B.; Schaaf, P.; Ball, V. Dynamic aspects of films prepared by a sequential deposition of species: Perspectives for smart and responsive materials. Adv. Mater. 2011, 23, 1191–1221. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Sun, J.Q. Fabrication of macroporous films with closed honeycomb-like pores from exponentially growing layer-by-layer assembled polyelectrolyte multilayers. Chem. Asian J. 2014, 9, 2063–2067. [Google Scholar] [CrossRef] [PubMed]
- Shibraen, M.H.M.A.; Yagoub, H.; Zhang, X.J.; Xu, J.; Yang, S.G. Anti-fogging and anti-frosting behaviors of layer-by-layer assembled cellulose derivative thin film. Appl. Surf. Sci. 2016, 370, 1–5. [Google Scholar] [CrossRef]
- Pavlukhina, S.; Sukhishvili, S. Polymer assemblies for controlled delivery of bioactive molecules from surfaces. Adv. Drug Deliv. Rev. 2011, 63, 822–836. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.X.; Su, C.; Zhang, X.J.; Yin, W.J.; Xu, J.; Yang, S.G. Reversible swelling-shrinking behavior of hydrogen-bonded free-standing thin film stabilized by catechol reaction. Langmuir 2015, 31, 5147–5154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, M.; Tian, L.L.; Guan, Y.; Zhang, Y.J. Release of polyphenolic drugs from dynamically bonded layer-by-layer films. ACS Appl. Mater. Interfaces 2013, 5, 3541–3548. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.D.; Chen, J.; Tian, H.Y.; Chen, X.S.; Sun, J.Q. Layer-by-layer assembled polymeric films for differential release of dual drugs. Chem. J. Chin. Univ. 2015, 11, 2342–2348. [Google Scholar]
- Chen, X.C.; Ren, K.F.; Zhang, J.H.; Li, D.D.; Zhao, E.; Zhao, Z.J.; Xu, Z.K.; Ji, J. Humidity-triggered self-healing of microporous polyelectrolyte multilayer coating for hydrophobic drug delivery. Adv. Funct. Mater. 2015, 25, 7470–7477. [Google Scholar] [CrossRef]
- Ma, S.M.; Qi, X.C.; Cao, Y.G.; Yang, S.G.; Xu, J. Hydrogen bond detachment in polymer complexes. Polymer 2013, 54, 5382–5390. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Guan, Y.; Yang, S.G.; Xu, J.; Han, C.C. Fabrication of hollow capsules based on hydrogen bonding. Adv. Mater. 2003, 15, 832–835. [Google Scholar] [CrossRef]
- Li, J.F.; Wang, Z.L.; Wen, L.G.; Nie, J.; Yang, S.G.; Xu, J.; Cheng, S.Z.D. Highly elastic fibers made from hydrogen-bonded polymer complex. ACS Macro Lett. 2016, 5, 814–818. [Google Scholar] [CrossRef]
- Zhuk, A.; Pavlukhina, S.; Sukhishvili, S.A. Hydrogen-bonded layer-by-layer temperature-triggered release films. Langmuir 2009, 25, 14025–14029. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Johnston, A.P.R.; Such, G.K.; Zelikin, A.N.; Caruso, F. Next generation, sequentially assembled ultrathin films: Beyond electrostatics. Chem. Soc. Rev. 2007, 36, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Archer, W.L. Determination of hansen solubility parameters for selected cellulose ether derivatives. Ind. Eng. Chem. Res. 1991, 30, 2292–2298. [Google Scholar] [CrossRef]
- Wang, B.; Yang, P.; Li, Y.Q.; He, Y.S.; Zhu, R.Q.; Gu, Y. Blends of polybenzoxazine/poly(acrylic acid): Hydrogen bonds and enhanced performances. Polym. Int. 2017, 66, 1159–1163. [Google Scholar] [CrossRef]
- Raposo, M.; Pontes, R.S.; Mattoso, L.H.C.; Oliveira, O.N., Jr. Kinetics of adsorption of poly(o-methoxyaniline) self-assembled films. Macromolecules 1997, 30, 6095–6101. [Google Scholar] [CrossRef]
- Pontes, R.S.; Raposo, M.; Camilo, C.S.; Dhanabalan, A.; Ferreira, M.; Oliveira, O.N., Jr. Non-equilibrium adsorbed polymer layers via hydrogen bonding. Phys. Status Solidi A 1999, 173, 41–50. [Google Scholar] [CrossRef]
- Raposo, M.; Oliveira, O.N., Jr. Energies of adsorption of poly(o-methoxyaniline) layer-by-layer films. Langmuir 2000, 16, 2839–2844. [Google Scholar] [CrossRef]
- Chen, F.L.; Pearce, E.M.; Kwei, T.K. Intermacromolecular complexes by in situ polymerization. Polymer 1988, 29, 2285–2289. [Google Scholar] [CrossRef]
- Matsuda, Y.; Takatsuji, K.; Shiokawa, Y.; Kikuchi, M.; Kidoaki, S.; Takahara, A.; Tasaka, S. Characterization of complexes formed by mixing aqueous solutions of poly(2-ethyl-2-oxazoline) and poly(methacrylic acid) with a wide range of concentrations. Polymer 2013, 54, 1896–1904. [Google Scholar] [CrossRef]
- Yang, S.G.; Ma, S.M.; Wang, C.Y.; Xu, J.; Zhu, M.F. Polymer complexation by hydrogen bonding at the interface. Aust. J. Chem. 2014, 67, 11–21. [Google Scholar] [CrossRef]
- Sundaramurthy, A.; Vergaelen, M.; Maji, S.; Auzély-Velty, R.; Zhang, Z.Y.; de Geest, B.G.; Hoogenboom, R. Hydrogen bonded multilayer films based on poly(2-oxazoline)s and tannic acid. Adv. Healthc. Mater. 2014, 10, 2040–2047. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca Antunes, A.B.; Dierendonck, M.; Vancoillie, G.; Remon, J.P.; Hoogenboom, R.; Geest, B.D. Hydrogen bonded polymeric multilayer films assembled below and above the cloud point temperature. Chem. Commun. 2013, 10, 1039–1041. [Google Scholar] [CrossRef] [PubMed]
- Kempe, K.; Ng, S.L.; Noi, K.F.; Müllner, M.; Gunawan, S.T.; Caruso, F. Clickable poly(2-oxazoline) architectures for the fabrication of low-fouling polymer capsules. ACS Macro Lett. 2013, 2, 1069–1072. [Google Scholar] [CrossRef]
- Kempe, K.; Ng, S.L.; Gunawan, S.T.; Noi, K.F.; Caruso, F. Intracellularly degradable hydrogen-bonded polymer capsules. Adv. Funct. Mater. 2014, 24, 6187–6194. [Google Scholar] [CrossRef]
- Li, Y.; Pan, T.; Ma, B.; Liu, J.; Sun, J. Healable antifouling films composed of partially hydrolyzed poly(2-ethyl-2-oxazoline) and poly(acrylic acid). ACS Appl. Mater. Interfaces 2017, 9, 14429–14436. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ozaki, Y.; Nakashima, K. Infrared. Raman, and Near-Infrared spectroscopic evidence for the coexistence of various hydrogen-bond forms in poly(acrylic acid). Macromolecules 1997, 30, 1111–1117. [Google Scholar] [CrossRef]
- Koenig, J.F.; Martel, D. Applying UV-Vis spectroscopy to step-by-step molecular self-assembly on surface: Does it bring pertinent information. Thin Solid Films 2008, 516, 3865–3872. [Google Scholar] [CrossRef]
- Yang, S.G.; Tan, S.X.; Zhang, Y.J.; Xu, J.; Zhang, X.L. Fabry-Pérot fringes of hydrogen-bonded assembly films. Thin Solid Films 2008, 516, 4018–4024. [Google Scholar] [CrossRef]
- Guan, Y.; Yang, S.G.; Zhang, Y.J.; Xu, J.; Han, C.C.; Kotov, N.A. Fabry-Perot fringes and their application to study the film growth, chain rearrangement, and erosion of hydrogen-bonded PVPON/PAA films. J. Phys. Chem. B 2006, 110, 13484–13490. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Decher, G.; Möhwald, H. Assembly, structural characterization, and thermal behavior of layer-by-layer deposited ultrathin films of poly(vinyl sulfate) and poly(allylamine). Langmuir 1993, 9, 481–486. [Google Scholar] [CrossRef]
- Mjahed, H.; Porcel, C.; Senger, B.; Chassepot, A.; Netter, P.; Gillet, P.; Decher, G.; Voegel, J.C.; Schaaf, P.; Benkirane-Jessel, N.; et al. Micro-stratified architectures based on successive stacking of alginate gel layers and poly(l-lysine)-hyaluronic acid multilayer films aimed at tissue engineering. Soft Matter 2008, 4, 1422–1429. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Shuttleworth, P.S. Layer-by-Layer assembly of biopolyelectrolytes onto thermo/pH-responsive micro/nano-gels. Materials 2014, 7, 7472–7512. [Google Scholar] [CrossRef] [PubMed]
- Boudou, T.; Crouzier, T.; Auzély-Velty, R.; Glinel, K.; Picart, C. Internal composition versus the mechanical properties of polyelectrolyte multilayer films: The influence of chemical cross-linking. Langmuir 2009, 25, 13809–13819. [Google Scholar] [CrossRef] [PubMed]
- Picart, C.; Mutterer, J.; Richert, L.; Luo, Y.; Prestwich, G.D.; Schaaf, P.; Voegel, J.C.; Lavalle, P. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl. Acad. Sci. USA 2002, 99, 12531–12535. [Google Scholar] [CrossRef] [PubMed]
- Abdelkebir, K.; Gaudiere, F.; Morin-Grognet, S.; Coquerel, G.; Labat, B.; Atmani, H.; Ladam, G. Evidence of different growth regimes coexisting within biomimetic Layer-by-Layer films. Soft Matter 2011, 7, 9197–9205. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Ferreira, Q.; Ribeiro, P.A.; Raposo, M. Villain’s fractal growth of poly[1-[4-(3-carboxy-4-hydroxyphenylazo) benzenesulfonamido]-1,2-ethanediyl. sodium salt] J-aggregates onto layer-by-layer films and its effect on film absorbance spectrum. J. Appl. Phys. 2013, 113, 243508. [Google Scholar] [CrossRef]
- Haynie, D.T.; Cho, E.; Waduge, P. “In and out diffusion” hypothesis of exponential multilayer film buildup revisited. Langmuir 2011, 27, 5700–5704. [Google Scholar] [CrossRef] [PubMed]
- Fetters, L.J.; Lohse, D.J.; Richter, D.; Witten, T.A.; Zirkel, A. Connection between polymer molecular weight, density, chain dimension, and melt viscoelastic properties. Macromolecules 1994, 27, 4639–4647. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Retrospective on the future of polyelectrolyte multilayers. Langmuir 2009, 25, 14007–14010. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.G.; Zhang, Y.J.; Zhang, X.Y.; Guan, Y.; Xu, J.; Zhang, X.L. From cloudy to transparent: Chain rearrangement in hydrogen-bonded layer-by-layer assembled films. Chem. Phys. Chem. 2007, 8, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Ambreen, J.; Siddiq, M. Effect of arm number of poly(acrylic acid) on cloud point temperature of poly(2-ethyl-2-oxazoline). J. Polym. Res. 2014, 21, 608–615. [Google Scholar] [CrossRef]
- Quinn, J.F.; Caruso, F. Facile tailoring of film morphology and release properties using layer-by-layer assembly of thermoresponsive materials. Langmuir 2004, 20, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.H.; Yang, S.G.; Li, Y.F.; Xu, X.; Xu, J. Effect of temperature on the build-up and post hydrothermal processing of hydrogen-bonded PVPON/PAA film. Soft Matter 2011, 7, 9435–9443. [Google Scholar] [CrossRef]
- Choi, J.; Rubner, M.F. Influence of the degree of ionization on weak polyelectrolyte multilayer assembly. Macromolecules 2005, 38, 116–124. [Google Scholar] [CrossRef]
- Yang, S.G.; Zhang, Y.J.; Zhang, X.L.; Xu, J. The influence of pH on a hydrogen-bonded assembly film. Soft Matter 2007, 3, 463–469. [Google Scholar] [CrossRef]
- Delongchamp, D.M.; Hammond, P.T. Highly ion conductive poly(ethylene oxide)-based solid polymer electrolytes from hydrogen bonding layer-by-layer assembly. Langmuir 2004, 20, 5403–5411. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Chang, Y.; Yoon, J.-S.; Kim, C.; Kwon, I.C.; Kim, Y.-H.; Jeong, S.Y. Synthesis and micellar characterization of amphiphilic diblock copolymers based on poly(2-ethyl-2-oxazoline) and aliphatic polyesters. Macromolecules 1999, 32, 1847–1852. [Google Scholar] [CrossRef]
- Izquierdo, A.; Ono, S.S.; Voegel, J.-C.; Schaaf, P.; Decher, G. Dipping versus spraying: Exploring the deposition conditions for speeding up layer-by-layer assembly. Langmuir 2005, 21, 7558–7567. [Google Scholar] [CrossRef] [PubMed]
- Kharlampieva, E.; Sukhishvili, S.A. Hydrogen-bonded layer-by-layer polymer films. Polym. Rev. 2006, 46, 377–395. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Sun, J.; Zhang, X.; Shen, D.; Yang, S. Hydrogen-Bonded Polymer Complex Thin Film of Poly(2-oxazoline) and Poly(acrylic acid). Polymers 2017, 9, 363. https://doi.org/10.3390/polym9080363
Su C, Sun J, Zhang X, Shen D, Yang S. Hydrogen-Bonded Polymer Complex Thin Film of Poly(2-oxazoline) and Poly(acrylic acid). Polymers. 2017; 9(8):363. https://doi.org/10.3390/polym9080363
Chicago/Turabian StyleSu, Chao, Jiaxing Sun, Xuejian Zhang, Duan Shen, and Shuguang Yang. 2017. "Hydrogen-Bonded Polymer Complex Thin Film of Poly(2-oxazoline) and Poly(acrylic acid)" Polymers 9, no. 8: 363. https://doi.org/10.3390/polym9080363